Abstract
The membrane-distal region of the cytoplasmic domain of human granulocyte colony-stimulating factor receptor (G-CSF-R) contains four conserved tyrosine residues: Y704, Y729, Y744, and Y764. Three of these (Y729, Y744, and Y764) are located in the C-terminal part of G-CSF-R, previously shown to be essential for induction of neutrophilic differentiation. To determine the role of the tyrosines in G-CSF–mediated responses, we constructed tyrosine-to-phenylalanine (Y-to-F) substitution mutants and expressed these in a differentiation competent subclone of 32D cells that lacks endogenous G-CSF-R. We show that all tyrosines can be substituted essentially without affecting the differentiation signaling properties of G-CSF-R. However, substitution of one specific tyrosine, ie, Y764, markedly influenced proliferation signaling as well as the timing of differentiation. 32D cells expressing wild-type (WT) G-CSF-R (or mutants Y704F, Y729F, or Y744F) proliferated in G-CSF–containing cultures until day 8 and then developed into mature neutrophils. In contrast, 32D/Y764F cells arrested in the G1 phase of the cell cycle within 24 hours and showed complete neutrophilic differentiation after 3 days of culture. This resulted in an average 30-fold reduction of neutrophil production as compared with the 32D/WT controls. Importantly, G-CSF–mediated activation of Shc, p21Ras and the induction of c-myc were severely reduced by substitution of Y764. These findings indicate that Y764 of G-CSF-R is crucial for maintaining the proliferation/differentiation balance during G-CSF–driven neutrophil development and suggest a role for multiple signaling mechanisms in maintaining this balance.
GRANULOCYTE colony-stimulating factor (G-CSF) is a 20- to 25-kD glycoprotein secreted by bone marrow stroma cells, macrophages, fibroblasts, and endothelial cells. G-CSF stimulates the proliferation, survival, and differentiation of myeloid progenitor cells towards neutrophilic granulocytes.1,2G-CSF–deficient mice show chronic neutropenia and a reduced granulopoietic response to infections, indicating that G-CSF plays an essential role in the regulation of granulopoiesis in both steady-state and stress conditions.3 The biological effects of G-CSF are mediated through a cell-surface receptor that is a member of the hematopoietin or class I cytokine receptor superfamily and that forms homodimeric complexes upon ligand binding.4 5
Like other members of the hematopoietin receptor superfamily, the G-CSF receptor (G-CSF-R) lacks intrinsic tyrosine kinase activity but activates cytoplasmic tyrosine kinases, in particular of the Jak family.2,5,6 Jaks associate with the membrane-proximal cytoplasmic region of the hematopoietin receptors and become activated on ligand-induced receptor dimerization.7,8 Jak activation leads to tyrosine phosphorylation of the STAT (signal transducer and activator of transcription) proteins, which form homodimers and/or heterodimers, translocate to the nucleus, and activate target genes by interaction with specific DNA sequences. G-CSF stimulation results in the activation of Jak1, Jak2, STAT1, STAT3, and STAT5.8-11
Tyrosine kinase activity induced by G-CSF also results in the rapid phosphorylation of four conserved cytoplasmic tyrosines (Y) of the G-CSF-R protein (Y704, Y729, Y744, and Y764) located in the region distal to the conserved box 2 sequence.9,12 These phosphotyrosines form potential binding sites for signaling molecules that contain src homology 2 (SH2) domains.13 For instance, Y704 of G-CSF-R, fitting the YXXQ consensus sequence for SH2-STAT3 binding, is involved in the recruitment and activation of STAT3.14,15 Activation of Shc and SHP-2 (Syp), SH2-containing signaling intermediates of the p21Ras pathway, also requires recruitment via tyrosine residues of G-CSF-R.16Notably, this depends on binding and activation of Jak kinases via the membrane-proximal region.17
To accomplish neutrophilic differentiation in murine myeloid cell lines (32D, L-GM, or FDCP1), signals provided by the C-terminal region of G-CSF-R, spanning approximately 100 amino acids, are indispensible.18 19 This so-called differentiation domain of G-CSF-R contains three of the four cytoplasmic tyrosines (Y729, Y744, and Y764). To what extent the cytoplasmic tyrosines of G-CSF-R contribute to G-CSF–mediated proliferation and differentiation induction in myeloid cells has not been established.
In this study, we examined the consequences of tyrosine-to-phenylalanine (Y-to-F) substitutions in the cytoplasmic domain of G-CSF-R for the transduction of proliferation and differentiation signals in differentiation competent 32D cells. We show that all tyrosines can be replaced without affecting G-CSF–induced differentiation. Substitution of Y704, Y729, or Y744 had no effect on proliferation signaling. In contrast, mutant Y764F failed to support G-CSF–induced cell cycle progression from the G1 to the S phase, resulting in accelerated differentiation and significantly reduced net production of mature neutrophils. Strikingly, we found that activation of Shc, p21Ras and induction of c-myc are all mediated via Y764 of G-CSF-R, indicative of a potential role of these signaling molecules in maintaining the proliferation/differentiation balance in neutrophil development.
MATERIALS AND METHODS
G-CSF-R constructs and transfectants.
Human G-CSF-R cDNA was cloned in the eukaryotic expression vector LNCX.20 Polymerase chain reaction techniques were used to generate the Y-to-F mutants Y704F, Y729F, Y744F, and Y764F, as described previously.16 A subline of the interleukin-3 (IL-3)–dependent murine myeloid cell line 32D,21 called 32D.C10,22 was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10 ng/mL of murine IL-3. The LNCX expression constructs were linearized by Pvu I digestion and transfected into 32D.C10 cells by electroporation. After 48 hours of incubation, cells were selected with G418 (GIBCO-BRL, Breda, The Netherlands) at a concentration of 0.8 mg/mL. Multiple clones were expanded for further analysis. To determine G-CSF-R expression levels, cells were incubated at 4°C for 60 minutes with 10 μg/mL of biotinylated mouse antihuman G-CSF-R monoclonal antibody LMM741 (PharMingen, San Diego, CA). After washing, cells were treated at 4°C for 60 minutes with 5 μg/mL of phycoerythrin (PE)-conjugated streptavidin. Samples were analyzed by flow cytometry using a FACSCAN (Becton Dickinson, San Jose, CA).
Cell proliferation and morphological analysis.
To determine proliferation, cells were incubated at an initial density of 2 × 105 cells/mL in 10% FCS/RPMI medium supplemented with 100 ng/mL of human G-CSF, 10 ng/mL of murine IL-3, or without growth factors. The medium was replenished every 2 to 4 days, and the cell densities were adjusted to 2 to 4 × 105cells/mL. Viable cells were counted on the basis of trypan blue exclusion. To analyze the morphologic features, cells were spun onto glass slides and examined after May-Grünwald-Giemsa staining.
Cell cycle analysis.
For flow cytometric analysis of DNA content, cells were collected by centrifugation and resuspended in 0.1% sodium citrate containing 50 μg/mL of propidium iodide. The fluorescence of the stained cells was measured using a FACSCAN (Becton Dickinson). The Cell Fit program was used to determine the percentages of cells in the different phases of the cell cycle.
Shc immunoprecipitation.
p21Ras activation assay.
Cells (1 × 107) were deprived of serum and growth factors and labeled by incubation for 3 hours in phosphate-free Dulbecco's modified Eagle's medium containing 100 μCi/mL of carrier-free [32P]orthophosphate. Subsequently, the cells were stimulated for 5 minutes at 37°C with human G-CSF (1 μg/mL), murine IL-3 (1 μg/mL), or without factors (control). Cell lysis, immunoprecipitation of p21Ras with monoclonal antibody Y13-259, and thin-layer chromatography were then performed as previously described.24 GTP binding to p21Ras was expressed as a percentage of total p21Ras-bound guanine nucleotide (GTP + GDP) determined with a phosphorimager.
Analysis of c-myc expression.
Cells were deprived of serum and growth factors for 4 hours and subsequently stimulated with human G-CSF (1 μg/mL) or murine IL-3 (1 μg/mL). At several time points, RNA was extracted from the cells using the Ultraspec-II RNA isolation system (Biotecx Laboratories Inc, Houston, TX). Agarose-formaldehyde gel electrophoresis and transfer to filters (Hybond; Amersham Life Sciences, Amersham, UK) was performed using standard procedures. As probes, a 1.4-kbEcoRI-HindIII fragment comprising the entire coding sequence of murine c-myc and a 777-bpHindIII-EcoRI human GAPDH fragment (control) were 32P-labeled by random priming (Boehringer, Mannheim, Germany).
RESULTS
Experimental model.
Tyrosine-to-phenylalanine substitution mutants of G-CSF-R are depicted in Fig 1A. Expression vectors encoding the various G-CSF-R cDNAs were introduced into a subline of the IL-3–dependent murine myeloid cell line 32D, called 32D.C10, that do not express endogenous G-CSF-R. In 32D.C10 cells transfected with the wild-type (WT) G-CSF-R cDNA, G-CSF induces transient proliferation followed by terminal neutrophilic differentiation after 8 to 11 days of culture.22 Expression levels of the different G-CSF-R proteins in the transfected 32D.C10 cells were determined by flow cytometry using G-CSF-R antibodies (Fig1B). Several independent clones of each mutant with approximately equivalent G-CSF-R levels were selected for further analysis.
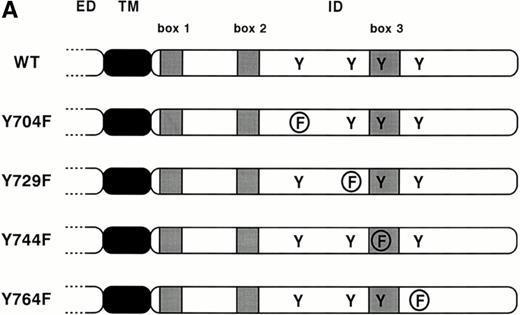
The G-CSF-R mutants. (A) Schematic diagram of the cytoplasmic domains of the G-CSF-R proteins. Boxes 1, 2, and 3 denote subdomains conserved in several members of the hematopoietin receptor superfamily. ED, extracellular domain; TM, transmembrane domain; ID, intracellular domain; Y, tyrosine; F, phenylalanine. (B) Flow cytometric analysis of G-CSF-R expressio on 32D.C10 transfectants. Cells were either stained with biotinylated anti–G-CSF-R antibodies followed by PE-conjugated streptavidin (solid lines) or with PE-conjugated streptavidin alone (dotted lines).
The G-CSF-R mutants. (A) Schematic diagram of the cytoplasmic domains of the G-CSF-R proteins. Boxes 1, 2, and 3 denote subdomains conserved in several members of the hematopoietin receptor superfamily. ED, extracellular domain; TM, transmembrane domain; ID, intracellular domain; Y, tyrosine; F, phenylalanine. (B) Flow cytometric analysis of G-CSF-R expressio on 32D.C10 transfectants. Cells were either stained with biotinylated anti–G-CSF-R antibodies followed by PE-conjugated streptavidin (solid lines) or with PE-conjugated streptavidin alone (dotted lines).
Mutation of Y764 of G-CSF-R inhibits proliferation but accelerates neutrophilic differentiation.
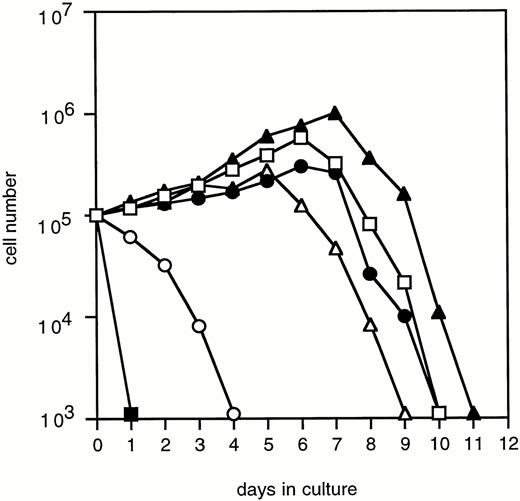
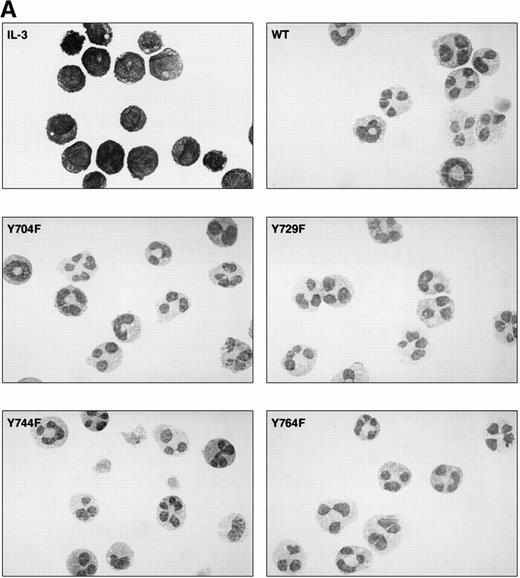
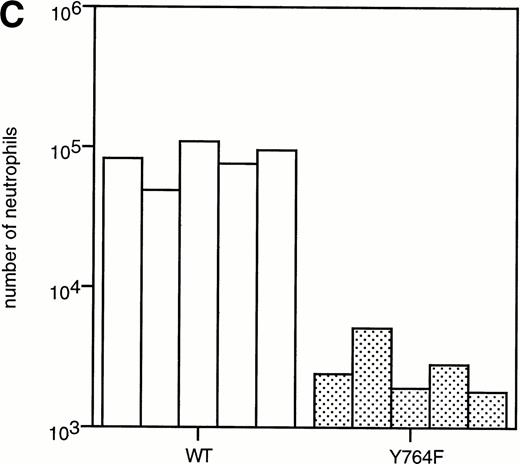
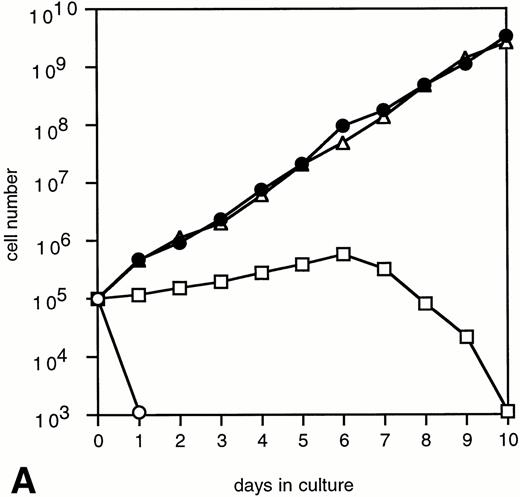
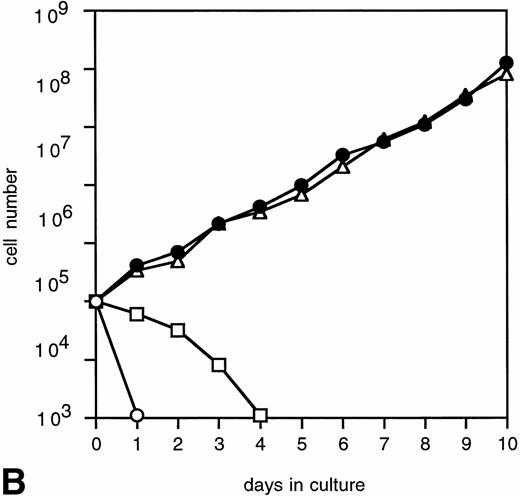
To determine the abilities of WT and mutant G-CSF-R to induce proliferation and neutrophilic differentiation, 32D.C10 transfectants were switched from IL-3– to G-CSF–containing medium after extensive washing to remove residual IL-3. The experiments described below were performed and repeated on at least three independent clones of each mutant. Without IL-3 or G-CSF, all transfectants died within 1 day and showed no signs of neutrophilic differentiation. Parental 32D.C10 cells and cells transfected with empty LNCX vector also died within 1 day in G-CSF–containing medium. The 32D.C10 cells expressing WT G-CSF-R (32D/WT) proliferated in response to G-CSF for 6 to 8 days (Fig 2). After 8 to 11 days, 32D/WT cells developed into terminally differentiated neutrophils, showing an enlarged cytoplasm-to-nucleus ratio, neutrophilic cytoplasm, lobulated nuclei, granules, and expression of the murine neutrophil-specific surface antigen GR-1 (Fig 3A and B and data not shown). Similar results were obtained with 32D/Y704F, 32D/Y729F, and 32D/Y744F cells. In contrast, 32D/Y764F cells did not proliferate in G-CSF–containing medium and showed terminal differentiation after 2 to 4 days, instead of 8 to 11 days. On average, this inappropriate balance of proliferation/differentiation resulted in a 30-fold reduced production of neutrophils as compared with 32D/WT cells (Fig 3C). Furthermore, 3H-thymidine uptake assays after G-CSF stimulation showed that induction of DNA synthesis by mutant Y764F was severely reduced on day 1 and absent on day 2 of culture (data not shown). Stimulation of 32D/WT and 32D/Y764F cells with the combination of IL-3 and G-CSF resulted in proliferation rates similar to those obtained with IL-3 alone (Fig 4). In the presence of IL-3, G-CSF did not induce neutrophilic differentiation, indicating that IL-3–induced proliferation completely overrules the differentiation signaling by WT G-CSF-R and by mutant Y764F.
Proliferation of 32D.C10 transfectants in response to G-CSF. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (□) WT; (•) Y704F; (▵) Y729F; (▴) Y744F; (○) Y764F; (▪) LNCX.
Proliferation of 32D.C10 transfectants in response to G-CSF. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (□) WT; (•) Y704F; (▵) Y729F; (▴) Y744F; (○) Y764F; (▪) LNCX.
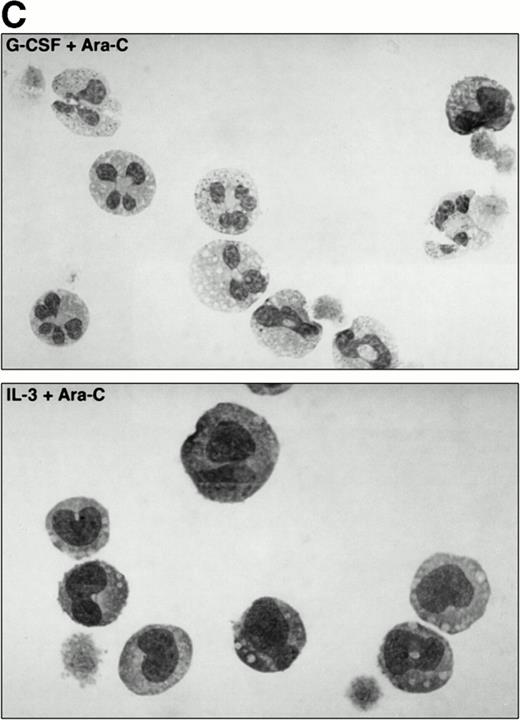
Neutrophilic differentiation of 32D.C10 transfectants in response to G-CSF. (A) Morphology of 32D.C10 transfectants maintained in IL-3–containing medium (IL-3) or cultured for 8 to 11 days (WT, Y704F, Y729F, and Y744F) or for 3 days (Y764F) in the presence of G-CSF (May-Grünwald-Giemsa staining; original magnification × 630). (B) The percentages of terminally differentiated 32D.C10 transfectants cultured in G-CSF–containing medium. At least 200 cells were scored at the indicated times. (□) WT; (•) Y704F; (▵) Y729F; (▴) Y744F; (○) Y764F. (C) Net amount of mature neutrophils derived from 1 × 105 32D/WT and 32D/Y764F cells cultured in G-CSF–containing medium for 8 or 3 days, respectively. Data from five independent clones are shown.
Neutrophilic differentiation of 32D.C10 transfectants in response to G-CSF. (A) Morphology of 32D.C10 transfectants maintained in IL-3–containing medium (IL-3) or cultured for 8 to 11 days (WT, Y704F, Y729F, and Y744F) or for 3 days (Y764F) in the presence of G-CSF (May-Grünwald-Giemsa staining; original magnification × 630). (B) The percentages of terminally differentiated 32D.C10 transfectants cultured in G-CSF–containing medium. At least 200 cells were scored at the indicated times. (□) WT; (•) Y704F; (▵) Y729F; (▴) Y744F; (○) Y764F. (C) Net amount of mature neutrophils derived from 1 × 105 32D/WT and 32D/Y764F cells cultured in G-CSF–containing medium for 8 or 3 days, respectively. Data from five independent clones are shown.
Proliferation of 32D/WT (A) and 32D/Y764F (B) cells in response to G-CSF and/or IL-3. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (○) Without cytokines; (□) G-CSF; (•) G-CSF + IL-3; (▵) IL-3.
Proliferation of 32D/WT (A) and 32D/Y764F (B) cells in response to G-CSF and/or IL-3. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (○) Without cytokines; (□) G-CSF; (•) G-CSF + IL-3; (▵) IL-3.
Y764 of G-CSF-R is essential for G-CSF–induced cell cycle progression from G1 to S phase.
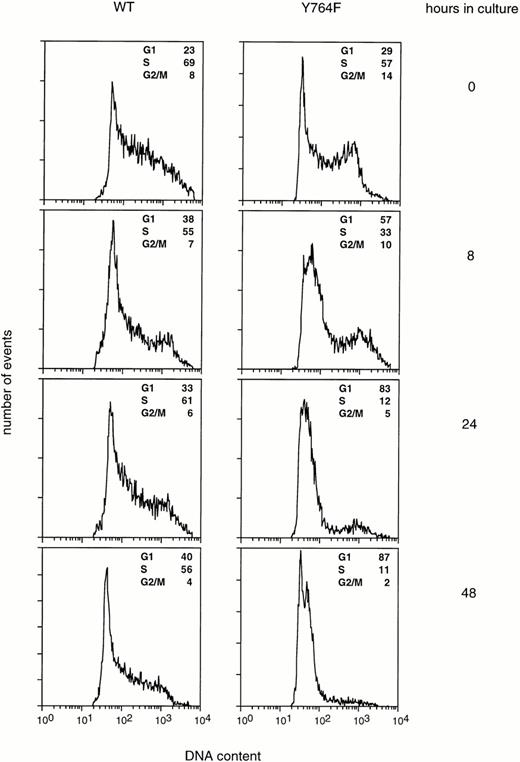
Cell cycle profiles of 32D/WT and 32D/Y764F cells were analyzed by flow cytometry (Fig 5). In the presence of IL-3, the majority of 32D/WT and 32D/Y764F cells were in S phase. After transfer to G-CSF–containing medium, the cell cycle distribution of 32D/WT cells changed only slightly during the first 48 hours, in agreement with the observation that the cells continued to proliferate at this stage of culture (Fig 2). In contrast, the number of 32D/Y764F cells in S phase had already significantly decreased 8 hours after the switch to G-CSF. After 48 hours, 87% of cells were in G1 and only 11% in S phase, with 35% of the total showing terminal neutrophilic differentiation. These results indicate that mutation of Y764 abrogates G-CSF–mediated cell cycle progression from the G1 to the S phase.
Cell cycle distribution of 32D/WT and 32D/Y764F cells at various time points after transfer to G-CSF–containing medium. The cells were stained with propidium iodide, and their DNA contents were analyzed by flow cytometry. The percentages of cells in the G1, S, and G2/M phases of the cell cycle are given in the upper right of each panel.
Cell cycle distribution of 32D/WT and 32D/Y764F cells at various time points after transfer to G-CSF–containing medium. The cells were stained with propidium iodide, and their DNA contents were analyzed by flow cytometry. The percentages of cells in the G1, S, and G2/M phases of the cell cycle are given in the upper right of each panel.
The WT G-CSF-R also induces accelerated differentiation in G1-arrested cells.
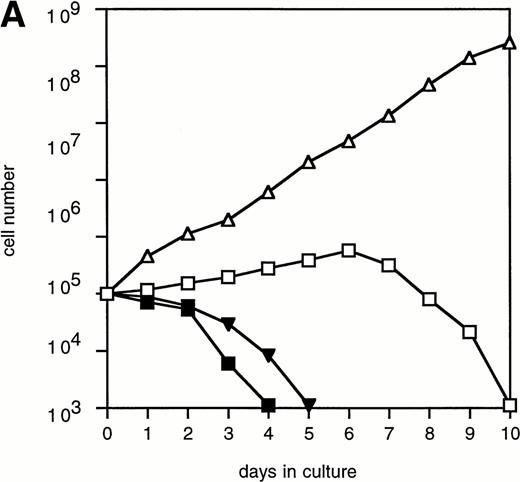
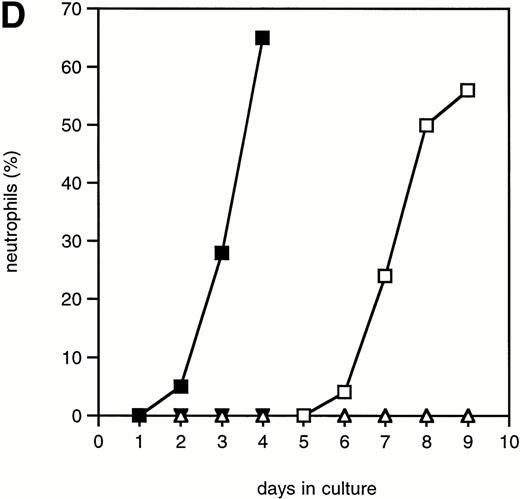
To investigate whether accelerated neutrophilic differentiation in G-CSF–stimulated 32D/Y764F cells is the direct consequence of the lack of proliferation, we cultured 32D/WT cells in the presence of the cell cycle inhibitor cytosine arabinoside (Ara-C). Concentrations of Ara-C required to inhibit G-CSF– and IL-3–mediated proliferation with minimal cytotoxicity were 10−6 M and 10−5 mol/L, respectively (data not shown). At these concentrations of Ara-C, cells cultured in IL-3– or G-CSF–containing medium gradually lost viability and died after 4 to 5 days (Fig 6A). Cell cycle analysis showed that the cells were arrested in G1 (Fig 6B). In the G-CSF–containing cultures, terminal neutrophilic differentiation occurred after 2 to 4 days in the presence and after 7 to 9 days in the absence of Ara-C (Fig6C and D). Thus, 32D/WT cells arrested in G1 by Ara-C also show accelerated differentiation in response to G-CSF. In contrast, IL-3 did not induce neutrophilic differentiation in G1-arrested 32D/WT cells (Fig 6C). Essentially similar results were obtained using hydroxyurea as a cell cycle inhibitor (data not shown).
The effect of Ara-C on proliferation and differentiation of 32D/WT cells. (A) G-CSF– and IL-3–dependent proliferation of 32D/WT cells in the absence or presence of Ara-C. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (□) G-CSF; (▪) G-CSF + Ara-C; (▵) IL-3; (▾) IL-3 + Ara-C. (B) Cell cycle distribution of 32D/WT cells cultured for 3 days in G-CSF– or IL-3–containing medium in the absence or presence of Ara-C. The percentages of cells in the G1, S, and G2/M phases of the cell cycle are given in the upper right of the panels. (C) Morphology of 32D/WT cells cultured for 3 days in G-CSF– or IL-3–containing medium in the presence of Ara-C (May-Grünwald-Giemsa staining; original magnification × 630). (D) The percentages of terminally differentiated 32D/WT cells cultured in G-CSF– or IL-3–containing medium in the absence or presence of Ara-C. At least 200 cells were scored at the indicated times. Symbols are the same as in (A).
The effect of Ara-C on proliferation and differentiation of 32D/WT cells. (A) G-CSF– and IL-3–dependent proliferation of 32D/WT cells in the absence or presence of Ara-C. The numbers of viable cells were determined on the basis of trypan blue exclusion at the indicated times. (□) G-CSF; (▪) G-CSF + Ara-C; (▵) IL-3; (▾) IL-3 + Ara-C. (B) Cell cycle distribution of 32D/WT cells cultured for 3 days in G-CSF– or IL-3–containing medium in the absence or presence of Ara-C. The percentages of cells in the G1, S, and G2/M phases of the cell cycle are given in the upper right of the panels. (C) Morphology of 32D/WT cells cultured for 3 days in G-CSF– or IL-3–containing medium in the presence of Ara-C (May-Grünwald-Giemsa staining; original magnification × 630). (D) The percentages of terminally differentiated 32D/WT cells cultured in G-CSF– or IL-3–containing medium in the absence or presence of Ara-C. At least 200 cells were scored at the indicated times. Symbols are the same as in (A).
Activation of Shc and p21Ras and expression of c-myc are mediated via Y764 of G-CSF-R.
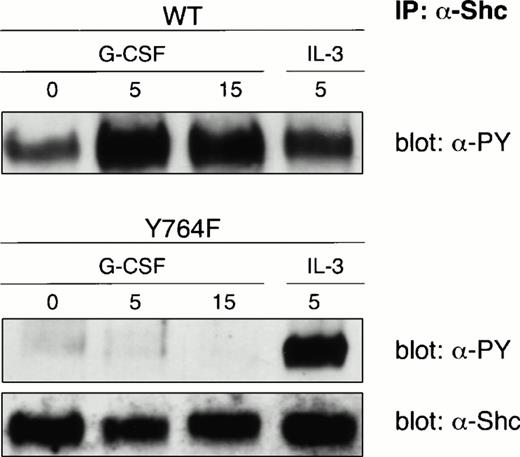
We previously showed in lymphoid BAF/B03 cells that Shc/Grb2 and SHP-2/Grb2 complexes, implicated in activation of p21Ras by a variety of receptor systems, are both activated by G-CSF-R.16Multiple tyrosines in G-CSF-R mediate the formation of SHP-2/Grb2 complexes, but Shc activation and Shc/Grb2 association critically depend on Y764 of G-CSF-R.16 We first confirmed that Y764 is also essential for G-CSF–induced Shc activation in myeloid 32D.C10 transfectants (Fig 7). Ras-loading assays indicated that activation of WT G-CSF-R resulted in an approximately eightfold increase of p21Ras-GTP as compared with nontreated controls (Fig 8, middle panel). In contrast, activation of mutant Y764F induced a marginal increase of p21Ras-GTP over background levels (Fig 8, right panel). Control cells transfected with empty LNCX vector showed no activation of p21Ras in response to G-CSF (Fig 8, left panel).
Shc immunoprecipitation on lysates from 32D/WT and 32D/Y764F cells. Serum- and growth factor-deprived cells were stimulated for 5 or 15 minutes at 37°C with G-CSF or IL-3. The blots were hybridized with antiphosphotyrosine antibodies and reprobed with anti-Shc antibodies to confirm equal loading of Shc.
Shc immunoprecipitation on lysates from 32D/WT and 32D/Y764F cells. Serum- and growth factor-deprived cells were stimulated for 5 or 15 minutes at 37°C with G-CSF or IL-3. The blots were hybridized with antiphosphotyrosine antibodies and reprobed with anti-Shc antibodies to confirm equal loading of Shc.
Activation of p21Ras in 32D.C10 transfectants. Serum- and growth factor-deprived cells were labeled with [32P]orthophosphate and subsequently stimulated for 5 minutes at 37°C without factor (−), with G-CSF, or with IL-3. Cells were lysed and p21Ras was collected by immunoprecipitation. Nucleotides bound to p21Ras were eluted and separated by thin-layer chromatography. The positions of the GTP and GDP standards are indicated. The level of p21Ras-GTP, expressed as a percentage of total nucleotide bound to p21Ras (GTP + GDP), is given under each lane.
Activation of p21Ras in 32D.C10 transfectants. Serum- and growth factor-deprived cells were labeled with [32P]orthophosphate and subsequently stimulated for 5 minutes at 37°C without factor (−), with G-CSF, or with IL-3. Cells were lysed and p21Ras was collected by immunoprecipitation. Nucleotides bound to p21Ras were eluted and separated by thin-layer chromatography. The positions of the GTP and GDP standards are indicated. The level of p21Ras-GTP, expressed as a percentage of total nucleotide bound to p21Ras (GTP + GDP), is given under each lane.
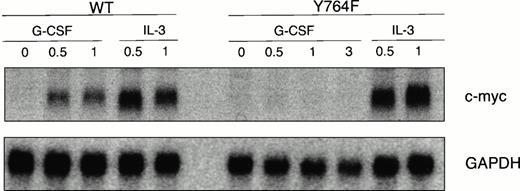
Because it has recently been established that Shc is involved in the induction of c-myc expression via a novel Grb2- and p21Ras-independent mechanism,25 26 we also investigated the ability of G-CSF-R mutant Y764 to activate c-myc. We found that G-CSF–induced expression of c-myc was severely reduced in 32D/Y764F cells as compared with 32D/WT cells, whereas responses to IL-3 had not changed (Fig 9).
Induction of c-myc expression in 32D/WT and 32D/Y764F cells. Serum- and growth factor-deprived cells were stimulated for 0.5, 1, or 3 hours at 37°C with G-CSF or IL-3. Total RNA (10 μg) was analyzed by Northern blot hybridization using a32P-labeled c-myc probe. The blot was reprobed withGAPDH to confirm equal loading.
Induction of c-myc expression in 32D/WT and 32D/Y764F cells. Serum- and growth factor-deprived cells were stimulated for 0.5, 1, or 3 hours at 37°C with G-CSF or IL-3. Total RNA (10 μg) was analyzed by Northern blot hybridization using a32P-labeled c-myc probe. The blot was reprobed withGAPDH to confirm equal loading.
DISCUSSION
Previously, we have investigated the contribution of the cytoplasmic tyrosine residues of G-CSF-R to signaling using BAF/B03 cell transfectants expressing Y-to-F substitution mutants.15,16These studies provided information on the specific involvement of these tyrosines in the activation of signaling substrates of the Jak/STAT and p21Ras signaling pathways. However, replacement of the tyrosines did not affect the proliferation signaling abilities of G-CSF-R. This was not unexpected, because earlier work had shown that truncated forms of G-CSF-R, which lack all tyrosine residues and which fail to mediate proliferation in myeloid 32D or L-GM cells, still efficiently transduced proliferation signals in BAF/B03 cells.18 27Apparently, proliferation control mechanisms mediated via regions C-terminal of the box 2 consensus domain are bypassed in BAF/B03 cells. This, combined with their inability to differentiate towards the myeloid lineage, makes BAF/B03 cells not suitable for studying the coupled proliferation/differentiation response to G-CSF.
We reported here on four observations relevant to the balanced proliferation and differentiation response to G-CSF in differentiation competent 32D cells. (1) A single tyrosine (Y764) of human G-CSF-R plays a key role in maintaining this balance by activation of mechanisms that control cell cycle progression at the level of G1 to S transition. (2) Shc, p21Ras, and Myc are activated via this tyrosine. (3) Inhibition of proliferation does not prevent but accelerates terminal differentiation. (4) Enforced cell cycle arrest does not induce terminal neutrophilic differentiation in the absence of G-CSF.
Activation of p21Ras via Y764 of G-CSF-R is likely to play a major role in G-CSF–induced proliferative responses. Previous studies provided evidence for the involvement of p21Ras in IL-3– or G-CSF–induced cell cycle progression in myeloid cells. Expression of a dominant inhibitory mutant of p21Ras blocked G-CSF–mediated proliferation of 32Dcl3 cells and caused a G1 arrest.28 Further, enforced expression of constitutively active Raf-1, a downstream target of p21Ras, promoted cell cycle progression, whereas c-raf antisense oligonucleotides inhibited G-CSF–induced proliferation of 32Dcl3 cells.29 30
In addition to p21Ras, Myc has been implicated in the control of proliferation of myeloid cells. Depending on the cell system, enforced expression of c-myc either induced cell cycle progression or premature apoptosis, indicative of the dualistic role played by Myc in controlling these processes.31-33 Conversely, inhibition ofc-myc with antisense oligonucleotides inhibited proliferation and permitted differentiation in HL-60 cells.34Importantly, p21Ras and Myc have recently been found to collaborate in activation of cyclinE/cdk2 complexes.35 Thus, it is likely that the activation of p21Ras and induction of c-myc expression via Y764 of G-CSF-R both play a crucial role in the control of cell cycle progression and thereby in the timing of neutrophilic differentiation. Finally, Y763 of murine G-CSF-R (analogous to human Y764) has been implicated in yet another signaling mechanism, ie, involving activation of JNK/SAPK.36 Although the significance of this finding for the regulation of cell cycle progression is not yet clear, it underscores the significance of this tyrosine residue for G-CSF-R function.
The docking protein Shc is activated by different members of the hematopoietin receptor superfamily. By virtue of its binding via Y317 to Grb2, Shc has been implicated primarily in the activation of p21Ras, Raf-1, and ERK1/ERK2.37 Recently, a second mechanism of Shc-mediated signaling was discovered.25,26 This route requires residues Y239/240 instead of Y317 of the Shc protein and involves expression of the c-myc gene. Intriguingly, depending on the receptor type and cell system, distinct cellular responses have been associated with Shc. For instance, Inhorn et al38showed that activation of Shc by granulocyte-macrophage colony-stimulating factor (GM-CSF) is mediated via Y750 of the GM-CSF receptor β-chain and that site-directed mutagenesis of this tyrosine results in reduced cellular viability of BAF/B03 transfectants in response to GM-CSF. On the other hand, overexpression of Shc proteins in GM-CSF–dependent TF-1 cells increases the proliferative response to GM-CSF, in agreement with a role for Shc in mitogenic signaling.39 Finally, Y599 of the c-Mpl receptor has been implicated in Shc phosphorylation and macrophage differentiation in WEHI3B-D+ cells.40 With regard to the role of Shc in G-CSF-R function, our data are most consistent with a model in which two pathways, Shc to p21Ras and Shc to Myc, both activated via Y764 of G-CSF-R, are involved in the regulation of G-CSF–driven cell cycle progression but are not required for execution of the neutrophilic differentiation program.
Previously, we detected point mutations in the G-CSF-R gene in patients with severe congenital neutropenia who showed disease progression to acute myeloid leukemia.22,41 These mutations introduce premature stop codons between amino acids 715 and 731 and result in the deletion of the C-terminal cytoplasmic region of the receptor that is essential for the induction of neutrophilic differentiation.18,19,22 Contrary to the transient proliferative responses provided by WT G-CSF-R, activation of G-CSF-R mutants lacking the C-terminal differentiation domain resulted in sustained and enhanced proliferation in myeloid cells. Paradoxically, such truncated G-CSF-R were found to activate p21Ras and c-mycat levels comparable to WT G-CSF-R, despite the fact that they lack Y764 (De Koning et al, unpublished data). The mechanisms by which this is achieved are not yet clear, but do not appear to involve Shc, because Shc is not detectably activated by these truncated forms of G-CSF-R.16 Most likely, activation of p21Ras is mediated via SHP-2/Grb2 complexes that bind to Y704.16 As yet, we have no data to explain why these alternative mechanisms are not activated by G-CSF-R mutant Y764. One possibility is that this is due to negative interference, caused by the configuration of full-length G-CSF-R.
We found that mutation of Y704, Y729, Y744, or Y764 did not affect the ability of G-CSF-R to transduce differentiation signals in 32D.C10 cells. In contrast, Yoshikawa et al12 reported that substitution of Y703 or Y728 of the murine G-CSF-R (analogous to human Y704 and Y729, respectively) prevented G-CSF–induced neutrophilic differentiation in L-GM-1 transfectants. It is relevant to note that, although the cells used by Yoshikawa et al12 showed expression of myeloperoxidase transcripts in response to G-CSF, cytologically the cells did not show the characteristics of terminally mature neutrophils as observed in our study. A further complication is that the experiments with the L-GM-1 cells were performed with pools of five or six clones for each mutant.12 In such pools, outgrowth of one or more clones with growth advantage can easily take place. This could result in the selective loss of G-CSF–mediated growth arrest and, consequently, the absence of neutrophilic differentiation, as observed by Yoshikawa et al12 in the pools of the Y703F and Y728F transfectants. In M1 cells, differentiation in response to G-CSF was reduced by mutation of Y744 of G-CSF-R and, to a lesser extent, by mutation of Y704 and Y729.42 A crucial difference between the M1 and the 32D.C10 cell models is that M1 cells expressing WT G-CSF-R differentiate into macrophages, instead of neutrophils. Obviously, specific signaling molecules required for macrophage differentiation may differ from those involved in neutrophilic differentiation. Therefore, the tyrosine residues of G-CSF-R essential for macrophage differentiation in the M1 model need not have any relevance to neutrophilic differentiation. More recently, we observed that G-CSF also induces complete neutrophilic differentiation in 32D cells expressing the triple mutant Y729/744/764F, indicating that the tyrosines in the C-terminal part of G-CSF-R are not essential for differentiation induction (De Koning et al, unpublished data).
Our findings strongly suggest that differentiation induction by WT G-CSF-R does not require active cell cycling. Rather, downmodulation of G-CSF–mediated proliferation and accumulation in G1 appeared a prerequisite for neutrophilic differentiation (Figs 5 and6).43 44 Identification of the signaling substrates that are essential for differentiation and understanding of how these molecules influence the signals provided via Y764 of G-CSF-R will shed further light on how the balance between proliferation and differentiation is maintained at progressive stages of neutrophil development. This knowledge may provide important clues as to how this balance may be affected in diseases characterized by a disturbed production of neutrophils, such as severe congenital neutropenia and acute myeloid leukemia.
ACKNOWLEDGMENT
The authors thank Dr Kevin P. Foley (Fred Hutchinson Cancer Research Center, Seattle, WA) for providing murine c-myc cDNA, Karola van Rooyen for preparation of figures, and Drs Alister Ward and Marieke von Lindern for manuscript reading.
Supported by grants from the Dutch Cancer Society “Koningin Wilhelmina Fonds” and the Netherlands Organization for Scientific Research (NWO).
Address reprint requests to Ivo P. Touw, PhD, Institute of Hematology, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 8. Activation of p21Ras in 32D.C10 transfectants. Serum- and growth factor-deprived cells were labeled with [32P]orthophosphate and subsequently stimulated for 5 minutes at 37°C without factor (−), with G-CSF, or with IL-3. Cells were lysed and p21Ras was collected by immunoprecipitation. Nucleotides bound to p21Ras were eluted and separated by thin-layer chromatography. The positions of the GTP and GDP standards are indicated. The level of p21Ras-GTP, expressed as a percentage of total nucleotide bound to p21Ras (GTP + GDP), is given under each lane.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/6/10.1182_blood.v91.6.1924/2/m_blod4062008.jpeg?Expires=1767800293&Signature=L7huXRK-nokHIPDWtWCdCX0Fo-3hW3RB7ipaV-aAaANG91zdJNfIuH6ncpsCnklHgEUIm8y9ZNWaegu8p19lW171My0TwY4GcUu98vWltpg8-Mm76dFFHzkWjotz-6qDqFnMI77dM6nCrdvnruH0XyURjbKqj4xOYEnI20zVdCWuzjhvxorxtd4kNEVLOBws0wjIGyMMT7T7gXF2Ezj8ldAFsNqzQCtAvb3zK0p5w-Zh~Pcz6ltYJGOBUCqLmUZ6CPt9CuBRWIsbVwfsVrErSCQkE75efJDgnBPvnHY3LprRimlFTR3MZZYSzAUQpxA2ZWbGtHb~RQKU-8k3U91lWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal