Abstract
Steel factor (SLF), the ligand for the c-Kit receptor, protects hemopoietic progenitors and mast cells from apoptosis. We show here that protection of 32D-Kit cells or mast cells from apoptosis by SLF is abrogated through concurrent inhibition of Ca2+influx. In contrast, cell survival promoted by interleukin-3 is not affected by Ca2+ influx blockers. In the presence of blockers, increasing stimulation by SLF leads to greater levels of cell death in the population, indicating that it is the combination of activation by SLF with concurrent blockade of Ca2+ influx that results in apoptosis. The p815 mastocytoma, which expresses a mutated, constitutively active c-kit receptor, dies apoptotically in the presence of Ca2+ influx blockers alone. Ionomycin protects cells from SLF plus blocker-induced apoptosis, confirming specificity for Ca2+ ion blockade in cell death induction. Overexpression of bcl-2, which protects 32D-Kit cells from factor withdrawal, does not protect cells from apoptosis by SLF plus blocker. In contrast, caspase inhibitors YVAD-CHO, DEVD-FMK, and Boc-Asp-FMK protect cells from SLF plus blocker-induced death. These observations highlight the importance of SLF-stimulated Ca2+ influx in the protection of cells from apoptosis and demonstrate a new mechanism for inducing bcl-2 insensitive, caspase-dependent apoptosis through the combination of SLF stimulation with Ca2+ influx blockade.
HEMATOPOIETIC CELLS are protected from cell death by a variety of cytokines and growth factors. In the absence of these protective signals, cells undergo a series of morphologic and biochemical changes, including membrane blebbing, nuclear condensation, cell shrinkage, and DNA fragmentation, culminating within 12 to 48 hours in apoptotic or programmed cell death.1
One documented mechanism for the protection of cells from apoptosis is through the upregulation of bcl-2. The bcl-2 family of proteins are key regulators of apototic death consisting of both anti-apoptotic and pro-apoptotic members.2 Although the precise mechanism by which bcl-2 family members influence apoptosis is unknown, recent structural and biochemical evidence indicates that bcl-2 proteins perform multiple functions that may influence cell death pathways. These include physical interactions with numerous cytoplasmic proteins, formation of ion channels, and regulation of the permeability transition in mitochondria.3
For hematopoietic stem cells, myeloid progenitor cells, and mast cells, two factors that protect cells from apoptosis are Steel factor (SLF), the ligand for the c-Kit receptor tyrosine kinase, and interleukin-3 (IL-3).4-7 Upregulation of bcl-2 by IL-3 in myeloid cells has been well documented.8-10 In contrast, SLF has only been observed to upregulate bcl-2 in natural killer cells,11 indicating that the mechanism by which SLF protects myeloid cells from apoptosis may differ from that of IL-3.
One biochemical change that has been associated with the induction of apoptosis in a number of cell types is the deregulation of intracellular Ca2+ concentrations.12,13However, a general model explaining the role of Ca2+ in apoptosis remains elusive. Excessive intracellular Ca2+levels such as those induced by Ca2+ ionophore have been shown to induce apoptosis in a number of experimental systems.14,15 Apoptosis in splenocytes appears to involve a Ca2+-dependent endonuclease,16 and intracellular Ca2+ increases have been linked to apoptosis of both activated T-cell hybridomas17 and immature thymocytes.18 In contrast to these observations, some cells seem to be protected from apoptosis by Ca2+ influx. For instance, IL-3–dependent mast cells and cell lines are protected from growth factor withdrawal-mediated apoptosis by addition of Ca2+ ionophore,19 and programmed neuronal death is also suppressed by increases in intracellular Ca2+.20
SLF, unlike IL-3, stimulates the mobilization of Ca2+ from internal stores followed by influx of Ca2+ from the extracellular milieu.21 Given the importance of intracellular Ca2+ in the apoptotic process, we have investigated the effect of inhibitors of Ca2+ influx on cell survival promoted by SLF and IL-3. We show here that blockade of Ca2+ influx reverses the ability of SLF to protect cells from apoptosis, but does not affect cell viability promoted by IL-3. Notably, in the presence of Ca2+ influx blockers, higher concentrations of SLF induce greater levels of cell death, indicating that this form of apoptosis is dependent on cellular stimulation. We also show that overexpression of bcl-2 does not protect cells from the combination of SLF plus Ca2+ influx blockers, but caspase inhibitors provide significant protection. Our results therefore show a role for Ca2+ influx in SLF-mediated protection from cell death and identify a new mechanism for inducing caspase-mediated apoptosis through the combination of a growth signal with blockade of Ca2+ influx.
MATERIALS AND METHODS
Cells.
32D-Kit cells (gift from Dr Mark Minden, Toronto, Ontario, Canada) are an IL-3–dependent myelomonocytic cell line expressing c-kit.22 32D-Kit cells were grown in RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2% WEHI-3 supernatant, and 1 mg/mL G418 (GIBCO, Grand Island, NY). Bone marrow-derived mast cells (BMMC) were generated as described previously.23 They were cultured in OPTI-MEM (GIBCO) supplemented with 10% heat-inactivated FBS and 2% WEHI-3 supernatant as a source of IL-3. The P815 cell line is a murine mastocytoma.24 P815 cells were grown in RPMI supplemented with 10% heat-inactivated FBS. Bcl-2 gp+e retroviral producing NIH 3T3 packaging cells (gift from Dr Y. Ben-David, Toronto, Ontario, Canada) contain an LXSN-based retroviral vector expressing genes for both puromycin resistance and murine bcl-2. These were grown in Dulbecco's modified Eagle's medium (DMEM) and supplemented with 10% FBS and 2 μg/mL puromycin (Sigma, St Louis, MO). 32D-Kit-bcl-2 cells were generated by coculturing 32D-Kit cells with packaging cells for 24 hours. Nonadherent 32D-Kit cells were then removed, cultured for 48 hours, and then selected for bcl-2 overexpressing cells in the presence of 2 μg/mL puromycin. All cell cultures also contained 55 μmol/L β-mercaptoethanol and antibiotics (both Sigma).
Production of recombinant SLF.
Recombinant murine Steel Factor (SLF) was produced in soluble form inEscherichia coli using the pFLAG.ATS, IPTG-inducible secretion expression vector (InterScience, Markham, Ontario, Canada). This vector includes an eight amino acid N-terminal FLAG epitope. E coli containing the pFLAG.ATS.SLF plasmid were grown to an OD600 of 0.4 to 0.5 in Luria Broth at 37°C and induced overnight with 33 μg/L isopropyl-β-D-throgalactopyranoside (IPTG). A total of 1 mmol/L CaCl2 and 100 μmol/L phenylmethyl sulfonyl fluoride (PMSF) were added to the bacterial supernatant and FLAG-SLF was affinity-purified on a column of Anti-FLAG M1 mouse monoclonal antibodies covalently attached to agarose gel. FLAG-SLF was eluted with phosphate-buffered saline (PBS) + 2 mmol/L EDTA, collected, concentrated, dialyzed against PBS, and checked for purity by silver stain. Bioactivity of SLF was measured on both 32D-Kit cells and BMMC by incubating 2.5 × 104 cells in 96-well plates with varying concentrations of SLF for 36 hours, followed by a 6-hour pulse with 3H-thymidine. Incorporated radioactivity was determined by scintillation counting.
Other reagents.
Ca2+ channel blockers and ionomycin were obtained from Sigma. YVAD-CHO ICE protease inhibitor peptide was obtained from Amersham (Arlington Heights, IL). DEVD-FMK and Boc-Asp-FMK were both obtained from Enzyme Systems (Dublin, CA).
Cell death assays.
BMMC or 32D-Kit cells (2.5 × 104) were placed in 96-well flat-bottom plates in a volume of 0.1 mL RPMI containing 0.5% FBS. Cells were supplemented with either SLF or IL-3 plus Ca2+ channel blocker. The proportion of dead cells was determined after 18 or 24 hours in culture by counting cells that could or could not exclude Trypan blue.
Semisolid agar assays.
Cell death assays were plated as described above. After 18 hours of incubation, cells were washed once in RPMI containing 10% FBS and plated in 35-mm petri dishes along with 1 mg/mL G418, 25% WEHI-3 supernatant, and 0.3% agar (GIBCO). Colonies of 40 or more cells were counted after 7 days at 37°C.
Analysis of DNA content.
Cells (1.25 × 106) were incubated for 18 or 24 hours as described above. Cells were spun down and resuspended in Vindelöv's reagent (3.4 mmol/L Tris [pH 8], 75 μmol/L propidium iodide [from Sigma], 0.1% NP-40, 700 U/L RNAse [Sigma], and 10 mmol/L NaCl). Cells were then analyzed by flow cytometry.
Western blotting.
For the Bcl-2 blot, 1 × 106 32D-Kit and 32D-Kit-bcl-2 cells were washed in PBS; resuspended in TBS lysis buffer containing 1% NP-40, 10% glycerol, protease inhibitors (500 μmol/L sodium-orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mmol/L PMSF [all Sigma]); and incubated at 4°C for 20 minutes. Lysates were centrifuged at 12,000 rpm for 10 minutes, and the supernatant was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. The blot was blocked with 5% skim milk powder and 0.1% TWEEN-20 in PBS, probed with anti–bcl-2 antibodies (UBI, Lake Placid, NY) followed by a horseradish peroxidase-labeled goat antimouse secondary antibody (Jackson Immunoresearch, Mississauga, Ontario, Canada), and developed with chemiluminescence reagents (Amersham). To confirm equal protein loading, the blot was stripped by acid treatment and reprobed with an antiactin monoclonal antibody (Sigma) followed by the same horseradish peroxidase-labeled goat antimouse secondary antibody (Jackson) and developed with chemiluminescence reagents.
For the PARP cleavage blot, 2 × 106 32D-Kit cells were pretreated with combinations of 10 μmol/L econazole, SLF, IL-3, and Boc-Asp-FMK. Cells were washed in PBS, resuspended in a hypotonic lysis buffer (20 mmol/L HEPES, 10 mmol/L KCl, 1 mmol/L EDTA, and 1 mmol/L dithiothreitol) with protease inhibitors aprotinin and leupeptin (both at 1 μg/mL) and 1 mmol/L PMSF (all Sigma) and left at 4°C for 5 minutes before adding 0.1% NP40. Lysates were centrifuged at 6,000 rpm for 5 minutes at 4°C. The supernatant was removed and centrifuged again at 10,000 rpm for 10 minutes at 4°C. The nuclear pellets were resuspended in sample buffer containing 10% β-mercaptoethanol and 8 mol/L urea, boiled and sonicated for 10 seconds at a setting of 50%, resolved by 7.5% SDS-PAGE, and transferred to nitrocellulose. The blot was probed and developed as described above using a rabbit polyclonal anti-PARP antibody (UBI) that was raised against a synthetic peptide corresponding to the region C-terminal to the cleavage site of human PARP. The blot was then probed with a goat antirabbit horseradish peroxidase-labeled secondary antibody (Jackson) and then developed using chemiluminescence reagents.
RESULTS
Protection from apoptosis by SLF is reversed through blockade of Ca2+ influx.
Ca2+ influx has been observed to both induce and protect cells from apoptosis in different cell systems.14,15,19 To investigate the role of Ca2+ influx in protection from apoptosis, we determined the effect of Ca2+ influx blockers on cell viability supported by SLF, a growth factor that mobilizes Ca2+ or IL-3, a mitogenic cytokine that does not mobilize Ca2+.21,25 For these experiments, we used 32D-Kit cells, an IL-3–dependent murine myelomonocytic cell line that has been transfected with the c-kit receptor tyrosine kinase gene. Expression of c-kit in these cells makes them mitogenically responsive to SLF in vitro (Fig 1F) and renders them tumorigenic in vivo.22 These cells die apoptotically within 12 to 24 hours upon removal of factor.10
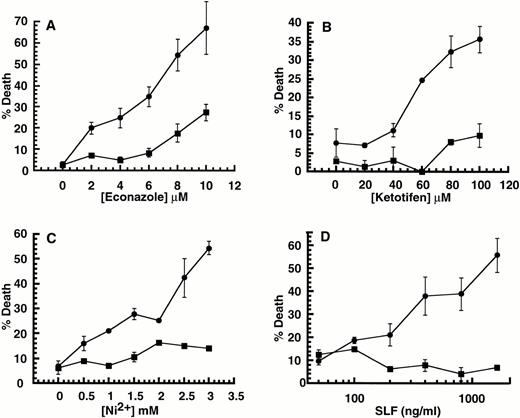
Effect of SLF or IL-3 with Ca2+ influx blockers on 32D-Kit cells. (A through D) 32D-Kit cells were incubated with either SLF (500 ng/mL in all cases) (circles) or IL-3 (25% WEHI-3 conditioned medium in all cases) (squares) or both SLF and IL-3 (triangles) with varying amounts of econazole or ketotifen. In the case of (A) and (C), the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. For (B) and (D), colony formation was determined by incubating cells in liquid culture with either SLF or IL-3 with econazole or ketotifen followed by 7 days of incubation of cells in semisolid medium. (E) 32D-Kit cells were incubated with varying amounts of SLF. (Circles) Plus 7.5 μmol/L econazole. (Squares) No econazole. (F) 32D-Kit cells were incubated with varying amounts of SLF for 36 hours followed by a 6-hour pulse with 3HdT thymidine, harvesting, and counting. Error bars represent the standard error determined from triplicate measurements.
Effect of SLF or IL-3 with Ca2+ influx blockers on 32D-Kit cells. (A through D) 32D-Kit cells were incubated with either SLF (500 ng/mL in all cases) (circles) or IL-3 (25% WEHI-3 conditioned medium in all cases) (squares) or both SLF and IL-3 (triangles) with varying amounts of econazole or ketotifen. In the case of (A) and (C), the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. For (B) and (D), colony formation was determined by incubating cells in liquid culture with either SLF or IL-3 with econazole or ketotifen followed by 7 days of incubation of cells in semisolid medium. (E) 32D-Kit cells were incubated with varying amounts of SLF. (Circles) Plus 7.5 μmol/L econazole. (Squares) No econazole. (F) 32D-Kit cells were incubated with varying amounts of SLF for 36 hours followed by a 6-hour pulse with 3HdT thymidine, harvesting, and counting. Error bars represent the standard error determined from triplicate measurements.
Although 32D-Kit cells are normally protected from apoptosis by SLF, we observed, using Trypan blue exclusion analysis, that this protective signal is converted to a death signal if the cells are co-incubated with the Ca2+ influx blockers econazole or ketotifen (Fig1A and C, circles). In contrast, death is not observed when 32D-Kit cells are incubated with Ca2+ influx blocker plus the mitogen IL-3 (Fig 1A and C, squares), and blockers do not accelerate cell death due to withdrawal of protective factors (not shown). Furthermore, simultaneous incubation of 32D-Kit cells with SLF, Ca2+ influx blockers, and IL-3 still results in cell death (Fig 1A, triangles), indicating that IL-3 is not protective for this particular death signal. Induction of cell death by SLF plus Ca2+ influx blocker increases with increasing concentrations of SLF (Fig 1E). Therefore, taken together, the effect of the Ca2+ influx blockers is not simply to counteract or neutralize the protective effect of SLF. Rather, they combine with high levels of SLF to induce cell death.
Failure to exclude Trypan blue is a useful but late cell death endpoint that may not always correlate with other measures of cell viability. We therefore also determined the effect of combining Ca2+influx blockers with SLF or IL-3 on the clonogenic capacity of cells. 32D-Kit cells were exposed to blockers with either SLF or IL-3 for 18 hours. The cells were then collected and plated in semisolid agar in the presence of IL-3, and colonies were counted 7 days later. As shown in Fig 1B and D, overnight exposure to econazole or ketotifen in the presence of IL-3 has little effect on the ability of cells to form colonies. In contrast, exposure to SLF plus ketotifen or econazole reduces clonogenicity by 10- or 1,000-fold, respectively. Therefore, the specific combination of SLF with Ca2+ influx blockers also results in clonogenic cell death.
Although 32D-Kit cells are factor-dependent, they grow continuously in culture and are tumorigenic in vivo. We wished to determine if the ability to induce cell death by SLF plus Ca2+ influx blockers could also be observed in nontransformed cells. We therefore determined the effect of combining Ca2+ influx blockers with SLF or IL-3 on murine BMMC, which are also protected from apoptosis and mitogenically stimulated by IL-3 and SLF. As shown in Fig 2A, B, and C, exposure of BMMC to the combination of Ca2+ influx blockers ketotifen, econazole, or Ni2+ with SLF but not IL-3 results in cell death. As with 32D-Kit cells, greater stimulation by SLF leads to greater levels of cell death in the population (Fig 2D). We therefore conclude that cell death induced by the combination of SLF plus Ca2+influx blockers is not limited to transformed cells such as 32D-Kit.
Effect of SLF or IL-3 with Ca2+ influx blockers on BMMC. (A through C) BMMC were incubated with either SLF (500 ng/mL in all cases) (circles) or IL-3 (25% WEHI-3 conditioned medium in all cases) (squares) or both SLF and IL-3 (triangles) with varying amounts of econazole, ketotifen, or Ni2+. (D) BMMC were incubated with IL-3 and varying amounts of SLF. (Circles) Plus 2 mmol/L Ni2+. (Squares) No Ni2+. In all cases, the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. Error bars represent the standard error determined from triplicate measurements.
Effect of SLF or IL-3 with Ca2+ influx blockers on BMMC. (A through C) BMMC were incubated with either SLF (500 ng/mL in all cases) (circles) or IL-3 (25% WEHI-3 conditioned medium in all cases) (squares) or both SLF and IL-3 (triangles) with varying amounts of econazole, ketotifen, or Ni2+. (D) BMMC were incubated with IL-3 and varying amounts of SLF. (Circles) Plus 2 mmol/L Ni2+. (Squares) No Ni2+. In all cases, the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. Error bars represent the standard error determined from triplicate measurements.
Ca2+ influx blockers alone induce cell death in P815 mastocytoma cells.
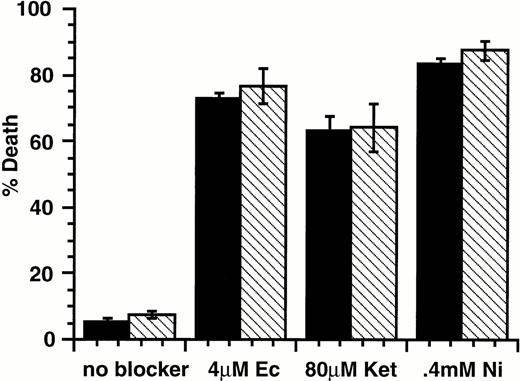
Our data show that stimulation of factor-dependent cells with SLF in the presence of Ca2+ influx blockers leads to cell death. There are many examples of cells that exhibit factor-independent growth due to expression of receptor tyrosine kinases with activating mutations. One example is the P815 mastocytoma,24 which exhibits constitutive tyrosine phosphorylation of the c-Kit protein and factor-independent growth.26 These properties have been attributed to an activating point mutation in the c-kit cytoplasmic domain.27 Our observations predict that these cells should be susceptible to death induced by Ca2+ influx blockers in the absence of SLF stimulus. As shown in Fig 3, treatment of P815 cells with Ca2+ channel blockers econazole, ketotifen, or Ni2+ leads to cell death in the presence or absence of added SLF. Thus, the constitutive signals in P815 cells are sufficient to combine with Ca2+ channel blockers to induce cell death.
Effect of SLF or IL-3 with Ca2+ influx blockers on P815 mastocytoma cells. P815 cells were incubated with either SLF (500 ng/mL; ▪) or IL-3 (25% WEHI-3 conditioned medium; □) with Ca2+ channel blockers econazole, ketotifen, or Ni2+. In all cases, the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. Error bars represent the standard error determined from triplicate measurements.
Effect of SLF or IL-3 with Ca2+ influx blockers on P815 mastocytoma cells. P815 cells were incubated with either SLF (500 ng/mL; ▪) or IL-3 (25% WEHI-3 conditioned medium; □) with Ca2+ channel blockers econazole, ketotifen, or Ni2+. In all cases, the proportion of dead cells was determined after 18 hours in culture by counting cells that could or could not exclude Trypan blue. Error bars represent the standard error determined from triplicate measurements.
BMMC and 32D-Kit–induced death is apoptotic.
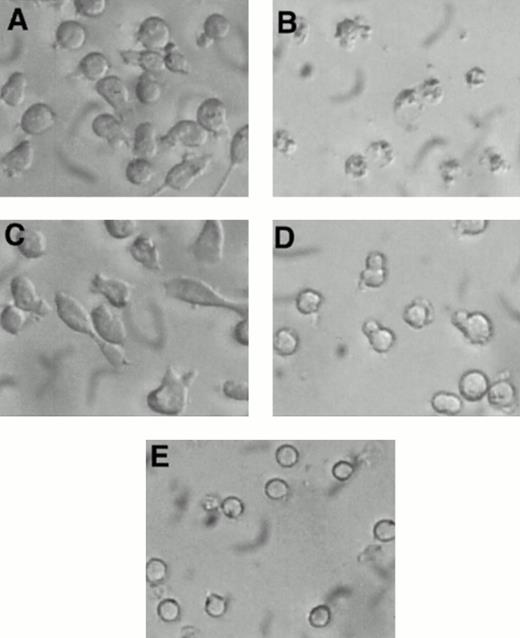
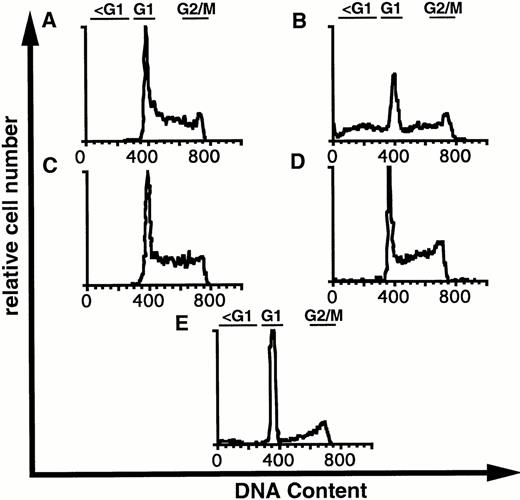
Visual inspection of 32D-Kit cells after treatment with SLF plus Ca2+ influx blockers showed some morphologic characteristics of apoptosis, including nuclear condensation and membrane blebbing (Fig 4B). However, to further substantiate that 32D-Kit and mast cells were undergoing apoptosis, DNA content, which characteristically fragments and decreases during apoptosis, was measured. As shown in Fig 5A, C, and D and summarized in Table 1, treatment of 32D-Kit cells with SLF or IL-3 alone or IL-3 with econazole for 18 hours did not generate a population of cells with subdiploid DNA content, whereas SLF plus econazole generated a large population of cells with subdiploid DNA (Fig 5B). 32D-Kit cells will undergo apoptosis when they are deprived of growth factor. After 18 hours of growth factor withdrawal, 32D-Kit cells exhibited a modest proportion of cells with subdiploid DNA content. This population is substantially increased after 24 hours of factor withdrawal (data not shown). Thus, the apoptotic process induced by Ca2+ influx blocker plus SLF is more rapid than the apoptosis observed from factor withdrawal.
Morphology of 32D-Kit cells incubated with factor and Ca2+ influx blockers. 32D-Kit cells were incubated for 18 hours with (A) SLF alone, (B) SLF + 8 μmol/L econazole, (C) IL-3 alone, or (D) IL-3 + 8 μmol/L econazole. (E) Cells were incubated with no added factor.
Morphology of 32D-Kit cells incubated with factor and Ca2+ influx blockers. 32D-Kit cells were incubated for 18 hours with (A) SLF alone, (B) SLF + 8 μmol/L econazole, (C) IL-3 alone, or (D) IL-3 + 8 μmol/L econazole. (E) Cells were incubated with no added factor.
DNA content of 32D-Kit cells incubated with factor and Ca2+ influx blockers. 32D-Kit cells were incubated for 18 hours with (A) SLF alone, (B) SLF + 8 μmol/L econazole, (C) IL-3 alone, or (D) IL-3 + 8 μmol/L econazole. (E) Cells were incubated with no added factor. Cells were then resuspended in Vindelövs solution containing propidium iodide and analyzed by flow cytometry.
DNA content of 32D-Kit cells incubated with factor and Ca2+ influx blockers. 32D-Kit cells were incubated for 18 hours with (A) SLF alone, (B) SLF + 8 μmol/L econazole, (C) IL-3 alone, or (D) IL-3 + 8 μmol/L econazole. (E) Cells were incubated with no added factor. Cells were then resuspended in Vindelövs solution containing propidium iodide and analyzed by flow cytometry.
Sub-G1 DNA Content of BMMC or 32D-Kit Cells Treated With Econazole Plus Factor
| . | Sub-G1 Content (%) . | |
|---|---|---|
| No Econazole . | 8 μmol/L Econazole . | |
| BMMC | ||
| IL-3 | 25.4 | 20.4 |
| SLF | 6.8 | 66.8 |
| Factor withdrawal | 61.8 | ND |
| 32D-Kit | ||
| IL-3 | 1.6 | 4.8 |
| SLF | 2.3 | 35.0 |
| Factor withdrawal | 9.2 | ND |
| . | Sub-G1 Content (%) . | |
|---|---|---|
| No Econazole . | 8 μmol/L Econazole . | |
| BMMC | ||
| IL-3 | 25.4 | 20.4 |
| SLF | 6.8 | 66.8 |
| Factor withdrawal | 61.8 | ND |
| 32D-Kit | ||
| IL-3 | 1.6 | 4.8 |
| SLF | 2.3 | 35.0 |
| Factor withdrawal | 9.2 | ND |
BMMC were incubated with 8 μmol/L econazole plus IL-3 (25% WEHI conditioned media), SLF (500 ng/mL), or no factor for 24 hours, stained with propidium iodide, and analyzed for DNA content. 32D-Kit cells were similarly incubated for 18 hours before analysis.
Abbreviation: ND, not determined.
Specificity for non–voltage-gated Ca2+ influx blockers.
Ca2+ influx after receptor activation is mediated by the opening of store-operated Ca2+ channels (SOC).28 It is likely that this channel is the target for inhibition, because the efficacy with which the three compounds, ketotifen, econazole, and Ni2+, induce cell death in combination with SLF correlates with their ability to inhibit the SOC.29 We have observed that the voltage-gated Ca2+ channel blockers verapamil and nifedipine are ineffective in inducing cell death when combined with SLF (not shown). This result suggests that the induction of cell death in combination with SLF is specific for non–voltage-gated Ca2+ influx blockers.
Ionomycin protects cells from SLF plus Ca2+ influx blocker induced apoptosis.
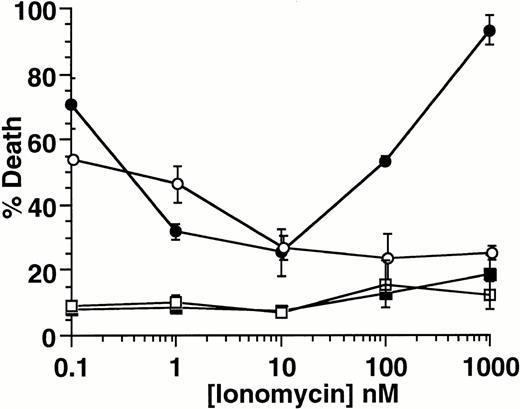
Other ion-blocking effects have been reported for both econazole and ketotifen.29 To determine if specific blockade of Ca2+ influx is critical for induction of cell death, we examined the effect of the calcium ionophore ionomycin, on induction of apoptosis. As shown in Fig 6, ionomycin protects 32D-Kit cells from SLF plus Ca2+ influx blocker-induced death in a concentration-dependent manner, with maximal protection at 10 nmol/L (solid circles). Given the specificity of ionomycin for Ca2+,30 these results indicate that it is the specific blockade of Ca2+ influx that is required for induction of apoptosis in combination with SLF. Importantly, we also observed that higher concentrations of ionomycin, which generate excessive levels of intracellular Ca2+, resulted in the restoration of cell death. This shows that, in the context of cell activation by SLF, both extremes of Ca2+influx can induce cell death.
Effect of ionomycin on SLF plus blocker-induced cell death. 32D-Kit (▪, •) or 32D-Kit-bcl-2 (□, ○) cells were incubated for 18 hours with 5 μmol/L econazole plus varying amounts of ionomycin in the presence of either SLF (circles) or IL-3 (squares). The proportion of dead cells was determined by Trypan Blue exclusion.
Effect of ionomycin on SLF plus blocker-induced cell death. 32D-Kit (▪, •) or 32D-Kit-bcl-2 (□, ○) cells were incubated for 18 hours with 5 μmol/L econazole plus varying amounts of ionomycin in the presence of either SLF (circles) or IL-3 (squares). The proportion of dead cells was determined by Trypan Blue exclusion.
Bcl-2 fails to protect 32D-Kit cells from SLF plus Ca2+influx blocker-induced apoptosis.
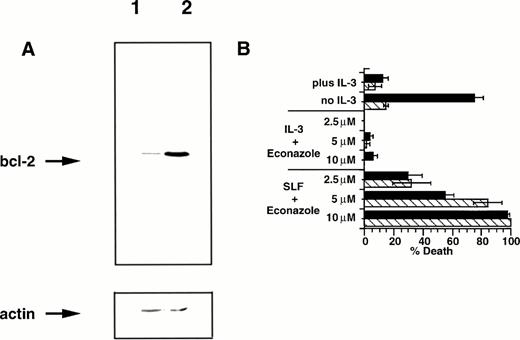
The bcl-2 family of proteins have been strongly linked to protection of cells from apoptosis induced by a wide variety of agents. Expression of bcl-2 is correlated with proliferating cells31,32 and is negatively regulated by the tumor suppressor p53,33 and overexpression of bcl-2 protects 32D cells from apoptotic death after factor withdrawal.10 Given the importance of bcl-2 in regulating susceptibility to apoptosis, we were interested in the effect that overexpression of this protein might have on the induction of apoptosis by SLF plus Ca2+ influx blocker. We therefore infected 32D-Kit cells with a retrovirus vector containing the bcl-2 gene,34 generated a bcl-2 overexpressing line (Fig 7A), and tested the cells for susceptibility to apoptosis. We confirmed that bcl-2 overexpression protects 32D-Kit cells from apoptosis induced by factor withdrawal (Fig7B). However, as also shown in Fig 7B, overexpression of bcl-2 in 32D-Kit cells fails to protect these cells from induction of apoptosis by SLF plus econazole. These observations show that induction of cell death by SLF plus blocker occurs in a bcl-2–independent manner.
Bcl-2 overexpression fails to protect 32D-Kit cells from SLF plus Ca2+ influx blocker. (A) Western blot of cell lysates from 32D-Kit (lane 1) or 32D-Kit-bcl-2 (lane 2) cells. Cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti–bcl-2 antibodies (top panel). The blot was then stripped and reprobed with an anti-actin monoclonal antibody to demonstrate equal protein loading (bottom panel). (B) 32D-Kit (▪) or 32D-Kit-bcl-2 (▨) cells were incubated for 18 hours with SLF or IL-3 plus 2.5, 5, or 10 μmol/L econazole, and the proportion of dead cells was determined by Trypan Blue exclusion. In additional cultures, IL-3 alone or no factor was added and cell viability was similarly determined.
Bcl-2 overexpression fails to protect 32D-Kit cells from SLF plus Ca2+ influx blocker. (A) Western blot of cell lysates from 32D-Kit (lane 1) or 32D-Kit-bcl-2 (lane 2) cells. Cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti–bcl-2 antibodies (top panel). The blot was then stripped and reprobed with an anti-actin monoclonal antibody to demonstrate equal protein loading (bottom panel). (B) 32D-Kit (▪) or 32D-Kit-bcl-2 (▨) cells were incubated for 18 hours with SLF or IL-3 plus 2.5, 5, or 10 μmol/L econazole, and the proportion of dead cells was determined by Trypan Blue exclusion. In additional cultures, IL-3 alone or no factor was added and cell viability was similarly determined.
32D-Kit-bcl-2 cells were similarly protected from apoptosis by SLF plus blocker with low levels of ionomycin (Fig 6); however, unlike the 32D-Kit cells, they did not demonstrate significant cell death at higher levels of ionomycin. Taken together, these results therefore confirm that bcl-2 can protect cells from both factor withdrawal and high levels of intracellular Ca2+ but is unable to protect cells from the combination of SLF plus Ca2+ influx blockers.
Caspase inhibitors protect cells from Ca2+ influx blocker plus SLF-induced death.
Members of the caspase family of intracellular proteases are common effectors of apoptotic death induced by a wide variety of agents. Current models of caspase involvement in apoptosis suggest that pro-apoptotic stimuli activate a cascade of proteases, with ICE-like caspases (caspase-1) acting upstream of CPP32-like caspases (caspase-3).35,36 ICE-like caspases show cleavage specificity for substrates with aspartate in the P1 position and hydrophobic amino acids in the P4 position. These enzymes are preferentially inhibited by tetrapeptides such as the aldehyde YVAD-CHO.37 In contrast, CPP32-like caspases preferentially cleave substrates with acidic amino acids in the P4 position and are inhibited by tetrapeptides such as DEVD-FMK and Boc-Asp-FMK.38 39 To determine if caspases were involved in the induction of apoptosis by SLF plus Ca2+ influx blocker, these caspase inhibitors were tested for their protective ability. We found that all three inhibitors protected 32D-Kit cells from apoptosis induced by the topoisomerase inhibitor etoposide (not shown). We also found that these inhibitors provided resistance to apoptosis induced by SLF plus econazole (Fig 8), indicating that apoptosis induced through the combination of SLF stimulation with a Ca2+ influx blocker is likely mediated by a caspase cascade involving both ICE-like and CPP32-like enzymes.
YVAD-CHO, DEVD-FMK, and Boc-Asp-FMK protect 32D-Kit cells from SLF plus econazole. 32D-Kit cells were incubated with either SLF (▪) or SLF plus 10 μmol/L econazole (□). The proportion of dead cells was evaluated by Trypan Blue exclusion after 18 hours in culture.
YVAD-CHO, DEVD-FMK, and Boc-Asp-FMK protect 32D-Kit cells from SLF plus econazole. 32D-Kit cells were incubated with either SLF (▪) or SLF plus 10 μmol/L econazole (□). The proportion of dead cells was evaluated by Trypan Blue exclusion after 18 hours in culture.
Cleavage of PARP protein is observed with SLF treatment plus Ca2+ influx blockade.
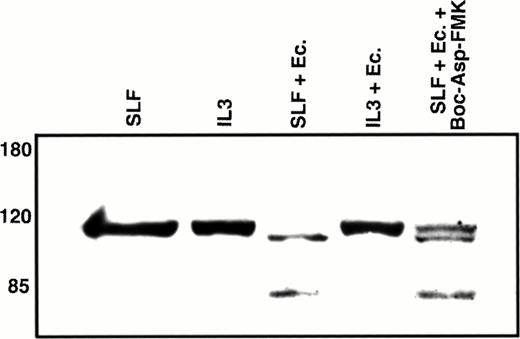
Poly(ADP-ribose) polymerase (PARP) is a nuclear repair enzyme that has also been implicated in transcription enhancement during preinitiation complex formation.40 A common endpoint in the caspase cascade is the cleavage of this 116-kD nuclear protein into an approximately 85-kD fragment.41-43 Because this cleavage event is likely mediated by the CPP32 subfamily of caspases and our results indicate that CPP32-like caspases are involved in the induction of apoptosis through the combination of SLF and Ca2+ influx blockers, we evaluated the effect of this treatment on PARP. We first confirmed that PARP cleavage is induced by exposure of 32D-Kit cells to etoposide (not shown). As shown in Fig 9, an approximately 85-kD PARP cleavage product is observed in nuclear extracts when 32D-Kit cells are treated with SLF plus econazole but is not detected in nuclear extracts from cells incubated with SLF alone, IL-3 alone, or IL-3 plus econazole. Coincubation of cells with Boc-Asp-FMK results in partial inhibition of PARP cleavage, in agreement with our data showing partial protection from apoptosis by this inhibitor. We therefore conclude that the combination of SLF plus Ca2+ influx blocker initiates a cascade involving ICE-like and CPP32-like caspases that ultimately results in cleavage of PARP.
Western blot analysis of nuclear PARP. 32D-Kit cells were incubated for 12 hours with either SLF (lane 1), IL-3 (lane 2), SLF plus 10 μmol/L econazole (lane 3), IL-3 plus 10 μmol/L econazole (lane 4), or SLF plus 10 μmol/L econazole plus 100 μmol/L Boc-Asp-FMK. Nuclear lysates from these cultures were separated by 7.5% SDS-PAGE, transferred to nitrocellulose, and probed with anti-PARP antibodies.
Western blot analysis of nuclear PARP. 32D-Kit cells were incubated for 12 hours with either SLF (lane 1), IL-3 (lane 2), SLF plus 10 μmol/L econazole (lane 3), IL-3 plus 10 μmol/L econazole (lane 4), or SLF plus 10 μmol/L econazole plus 100 μmol/L Boc-Asp-FMK. Nuclear lysates from these cultures were separated by 7.5% SDS-PAGE, transferred to nitrocellulose, and probed with anti-PARP antibodies.
DISCUSSION
An important property of many hematopoietic growth factors and cytokines is the protection of cells from apoptosis. We have shown here that blockade of Ca2+ influx reverses the ability of SLF but not IL-3 to protect cells from apoptotic death, highlighting the importance of Ca2+ influx in SLF-mediated cell survival. However, our data indicate that the effect of Ca2+ influx blockade extends beyond simple neutralization of the protective properties of Ca2+ influx. Because higher levels of SLF stimulus lead to greater levels of cell death and induction of cell death by SLF plus blockers occurs even in the presence of IL-3, this suggests that activation by SLF is an essential component of this form of apoptosis. Thus concurrent blockade of Ca2+ influx effectively converts the SLF-mediated protective signal into a death signal.
Several endpoints indicate that the cell death induced by the combination of SLF and Ca2+ influx blockers is apoptotic. These include specific morphologic characteristics such as membrane blebbing and nuclear condensation, loss of membrane integrity as indicated by failure to exclude Trypan Blue, DNA fragmentation, protection by caspase inhibitors, and PARP cleavage. Although many of these endpoints are also characteristic of apoptotic death caused by factor withdrawal,5 an important difference is that, whereas IL-3 or bcl-2 overexpression protects cells from factor withdrawal, they do not protect these cells from apoptosis induced by SLF plus Ca2+ influx blockade.
Other apoptotic signals have also been found to be bcl-2 insensitive.2 These signals include cell death induced by tumor necrosis factor, Fas activation, activation-induced cell death, and superantigen-mediated clonal deletion.44-48It is therefore possible that disruption of calcium influx in the context of activation may be an important component of such signals as well. In support of this possibility, Kovacs and Tsokos49showed that Fas activation inhibited anti-CD3-mediated Ca2+influx in T cells without affecting Ca2+ release from internal stores.
We found that ionomycin can protect cells from SLF plus blocker-induced cell death, supporting our contention that specific blockade of Ca2+ influx is required for apoptosis induction. Our observation that higher levels of ionomycin restores cell death shows that both extremes of Ca2+ influx can lead to apoptosis. However, in both cases, cell activation is required, because only minimal cell death is observed in the absence of SLF. As observed in other cell systems,2,50 we found that IL-3 or bcl-2 overexpression protected cells from death induced by high concentrations of ionomycin, highlighting the difference between apoptosis caused by SLF plus Ca2+ influx blockade and apoptosis caused by Ca2+ overload. In neurons, both low and high levels of intracellular Ca2+ have been shown to lead to apoptosis, whereas sustained levels of intracellular Ca2+ generated through chronic depolarization lead to protection from apoptotic death.51,52 These observations are consistent with our data and may therefore indicate that both high and low levels of intracellular Ca2+ can affect the balance between survival and apoptosis in a variety of cell types. One possible mediator of high Ca2+ death may be the Ca2+-dependent phosphatase calcineurin, which has been shown to potentiate apoptosis in T cells53 and B cells.54
Our observation that caspase inhibitors protect cells from apoptosis induced by SLF stimulation plus Ca2+ influx blockade, coupled with the demonstration that PARP, a nuclear target of caspases is cleaved by this treatment, indicates that this form of apoptosis is effected through activation of a caspase cascade. However, the specific molecular events responsible for activating this cascade remain to be identified. SLF stimulation mobilizes Ca2+ through activation of the enzyme phospholipase C-γ (PLC-γ).55 56 It is therefore likely that PLC activation is a minimal requirement. One prediction based on our observations is that other signals that activate PLC should also be able to combine with Ca2+ influx blockers to induce apoptosis. Our preliminary observations indicate that this is indeed the case (J.L.G. and S.A.B., unpublished data).
Ketotifen is a well-known anti-allergy and anti-asthmatic drug that exhibits both histamine H1 receptor antagonism and inhibition of Ca2+ influx.57,58 Given the importance of Ca2+ influx for mast cell degranulation, inhibition of this process is one accepted mechanism for its anti-allergic activity. Our observation that this compound also induces apoptosis when coadministered with an activation signal such as SLF suggests that this may be another mechanism by which ketotifen mediates its anti-allergic effects. Because increasing signal leads to increased apoptosis, this form of cell death will be most effective when Ca2+ influx blockers are combined with highly activated receptors. In support of this possibility, we have observed that the p815 mastocytoma, which has a constitutively activated c-Kit receptor,26 27 dies apoptotically when exposed to Ca2+ influx blockers alone (Fig 3).
Other investigators have proposed Ca2+ influx blockers as antiproliferative agents.59-64 Our data suggest that these compounds will be most effective when coupled with a signal such as SLF that mobilizes Ca2+. Therefore, the combination of Ca2+ influx blockers with such activating signals may have potent, but specific, antiproliferative or anti-inflammatory properties.
ACKNOWLEDGMENT
The authors thank M. Minden for the 32D-Kit cells, Y. Ben-David for the bcl-2 retrovirus-producing cells, and R. Chow for technical assistance.
Supported by grants from the National Cancer Institute of Canada and the Leukemia Research Fund of Canada to S.A.B.
Address reprint requests to Stuart A. Berger, PhD, Wellesley Hospital Research Institute, 160 Wellesley St E, Toronto, Ontario, Canada M4Y 1J3.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal