To the Editor:
The p16INK4A and p15INK4B genes encode proteins negatively regulating the cell cycle by inhibition of cyclin-dependent kinase 4 and 6, which control progression of cells from G1 to S phase.1 Homozygous deletion of the p16INK4A or p15INK4B genes has been detected frequently in acute lymphoblastic leukemia (ALL), especially in T-cell ALL, and may contribute to development of cancer.2 A subset of T-ALL with expression of pan-T antigen CD7 on the leukemic blasts but without sheep erythrocyte rosette (ER) formation of the cells, traditionally called pre-T ALL, was shown to differ significantly from ER-positive T-ALL in clinical characteristics. The pan-T antigen CD2 is sheep erythrocyte receptor protein that is associated with ER formation.3 Recently, CD2 antigen expression on leukemic cells was found to be a predictor of event-free survival after chemotherapy for T-lineage ALL.4 It is not known whether there is difference in the incidence of p16INK4A/p15INK4B gene deletion between CD2+ and CD2− T-ALL.
Leukemic cells from 125 ALL patients diagnosed according to FAB criteria were studied for deletion of p16INK4A/p15INK4B genes by Southern blot analyses. Ninety-five patients had B-lineage ALL and 30 had T-lineage ALL, in which 18 expressed CD2 antigen on the leukemic blasts (CD2+) and 12 did not (CD2−). Patients with CD7+ minimally differentiated AML, M0 subtype5 were carefully excluded. The p16 exon 2 and exon 1 probes were obtained by polymerase chain reaction (PCR) of normal human DNA. The primers used for exon 2 amplification were 5′-GGAAATTGGAAACTGGAAGC-3′ (sense) and 5′-TCA TCA GTCCTCACCTGAG-3′ (antisense), and those for exon 1 were 5′-GAAGAAAGAGGAGGGGCTG-3′ (sense) and 5′-GCGCTACCTGATTCCAATTC-3′ (antisense) as described by Kamb et al.2 The amplification products were cloned into the PCR II vector and used as probes for Southern blotting analyses. Specificity of DNA inserts was confirmed by sequencing. The probes were32P-labeled by random priming. The p16 exon 2 probe can also recognize p15 exon 2, which is 90% identical to the p16 gene. Southern blot experiments were performed on EcoRI-digested genomic DNA as described previously.6 DNA from two cell lines K562 and HL60, the former having homozygous deletion and the latter having heterozygous deletion of both p15 and p16 genes,7 was included in all studies for better interpretation of the results. DNA loading was verified by hybridization of the membrane with a p16 probe and a retinoic acid receptor α cDNA probe simultaneously. All the analyses were repeated at least once to confirm the findings. Rearrangements of T-cell receptor (TCR) β-chain gene were also studied on leukemic cells from patients with T-ALL.
Homozygous deletions of the p16 gene were detected in 18 patients (19%) with B-lineage ALL and in 14 (47%) with T-ALL, and those of the p15 gene in 13 (14%) B-lineage ALL and in 7(23%) T-ALL (Fig1). Four B-lineage ALL and one T-ALL showed heterozygous deletion of the p16 gene, and three B- lineage ALL and two T-ALL had heterozygous deletion of the p15 gene. Patients with CD2+ T-ALL had a significantly higher incidence of homozygous deletion of the p16 gene than those with CD2−T-ALL (12 out of 18 or 67% v 2 out of 12 or 17%), but no significant difference was found in the occurrence of p15 gene deletion in these two subgroups of patients. Twenty-two T-ALL patients had adequate DNA for TCR β-chain gene analyses. Twelve (92%) of 13 CD2+ T-ALL showed rearrangements of the TCR β-chain gene, whereas 3 (33%) of 9 CD2− T-ALL did so. Among 15 patients with TCR β-chain gene rearrangements, 6 had homozygous deletion of the p16 gene, whereas none of 7 patients with germline configuration of TCR β-chain gene showed this change.
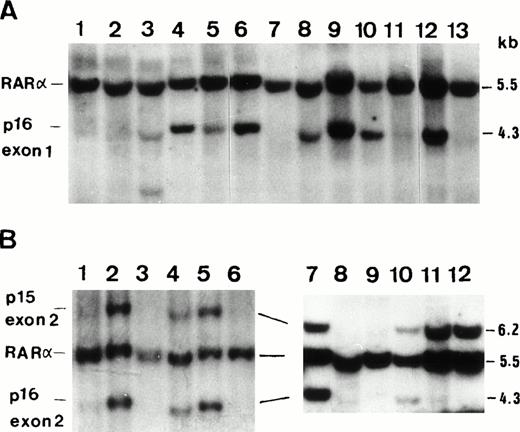
(A) A representative film of Southern blot analysis onEcoRI-digested DNA from ALL patients showing homozygous deletion of p16 exon 1 on lanes 1, 2, 11, and 14; heterozygous deletion on lane 5; and rearrangement on lane 3. Normal control was shown on lane 6, K562 cell line on lane 7 (homozygous deletion), and HL60 on lane 8 (heterozygous deletion) for comparison. A retinoic acid receptor α (RARα) probe was used with p16 exon 1 probe simultaneously to clarify DNA quality and amount. (1B) Two representative films of Southern blot analysis using p16 exon 2 probe and EcoRI restriction enzyme showing homozygous deletion of p15 exon 2 on lanes 1, 6, 8, and 9 and that of p16 exon 2 on lanes 1, 6, 8, 9, 11, and 12. Normel control was shown on lane 2, K562 cell line on lane 3 (homozygous deletion of both genes), and HL60 on lane 4 (hererozygous deletion).
(A) A representative film of Southern blot analysis onEcoRI-digested DNA from ALL patients showing homozygous deletion of p16 exon 1 on lanes 1, 2, 11, and 14; heterozygous deletion on lane 5; and rearrangement on lane 3. Normal control was shown on lane 6, K562 cell line on lane 7 (homozygous deletion), and HL60 on lane 8 (heterozygous deletion) for comparison. A retinoic acid receptor α (RARα) probe was used with p16 exon 1 probe simultaneously to clarify DNA quality and amount. (1B) Two representative films of Southern blot analysis using p16 exon 2 probe and EcoRI restriction enzyme showing homozygous deletion of p15 exon 2 on lanes 1, 6, 8, and 9 and that of p16 exon 2 on lanes 1, 6, 8, 9, 11, and 12. Normel control was shown on lane 2, K562 cell line on lane 3 (homozygous deletion of both genes), and HL60 on lane 4 (hererozygous deletion).
T-ALL has been reported to show homozygous deletion of the p16 gene in 15% to 77% of cases,8-10 but the incidence of the gene deletion in subgroups of T-ALL is totally unknown. The causes of wide difference in the frequency of the p16 gene deletion are unclear. It might be caused by geographic or racial differences, but different diagnostic criteria for T-ALL in various institutions might explain the phenomenon, too. Patients with CD7+ AML, M0 subtype, in which the leukemic cells showed negative reaction for myeloperoxidase but positive staining for myeloid antigens CD13/CD33, might be included in some studies if myeloid markers were not analyzed in immunophenotyping. On the other hand, some CD2− T-ALL might be excluded in other studies if T-ALL was defined as ALL expressing at least two of the T-cell antigens CD2, CD5, and CD7.9 In the former condition, the frequency of the p16 gene deletion might be underestimated because AML rarely showed this gene deletion, whereas in the latter, it might be overestimated because CD2+ T-ALL had a higher incidence of the p16 gene deletion than CD2− ALL as shown in this report. CD2−T-ALL patients in this study had a similar age distribution (ranging from 2 to 40 years with a median of 10 years) as that of CD2+ T-ALL patients (2 to 24 years with a median of 13 years) and showed male predominance (8 men and 4 women). All 10 patients who had analyses for myeloid antigen expression on leukemic cells showed absence of CD13 and CD33 expression, excluding the diagnosis of CD7+ AML, M0 in these patients. Furthermore, 8 of 10 CD2− T-ALL patients who were treated with ALL-directed chemotherapeutic drugs obtained a complete remission.
The finding that T-ALL at a more immature stage of differentiation, as shown by absence of CD2 expression and/or of TCR β-chain gene rearrangement, had a much lower incidence of homozygous deletion of the p16 gene than more mature subtype suggested that the underlying mechanisms of leukemogenesis in these two subgroups of leukemia might be different. The controvercial results in the literature as to the prognostic implication of the p16 gene deletion8 9 may be further clarified by separate analyses on different subgroups of ALL.
ACKNOWLEDGMENT
Supported in part by National Science Council Grant No. NSC 86-2314-B002-177 and NSC 87-2314-B002-074.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal