Abstract
There are three major classes of human Fcγ receptors (FcγRI, FcγRII, and FcγRIII) and various isoforms of each class are capable of mediating phagocytosis. FcγRIIA is an unusual Fcγ receptor in that it transmits a phagocytic signal in the absence of an additional receptor subunit. The cytoplasmic domain of FcγRIIA contains a conserved motif containing two copies of the sequence YXXL. The tyrosines (Y) within the motif are phosphorylated after receptor crosslinking and the integrity of these conserved sequences is required for efficient phagocytosis. The FcγRIIB receptors, FcγRIIB1 and FcγRIIB2, contain one copy of the cytoplasmic YXXL sequence and do not transmit a phagocytic signal. In B cells, FcγRIIB negatively regulates B-cell activation by the B-cell antigen receptor. Human macrophages express both FcγRIIA and FcγRIIB and while FcγRIIA mediates phagocytosis, the function of FcγRIIB in these cells is unknown. Using the epithelial/fibroblast-like cell line COS-1 as a model to examine the molecular events that regulate the phagocytosis of IgG-coated cells (EA), we investigated the effect of FcγRIIB on FcγRIIA signaling. FcγRIIB inhibited phagocytosis mediated both by FcγRIIA and by a chimeric FcγRIIA receptor containing the extracellular domain of FcγRI and the transmembrane and cytoplasmic domains of FcγRIIA. This inhibition occurred at an early signaling stage because tyrosine phosphorylation of the FcγRIIA cytoplasmic domain was inhibited after concurrent stimulation of these receptors with EA. FcγRIIB mutations showed the importance of the FcγRIIB YXXL for inhibition of FcγRIIA-mediated phagocytosis. Deletion of the FcγRIIB YXXL or conservative replacement of the YXXL tyrosine substantially reduced the inhibitory signal. FcγRIIB had a lesser inhibitory effect on phagocytosis by the Fcγ receptor FcγRIIIA, which requires a γ subunit to mediate a phagocytic signal. These results show that FcγRIIB negatively regulates phagocytic signaling by FcγRIIA and suggests that FcγRIIB plays a role in modulating FcγRIIA function in vivo.
PHAGOCYTOSIS PLAYS an important role in host defense against microbial infection and is a major function of monocytes and macrophages. Monocytes and macrophages induce a phagocytic signal through their cell-surface Fcγ receptors (FcγR) which detect and bind IgG coated cells through the constant (Fc) region of IgG.1-3 Human macrophages express receptors from all three FcγR classes: FcγRI, FcγRII, and FcγRIII. Although Fcγ receptors share similar structures, including homologous extracellular IgG binding domains, the individual Fcγ receptors differ significantly in their cytoplasmic regions. The divergence in the structures of the Fcγ receptor cytoplasmic domains largely accounts for their distinct functions.
The FcγRII class of receptors is encoded by three genes: FcγRIIA, FcγRIIB, and FcγRIIC.4 FcγRIIA is expressed on phagocytic cells such as monocytes and macrophages and is the only FcγR present on platelets. FcγRIIB receptors are expressed on monocytes and macrophages as well as on lymphoid cells (B cells and some subpopulations of T cells) and mast cells.5 The two major isoforms of FcγRIIB, FcγRIIB1, and FcγRIIB2 are identical except for a 19-amino acid insertion in the FcγRIIB1 cytoplasmic domain resulting from alternative splicing of the FcγRIIB gene.6 FcγRIIB isoforms are homologous to FcγRIIA in their extracellular and transmembrane regions, but the cytoplasmic domains of the FcγRIIB receptors share little homology with FcγRIIA. Because of the difficulty associated with examining individual Fcγ receptors in hematopoietic cells where several different FcγR classes may be expressed, we have used the fibroblast/epithelial-like cell line COS-1 as a model system to evaluate individual FcγR function. FcγRIIA, when transfected into these cells, can efficiently phagocytose antibody coated erythrocytes (EA).7
Much attention has focused on the presence of the pair of tyrosine containing sequences (YXXL) found within the cytoplasmic domains of most receptors of the Ig gene superfamily or their associated subunits.8-10 This immunoreceptor tyrosine-based activation motif (ITAM) is required for signal transduction through these receptors. FcγRIIA contains an ITAM-like consensus motif consisting of two YXXL sequences separated by 12 amino acids. Phosphorylation of the tyrosines (Y) within this motif is essential for FcγRIIA-mediated phagocytosis and the importance of the ITAM-like region in FcγRIIA-mediated signaling has been established by extensive mutation and deletion studies.10 Although FcγRIIB receptors do not contain a consensus ITAM in their cytoplasmic domain, they do contain one YXXL sequence (YSLL). This YSLL is contained within an ITIM sequence (for immunoreceptor tyrosine-based inhibitory motif). The ITIM sequence found in FcγRIIB was first studied in B lymphocytes where it inhibits B-cell receptor–mediated Ig production.11,12 When transfected into COS-1 cells, FcγRIIB isoforms do not mediate phagocytosis of opsonized cells although they bind EA avidly.13 These studies suggest that although FcγRIIB is expressed in cells of myeloid origin, it does not play a direct role in mediating phagocytosis in vivo.
Since FcγRIIB contains an ITIM sequence, we used our COS-1 cell model to examine the hypothesis that FcγRIIB may regulate FcγRIIA-mediated phagocytosis. Both FcγRIIB1 and FcγRIIB2 inhibited FcγRIIA-mediated phagocytosis whereas phagocytosis by the Fcγ receptor, FcγRIIIA, was only minimally reduced. Using a chimeric FcγRIIA receptor containing the cytoplasmic domain of FcγRIIA, we further showed that the FcγRIIB YSLL tyrosine is important for the inhibitory effect. Signaling through the FcγRIIA cytoplasmic domain and tyrosine phosphorylation of the FcγRIIA chimeric receptor are decreased after costimulation of the FcγRIIA chimera and FcγRIIB. These data indicate that one Fcγ receptor isoform, FcγRIIB, can regulate phagocytosis mediated by another monocyte/macrophage Fcγ receptor isoform, FcγRIIA.
MATERIALS AND METHODS
Construction of FcγRIIB mutants.
All cDNAs were expressed in the eukaryotic expression vector PRC/CMV (Invitrogen, San Diego, CA). Two-step overlap-extension polymerase chain reaction (PCR) using the appropriate oligonucleotide primers was used to construct the mutant cDNAs. Clones were subjected to DNA sequencing to verify the mutations. The following FcγRIIB mutants were constructed: Trun-B2 (FcγRIIB2 with the cytoplasmic domain deleted), B1-YF and B2-YF (FcγRIIB1 and FcγRIIB2 isoforms in which the tyrosine [Y] of the YSLL sequence has been mutated to phenylalanine [F]), B1ΔYSLL and B2ΔYSLL (FcγRIIB1 and FcγRIIB2 isoforms in which the YSLL sequence has been deleted), and B2-YETL and B2-YMTL (FcγRIIB2 in which the YSLL has been changed to YETL or YMTL, respectively).
Cell culture and COS-1 cell transfection.
COS-1 cells were cultured and maintained in Dulbecco's modified Eagle's medium (DMEM) containing glucose (4.5 mg/mL), glutamine (2 mmol/L), streptomycin (100 U/mL), penicillin (100 mg/mL), and 10% heat-inactivated fetal bovine serum. COS-1 cells were transiently transfected at 80% confluency (3 × 105 cells/well in a six-well plate) in DMEM culture medium containing diethylaminoethyl (DEAE)-dextran (750 μg/mL), chloroquine chloride (100 μmol/L), and 4.0 μg plasmid DNA per milliliter. After 3.5 hours of incubation at 37°C the transfection medium was removed and the cells incubated in a 10% solution of dimethyl sulfoxide in phosphate-buffered saline (PBS) for 2 minutes at room temperature. The cells were then washed twice with DMEM, overlaid with fresh medium, and incubated for 48 hours before analysis.
Binding and phagocytosis of IgG sensitized red blood cells (RBCs).
Antibody-coated sheep erythrocytes (Rockland, Gilbertsville, PA) (EA) were prepared in magnesium- and calcium-free PBS by incubating 109/mL sheep RBCs with an equal volume of the highest subagglutinating concentration of IgG rabbit–anti-sheep RBC antibody (Cappel Laboratories, West Chester, PA) as previously described.7 The medium was removed from the COS-1 transfectants and the cells overlaid with a large excess of EA (108 EA in 1.0 mL for 3 × 105 COS-1 cells) and incubated at 37°C for 30 minutes. Unbound EA were washed away with PBS, and EA binding to the transfected cells was determined. Extracellularly bound EA was then removed by a short (20 seconds) wash with 0.25% PBS. The cells were stained with Wright-Giemsa and the number of COS-1 cells with more than one internalized EA was determined in a blinded fashion by light microscopy. At least 300 cells were counted per experiment. Phagocytosis was expressed as phagocytic index (PI), the number of EA ingested per 100 COS-1 cells. The percent cells phagocytosing at least 1 EA was also determined. Levels of expression of the phagocytic chimeric receptors (I-IIA-IIA or α-γ-γ) were determined by flow cytometry.
Flow cytometry.
A total of 105 cells was incubated on ice for 30 minutes with anti-FcγRII monoclonal antibody (MoAb) IV.3 (for FcγRIIB staining) or anti-FcγRI MoAb 32.2 (for staining the chimeric FcγRIIA receptor containing the extracellular [EC] domain of FcγRI, I-IIA-IIA [EC-TM-CYT]). The cells were washed and labeled with fluorescein isothiocyanate–conjugated goat–anti-mouse F(ab′)2 IgG (TAGO Inc, Burlingame, CA) for 30 minutes on ice. After washing, the cells were fixed in a solution of 4% paraformaldehyde. Isotype controls were used for all antibodies and fluorescence was measured on a FACSTAR cytometer (Becton Dickinson, Mountain View, CA).
Immunoprecipitation and Western blotting.
After stimulation of FcγR-transfected COS-1 cells with EA at 37°C for 30 minutes, the cells were placed on ice to stop further phagocytosis. Externally bound EA was removed by hypotonic lysis and the COS-1 cell lysate obtained by the addition of 1.0 mL RIPA buffer (1% Triton X-100 [Sigma, St Louis, MO], 1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 158 mmol/L NaCl, 10 mmol/L Tris pH 7.2, 5 mmol/L NaEGTA, 1 mmol/L phenylmethylsulphonyl fluoride [PMSF], 1 mmol/L Na3VO4, 50 μg/mL leupeptin, and 10 μg/mL aprotinin) followed by incubation on ice for 30 minutes. Cleared lysates were immunoprecipitated with the appropriate antibody (10 μg/mL antiphosphotyrosine polyclonal antibody [PharMingen, San Diego, CA; cat. no. 14201A]). Immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblots were performed with antiphosphotyrosine MoAb 4G10 (UBI, Lake Placid, NY). Immunoblots were developed with horseradish peroxidase–conjugated goat–anti-mouse IgG (BioRad, Richmond, VA) and visualized by enhanced chemiluminescence (Amersham Corp, Arlington Heights, IL).
RESULTS
FcγRIIB inhibits FcγRIIA-mediated phagocytosis.
When transfected into COS-1 cells, the Fcγ receptor, FcγRIIA, mediates the phagocytosis of IgG-coated RBCs (EA).7 In contrast, two other members of this Fcγ receptor class, FcγRIIB1 and FcγRIIB2, do not phagocytose EA when expressed in COS-1 cells.13 In other cell types such as B cells, FcγRIIB receptors are known to inhibit certain signaling pathways.11 12 Therefore, we examined the possibility that the FcγRIIB receptors might inhibit the FcγRIIA signal for phagocytosis.
The FcγRIIB isoforms FcγRIIB1 or FcγRIIB2 were cotransfected with FcγRIIA into COS-1 cells. The cotransfected cells were incubated with EA and the extent of phagocytosis was compared with cells expressing FcγRIIA alone. Cotransfection of an FcγRIIB receptor had a marked inhibitory effect on the phagocytosis of EA by FcγRIIA. We observed a reduction of approximately 60% in both the phagocytic index and the % transfectants phagocytosing EA.
In these transfection experiments, MoAb 41H16, reported to differentiate between the His 131 variant of FcγRIIA and FcγRIIB, which has Arg at 131, did not clearly distinguish between FcγRIIA and FcγRIIB. Therefore, it was difficult using FcγRII MoAbs and flow cytometry to measure the expression of FcγRIIA in the presence of FcγRIIB. However, we have previously shown the importance of the cytoplasmic domain in transmitting the FcγRIIA phagocytic signal.10 Truncation of the FcγRIIA cytoplasmic domain eliminates its phagocytic function and disruption of the FcγRIIA cytoplasmic ITAM sequence, by substituting the tyrosines with phenylalanine, severely reduces or eliminates the receptor's phagocytic signal.10 To clearly determine the expression of FcγRIIA in the presence of FcγRIIB in our cotransfection experiments and to confirm that the decrease in phagocytosis was not caused by a change in FcγRIIA expression, we used a chimeric FcγRII receptor I-IIA-IIA (EC-TM-CYT). I-IIA-IIA contains both the transmembrane (TM) and cytoplasmic (CYT) domains of wild-type (WT) FcγRIIA but the extracellular (EC) domain of FcγRI and is therefore recognized by the anti-FcγRI MoAb 32.2. When expressed in COS-1 cells the I-IIA-IIA chimera mediated the phagocytosis of EA with an efficiency comparable to WT FcγRIIA.1,14 15 Therefore, the chimeric receptor I-IIA-IIA serves as a model for FcγRIIA, allowing examination of phagocytosis by the FcγRIIA cytoplasmic domain.
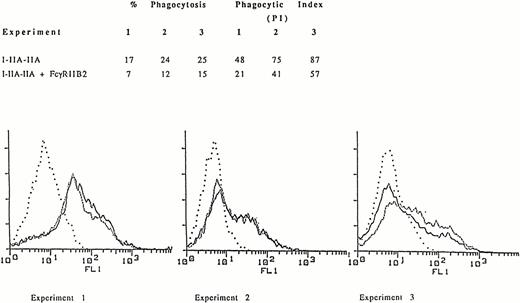
Similar to the observation with WT FcγRIIA, cotransfection of COS-1 cells with I-IIA-IIA and FcγRIIB2 resulted in a decrease in phagocytosis compared to COS-1 cells transfected with I-IIA-IIA alone (Fig 1A). In the three representative independent experiments shown, flow cytometry histograms demonstrate that similar levels of I-IIA-IIA were expressed in both the presence and absence of FcγRIIB (Fig 1B). Thus, the decrease in I-IIA-IIA phagocytosis observed in cells cotransfected with FcγRIIB is not caused by a change in the expression of the I-IIA-IIA receptor.
Phagocytosis of EA mediated by the chimeric receptor I-IIA-IIA in COS-1 cells expressing I-IIA-IIA alone or I-IIA-IIA plus FcγRIIB2. (A) The percent of cells that phagocytose EA and the phagocytic index (PI, number of ingested EA/100 transfected COS-1 cells) for three representative experiments are shown. (B) Expression of the chimeric receptor as determined by flow cytometry. Cells were stained with MoAb 32.2 to measure I-IIA-IIA expression in the presence (–––––) or absence (.........) of FcγRIIB2. (. . . .), Indicates transfectants stained with isotype control antibody. The expression of FcγRIIB was determined by flow cytometry after staining with MoAb IV.3. Mean fluorescence intensity (MFI) for FcγRIIB2 in experiments 1, 2, and 3 was 104, 91, and 80, respectively. The fluorescence histogram shows that the expression of I-IIA-IIA (MFI) in the presence or absence of FcγRIIB2 was virtually the same. Thus, FcγRIIB2 decreased phagocytosis by I-IIA-IIA without changing the cell-surface expression of I-IIA-IIA.
Phagocytosis of EA mediated by the chimeric receptor I-IIA-IIA in COS-1 cells expressing I-IIA-IIA alone or I-IIA-IIA plus FcγRIIB2. (A) The percent of cells that phagocytose EA and the phagocytic index (PI, number of ingested EA/100 transfected COS-1 cells) for three representative experiments are shown. (B) Expression of the chimeric receptor as determined by flow cytometry. Cells were stained with MoAb 32.2 to measure I-IIA-IIA expression in the presence (–––––) or absence (.........) of FcγRIIB2. (. . . .), Indicates transfectants stained with isotype control antibody. The expression of FcγRIIB was determined by flow cytometry after staining with MoAb IV.3. Mean fluorescence intensity (MFI) for FcγRIIB2 in experiments 1, 2, and 3 was 104, 91, and 80, respectively. The fluorescence histogram shows that the expression of I-IIA-IIA (MFI) in the presence or absence of FcγRIIB2 was virtually the same. Thus, FcγRIIB2 decreased phagocytosis by I-IIA-IIA without changing the cell-surface expression of I-IIA-IIA.
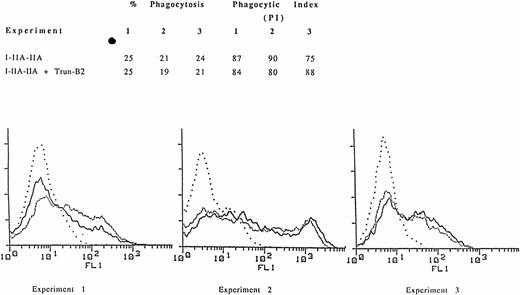
We constructed a mutant FcγRIIB, Trun-B2, which contains the WT extracellular and transmembrane regions of FcγRIIB2 but lacks the entire FcγRIIB cytoplasmic domain. This truncated FcγRIIB bound EA as efficiently as WT FcγRIIB. However, in contrast to cotransfection of WT FcγRIIB and I-IIA-IIA, cotransfection of Trun-B2 and I-IIA-IIA did not inhibit phagocytosis (Fig 2). These data indicate that the inhibition of phagocytosis mediated through the cytoplasmic domain of FcγRIIA by cotransfected FcγRIIB requires the FcγRIIB receptor cytoplasmic domain.
Phagocytosis mediated by the chimeric receptor I-IIA-IIA in COS-1 cells expressing the I-IIA-IIA chimera alone or the I-IIA-IIA chimera plus the FcγRIIB mutant lacking a cytoplasmic domain, Trun-B2. (A) The percent of cells phagocytosing EA and the phagocytic index for three representative experiments are shown. (B) Expression of the chimeric receptor I-IIA-IIA as determined by flow cytometry. Cells were stained with MoAb 32.2 to measure I-IIA-IIA expression in the presence (–––––) or absence (.........) of Trun-B2. (. . . .), Indicates transfectants stained with isotype control antibody. The expression (MFI) of I-IIA-IIA was the same in the presence or absence of Trun-B2 in each experiment. The expression of Trun-B2 was determined by staining with anti-FcγRII MoAb IV.3. MFI for Trun-B2 in experiments 1, 2, and 3 was 55, 44, and 60, respectively.
Phagocytosis mediated by the chimeric receptor I-IIA-IIA in COS-1 cells expressing the I-IIA-IIA chimera alone or the I-IIA-IIA chimera plus the FcγRIIB mutant lacking a cytoplasmic domain, Trun-B2. (A) The percent of cells phagocytosing EA and the phagocytic index for three representative experiments are shown. (B) Expression of the chimeric receptor I-IIA-IIA as determined by flow cytometry. Cells were stained with MoAb 32.2 to measure I-IIA-IIA expression in the presence (–––––) or absence (.........) of Trun-B2. (. . . .), Indicates transfectants stained with isotype control antibody. The expression (MFI) of I-IIA-IIA was the same in the presence or absence of Trun-B2 in each experiment. The expression of Trun-B2 was determined by staining with anti-FcγRII MoAb IV.3. MFI for Trun-B2 in experiments 1, 2, and 3 was 55, 44, and 60, respectively.
The FcγRIIB YSLL sequence is required for the inhibition of FcγRIIA-mediated phagocytosis.
A series of experiments was performed to examine the importance of FcγRIIB cytoplasmic sequences for inhibiting FcγRIIA-mediated phagocytosis. The FcγRIIB cytoplasmic domain contains a 13-amino acid region, the ITIM, which includes a YSLL sequence required for regulation of receptor signaling in B lymphocytes.11 That this sequence is also required for FcγRIIB-mediated inhibition of FcγRIIA phagocytic signaling was indicated in experiments using a deletion mutant of FcγRIIB (Table 1). Deletion of the FcγRIIB YSLL region (B2ΔYSLL) reduced the ability of FcγRIIB2 to inhibit phagocytosis by I-IIA-IIA. Replacement of the YSLL tyrosine (Y) with phenylalanine (F) (B2-YF) also reduced the inhibitory activity of FcγRIIB2. Flow cytometry analysis showed that the cell-surface expression of the I-IIA-IIA chimeric receptor was similar within each experiment and that the cell-surface expression of the FcγRIIB2 mutants and WT FcγRIIB2 was also similar within each experiment. These data indicate that FcγRIIB2 inhibition of the phagocytic signal mediated by the chimeric receptor I-IIA-IIA directly involves the FcγRIIB2 cytoplasmic YSLL sequence. The results of experiments with FcγRIIB1 were similar to those with FcγRIIB2. Costimulation with EA of I-IIA-IIA and FcγRIIB1 also resulted in inhibition of phagocytosis by I-IIA-IIA and deletion of the FcγRIIB1 YSLL (B1ΔYSLL) or mutation of the tyrosine (Y) to phenylalanine (F) (B1-YF) reduced the inhibitory effect of FcγRIIB1 (data not shown).
The Tyrosine-Containing Sequence Within the FcγRIIB Cytoplasmic Domain Is Required for the Inhibition of Phagocytosis by the Chimeric Receptor I-IIA-IIA
| . | PI . | MFI . | |
|---|---|---|---|
| α-FcγRI . | α-FcγRII . | ||
| Experiment 1 | |||
| I-IIA-IIA | 68 | 85 | — |
| I-IIA-IIA + B2 | 25 | 75 | 64 |
| I-IIA-IIA + B2-YF | 55 | 79 | 61 |
| I-IIA-IIA + B2ΔYSLL | 59 | 88 | 66 |
| Experiment 2 | |||
| I-IIA-IIA | 127 | 290 | — |
| I-IIA-IIA + B2 | 76 | 214 | 65 |
| I-IIA-IIA + B2-YF | 138 | 240 | 80 |
| I-IIA-IIA + B2ΔYSLL | 138 | 308 | 84 |
| Experiment 3 | |||
| I-IIA-IIA | 91 | 302 | — |
| I-IIA-IIA + B2 | 41 | 223 | -150 |
| I-IIA-IIA + B2-YF | 65 | 240 | -150 |
| I-IIA-IIA + B2ΔYSLL | 77 | 309 | -150 |
| Experiment 4 | |||
| I-IIA-IIA | 169 | 297 | — |
| I-IIA-IIA + B2 | 80 | 270 | 326 |
| I-IIA-IIA + B2-YF | 207 | 275 | 283 |
| I-IIA-IIA + B2ΔYSLL | 199 | 259 | 290 |
| . | PI . | MFI . | |
|---|---|---|---|
| α-FcγRI . | α-FcγRII . | ||
| Experiment 1 | |||
| I-IIA-IIA | 68 | 85 | — |
| I-IIA-IIA + B2 | 25 | 75 | 64 |
| I-IIA-IIA + B2-YF | 55 | 79 | 61 |
| I-IIA-IIA + B2ΔYSLL | 59 | 88 | 66 |
| Experiment 2 | |||
| I-IIA-IIA | 127 | 290 | — |
| I-IIA-IIA + B2 | 76 | 214 | 65 |
| I-IIA-IIA + B2-YF | 138 | 240 | 80 |
| I-IIA-IIA + B2ΔYSLL | 138 | 308 | 84 |
| Experiment 3 | |||
| I-IIA-IIA | 91 | 302 | — |
| I-IIA-IIA + B2 | 41 | 223 | -150 |
| I-IIA-IIA + B2-YF | 65 | 240 | -150 |
| I-IIA-IIA + B2ΔYSLL | 77 | 309 | -150 |
| Experiment 4 | |||
| I-IIA-IIA | 169 | 297 | — |
| I-IIA-IIA + B2 | 80 | 270 | 326 |
| I-IIA-IIA + B2-YF | 207 | 275 | 283 |
| I-IIA-IIA + B2ΔYSLL | 199 | 259 | 290 |
Four representative experiments are shown. The PI of I-IIA-IIA–mediated phagocytosis was determined in the absence or presence of FcγRIIB2 or its mutants. Within each experiment, expression of WT FcγRIIB and mutant FcγRIIB was similar and FcγRIIB mutants did not reduce the expression of I-IIA-IIA. B2, FcγRIIB2; B2-YF, FcγRIIB2 in which the tyrosine in the YSLL cytoplasmic region has been changed to phenylalanine; B2ΔYSLL, FcγRIIB2 in which the cytoplasmic YSLL sequence has been deleted. Different preparations of MoAbs were used in experiment 1 (anti-FcγRI) and experiment 4 (anti-FcγRII). α-FcγRI, anti-FcγRI (recognizes I-IIA-IIA); α-FcγRII, anti-FcγRII (recognizes FcγRIIB).
Not done.
Another Fcγ receptor that mediates a phagocytic signal is FcγRIIIA. We previously showed that the efficiency of FcγRIIIA-mediated signal transduction is due in part to the sequence of internal (XX) amino acids within the YXXL motifs of its associated γ-chain.16Therefore, we studied the role of the internal amino acids (XX) within the FcγRIIB YXXL cytoplasmic sequence. We observed that the specific YXXL internal amino acids (SL) of FcγRIIB do not appear to be important for regulating the FcγRIIA phagocytic signal. Changing the FcγRIIB YSLL to either YMTL or YETL (B2-YETL and B2-YMTL), thus creating YXXL sequences identical to those found in FcγRIIA or the Fcγ receptor γ-chain, did not alter the inhibitory activity of FcγRIIB (data not shown). Thus, the cytoplasmic tyrosine rather than the sequence of the internal XX amino acids within the YXXL of the ITIM is of primary importance for FcγRIIB inhibition of FcγRIIA-mediated phagocytosis.
FcγRIIB reduces tyrosine phosphorylation of FcγRIIA.
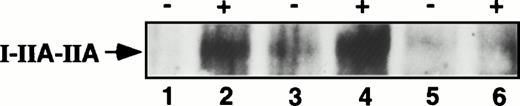
The cytoplasmic ITAM-like sequence is important for phagocytic signaling by FcγRIIA. FcγRIIA is tyrosine phosphorylated after receptor stimulation and this early signaling event is required for efficient phagocytosis.10 Disruption of the tyrosines within the ITAM-like region significantly inhibits Fcγ receptor-mediated phagocytosis in FcγRIIA-transfected COS-1 cells. In addition, treatment with inhibitors of tyrosine phosphorylation reduces FcγRIIA-mediated phagocytosis in monocytes and macrophages as well as in transfected COS-1 cells.10 Costimulation of the chimeric Fcγ receptor I-IIA-IIA and FcγRIIB2 in COS-1 cell transfectants resulted in decreased tyrosine phosphorylation of I-IIA-IIA (lane 6) compared with I-IIA-IIA alone (lane 2) (Fig3).
Antiphosphotyrosine immunoblot of the chimeric I-IIA-IIA receptor in COS-1 cells transfected with I-IIA-IIA alone, I-IIA-IIA plus FcγRIIB2, and I-IIA-IIA plus Trun-B2. Lanes 1 and 2, I-IIA-IIA transfectants; lanes 3 and 4, I-IIA-IIA plus Trun-B2 cotransfectants; lanes 5 and 6, I-IIA-IIA plus FcγRIIB2 cotransfectants. Transfected cells were either not stimulated (−) or stimulated (+) with EA. The arrow shows the position of the tyrosine phosphorylated I-IIA-IIA chimera (60 kD). The expression of I-IIA-IIA was similar in each transfection. FcγRIIB and Trun-B2 were also expressed at similar levels (MFI for FcγRIIB was 114 and 104 for Trun-B2).
Antiphosphotyrosine immunoblot of the chimeric I-IIA-IIA receptor in COS-1 cells transfected with I-IIA-IIA alone, I-IIA-IIA plus FcγRIIB2, and I-IIA-IIA plus Trun-B2. Lanes 1 and 2, I-IIA-IIA transfectants; lanes 3 and 4, I-IIA-IIA plus Trun-B2 cotransfectants; lanes 5 and 6, I-IIA-IIA plus FcγRIIB2 cotransfectants. Transfected cells were either not stimulated (−) or stimulated (+) with EA. The arrow shows the position of the tyrosine phosphorylated I-IIA-IIA chimera (60 kD). The expression of I-IIA-IIA was similar in each transfection. FcγRIIB and Trun-B2 were also expressed at similar levels (MFI for FcγRIIB was 114 and 104 for Trun-B2).
The antiphosphotyrosine blot in Fig 3 indicates that it is the contribution of the FcγRIIB2 cytoplasmic domain that is responsible for the inhibition of I-IIA-IIA tyrosine phosphorylation. In this blot we show that costimulation of I-IIA-IIA and the FcγRIIB2 mutant that is missing the cytoplasmic domain (Trun-B2) does not decrease tyrosine phosphorylation of I-IIA-IIA (lane 4). In contrast, costimulation of WT FcγRIIB2 markedly reduces tyrosine phosphorylation of I-IIA-IIA. In this experiment there is no significant difference between the expression levels of the WT and mutant (tailless) FcγRIIB receptors. I-IIA-IIA expression also does not change with the coexpression of the additional FcγRIIB receptors. Thus, stimulating FcγRIIB2 leads to both decreased tyrosine phosphorylation of I-IIA-IIA and decreased I-IIA-IIA–mediated phagocytosis.
Inhibition by FcγRIIB of phagocytosis by the Fcγ receptor FcγRIIIA.
The Fcγ receptor FcγRIIIA, which is expressed in such myeloid cells as macrophages, is also capable of mediating the phagocytosis of IgG coated cells. Unlike FcγRIIA-mediated phagocytosis, FcγRIIIA phagocytic signaling is mediated through an associated γ-chain subunit. In these experiments we made use of a chimeric molecule consisting of the EC domain of FcγRIIIA and the transmembrane (TM) and cytoplasmic (CYT) domains of the γ-chain, α-γ-γ (EC-TM-CYT). In contrast to I-IIA-IIA–mediated phagocytosis, phagocytosis of EA by α-γ-γ was only minimally decreased by costimulating FcγRIIB and α-γ-γ in COS-1 cell transfectants (Table 2).
The Effect of FcγRIIB1(B1) and FcγRIIB2 (B2) on Phagocytosis by the Chimeric Receptor FcγRIIIAα-γ-γ (EC-TM-CYT)
| . | PI . | MFI . | |
|---|---|---|---|
| α-FcγRIII . | α-FcγRII . | ||
| Experiment 1 | |||
| α-γ-γ | 140 | 261 | — |
| α-γ-γ + B1 | 127 | 227 | 104 |
| α-γ-γ + B2 | 132 | 203 | 118 |
| Experiment 2 | |||
| α-γ-γ | 210 | 169 | — |
| α-γ-γ + B1 | 198 | 140 | 102 |
| α-γ-γ + B2 | 228 | 130 | 99 |
| Experiment 3 | |||
| α-γ-γ | 139 | 146 | — |
| α-γ-γ + B1 | 103 | 149 | 149 |
| α-γ-γ + B2 | 117 | 224 | 179 |
| Experiment 4 | |||
| α-γ-γ | 76 | 127 | — |
| α-γ-γ + B1 | 63 | 128 | 128 |
| α-γ-γ + B2 | 66 | 124 | 127 |
| . | PI . | MFI . | |
|---|---|---|---|
| α-FcγRIII . | α-FcγRII . | ||
| Experiment 1 | |||
| α-γ-γ | 140 | 261 | — |
| α-γ-γ + B1 | 127 | 227 | 104 |
| α-γ-γ + B2 | 132 | 203 | 118 |
| Experiment 2 | |||
| α-γ-γ | 210 | 169 | — |
| α-γ-γ + B1 | 198 | 140 | 102 |
| α-γ-γ + B2 | 228 | 130 | 99 |
| Experiment 3 | |||
| α-γ-γ | 139 | 146 | — |
| α-γ-γ + B1 | 103 | 149 | 149 |
| α-γ-γ + B2 | 117 | 224 | 179 |
| Experiment 4 | |||
| α-γ-γ | 76 | 127 | — |
| α-γ-γ + B1 | 63 | 128 | 128 |
| α-γ-γ + B2 | 66 | 124 | 127 |
Four representative experiments are shown. The α-γ-γ chimera contains the EC of the FcγRIIIA α chain and the TM and CYT domains of the FcγRIIIA γ chain. B1 and B2 minimally inhibited α-γ-γ phagocytosis. Cell-surface expression of α-γ-γ was similar within each experiment and was not reduced by the expression of FcγRIIB. α-FcγRIII, anti-FcγRIII (recognizes α-γ-γ), α-FcγRII, anti-FcγRII (recognizes FcγRIIB).
DISCUSSION
We have observed that FcγRIIB inhibits the phagocytosis of IgG-coated cells mediated by the FcγRIIA cytoplasmic domain. In these studies we used a COS-1 cell-model system and a chimeric receptor, I-IIA-IIA, to examine phagocytosis mediated by the FcγRIIA cytoplasmic domain sequences. We previously determined that the phagocytic signals mediated by FcγRIIA and the chimeric receptor I-IIA-IIA are similar. This approach, using FcγRIIA in the form of a chimera, allows the expression of FcγRIIA to be distinguished from the expression of FcγRIIB in co-transfectants using MoAbs 32.2 and IV.3. MoAb 32.2 recognizes the EC of FcγRI and does not crossreact with the EC of FcγRIIB, and MoAb IV.3 recognizes the EC of FcγRII but not the EC of FcγRI in the chimera I-IIA-IIA.
The data indicate that the decrease in I-IIA-IIA–mediated phagocytosis is not caused by decreased expression of the phagocytic receptor I-IIA-IIA. In addition, WT FcγRIIB2 inhibits I-IIA-IIA phagocytosis whereas FcγRIIB2 cytoplasmic domain mutants, which are expressed at similar levels as WT FcγRIIB, do not inhibit phagocytosis by I-IIA-IIA (Table 1). Furthermore, because a large excess of ligand (>300 EA/COS-1 cell) was used to overlay the cells and because the extracellular domain of FcγRI (and I-IIA-IIA) has a higher affinity for IgG ligand than does the extracellular domain of FcγRIIB, competition for available EA cannot explain our findings.
Studies with FcγRIIB mutants showed that inhibition of phagocytosis requires an FcγRIIB cytoplasmic sequence, YSLL, within the 13-amino acid ITIM region. Of primary importance for the inhibitory signal is the presence of the YSLL tyrosine because its replacement with phenylalanine reduced the ability of FcγRIIB to inhibit phagocytosis mediated by the chimeric receptor I-IIA-IIA. We observed that the internal SL amino acids of the YSLL sequence are not critical for the inhibition because their replacement had no effect on the phagocytic signal.
Because I-IIA-IIA contains the complete FcγRIIA cytoplasmic domain responsible for mediating the phagocytic signal, the observations with I-IIA-IIA are pertinent for WT FcγRIIA. Costimulation of FcγRIIA and FcγRIIB likely alters signal transduction mediated by the FcγRIIA cytoplasmic domain. The mechanism of this interaction has not as yet been completely delineated, although in other signal transduction pathways, a role has been implicated for tyrosine phosphatases.17,18 It is likely that a cytosolic factor, such as a phosphatase, mediates the inhibition of phagocytosis by FcγRIIB. Inhibition by FcγRIIB of phagocytosis mediated by the FcγRIIA cytoplasmic domain occurs at an early stage after receptor activation, because tyrosine phosphorylation of the FcγRIIA cytoplasmic domain was reduced after costimulation of both the I-IIA-IIA chimera and FcγRIIB2. Receptor tyrosine phosphorylation, including FcγRIIA tyrosine phosphorylation, is an early step in signal transduction and is required for efficient FcγRIIA-mediated phagocytosis both in our COS-1 cell model system and in human monocytes and macrophages.7 10
FcγRIIB is known to play a role in inhibiting the proliferation of B cells.11,12 Recent work in B-cell and mast cell model systems has shown that this phenomenon is not restricted to B cells and has led to further understanding of the mechanism of the FcγRIIB inhibitory process.19-22 One observation emerging from work with rat basophilic leukemia (RBL) cells and B-lymphoma cell lines is that the FcγRIIB inhibitory signal may affect many receptors of the Ig gene superfamily that signal through ITAM sequences. The studies show that FcγRIIB inhibits serotonin release induced by costimulating FcεRI (a member of the Ig gene family) in mast cells. Serotonin release in RBL cells by chimeric receptors containing the cytoplasmic domain of the T-cell receptor ζ-chain or by FcγRIIA is also inhibited by FcγRIIB.22 Here we have shown that FcγRIIB can also inhibit an important FcγRIIA function of monocytes and macrophages.
Our observations suggest that a novel mechanism exists for the regulation of phagocytosis in phagocytic cells such as monocytes and macrophages, which express both FcγRIIA and FcγRIIB. Both receptors are members of the same Ig receptor gene family, recognize the same ligand, and are members of the same Fcγ receptor class (FcγRII). The extensive homology of the class II Fcγ receptor extracellular domains suggests that similar IgG ligands may both activate and inhibit FcγRIIA-mediated phagocytosis and it is likely that FcγRIIA-mediated phagocytosis is regulated by FcγRIIB in monocytes and macrophages. The extent of the phagocytic signal may depend on the relative expression of FcγRIIA and FcγRIIB and/or their affinity for IgG ligand. Both FcγRIIA and FcγRIIB recognize the Fc domain of most IgG molecules. However, it should be noted that there are some differences. For example, an FcγRIIA isoform (FcγRIIA-His-131)23 has high affinity for the IgG subclass IgG2. Thus, it is unlikely that FcγRIIA-mediated phagocytosis induced by IgG2 antibodies is regulated by FcγRIIB because IgG2 does not bind FcγRIIB.
A similar phenomenon has recently been observed in T cells. For example, T cells express two distinct receptors for recognizing major histocompatibility complex class 1 proteins, one that mediates a positive signal, the T-cell receptor (TCR), and a second receptor, NKB1, that mediates an inhibitory signal.24,25 The studies indicate that the negative NKB1 signal may inhibit tyrosine phosphorylation of the TCR CD3 ζ-chain. A similar situation also exists for another TCR system. The costimulatory receptor CD28 and the CTLA-4 receptor bind the same ligand and CTLA-4 appears to play a negative regulatory role in CD3/CD28-mediated T-cell activation.26-28 Also, we have recently observed that in polymorphonuclear leukocytes the Fcγ receptor FcγRIIIB, a glycan phosphoinositol-linked receptor protein, similarly can also negatively regulate signaling by FcγRIIA.29 This evidence for positive and inhibitory signals initiated by the same ligand through two distinct cell-surface receptors is similar to our observations with FcγRIIA and FcγRIIB and suggests that such regulation of receptor signaling may be operable in several cell systems.
We also examined the ability of FcγRIIB to regulate FcγRIIIA-mediated phagocytosis using the FcγRIIIA α chain and γ chain chimeric receptor α-γ-γ (EC-TM-CYT). Our results indicate that FcγRIIB inhibition of FcγRIIIA phagocytosis is less pronounced than that of FcγRIIA phagocytosis. This observation is of interest because FcγRIIIA mediates phagocytosis through a γ-chain subunit which is common to both FcγRIIIA and FcεRI.30 These data support other studies which suggest that FcγRIIA and FcγRIIIA differ in their requirements for phagocytosis and that they transmit a phagocytic signal through pathways that differ at some point.15 30 Taken together, the results suggest that not only do FcγRIIA and FcγRIIIA differ in their requirements for phagocytosis, but that their phagocytic signal is differentially regulated.
Thus, the data indicate that the FcγRIIB receptor can regulate phagocytosis transmitted by the FcγRIIA receptor. Because tyrosine phosphorylation of FcγRIIA is important for the phagocytic signal by FcγRIIA,2,10 13 decreased tyrosine phosphorylation induced by FcγRIIB is likely responsible for the inhibition of FcγRIIA-mediated phagocytosis.
Supported by National Institutes of Health Grants No. HL-27068 and AI-22193.
Address reprint requests to Sharon Hunter, PhD, 7 Silverstein, University of Pennsylvania School of Medicine, 3400 Spruce St, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal