Abstract
Targeted interleukin-2 (IL-2) therapy with a genetically engineered antidisialoganglioside GD2 antibody–IL-2 fusion protein induced a cell-mediated antitumor response that effectively eradicated established bone marrow and liver metastases in a syngeneic model of neuroblastoma. The mechanism involved is exclusively natural killer (NK) cell–dependent, because NK-cell deficiency abrogated the antitumor effect. In contrast, the fusion protein remained completely effective in the T-cell–deficient mice or immunocompetent mice depleted of CD8+ T cells in vivo. A strong stimulation of NK-cell activity was also shown in vitro. Immunohistology of the leukocytic infiltrate of livers from treated mice revealed a strong staining for NK cells but not for CD8+ T cells. The therapeutic effect of the fusion protein was increased when combined with NK-cell–stimulating agents, such as poly I:C or recombinant mouse interferon-γ. In conclusion, these data show that targeted delivery of cytokines to the tumor microenvironment offers a new strategy to elicit an effective cellular immune response mediated by NK cells against metastatic neuroblastoma. This therapeutic effect may have general clinical implications for the treatment of patients with minimal residual disease who suffer from T-cell suppression after high-dose chemotherapy but are not deficient in NK cells.
MORE THAN 50% of the patients with stage 4 neuroblastoma initially present with disseminated metastasis to distant organ sites, predominantly bone marrow, making effective treatment for this disease most challenging. Drastic therapeutic interventions with radiotherapy and/or high-dose chemotherapy, followed by allogeneic or autologous bone marrow transplantation,1 have resulted in only minor improvements in the overall survival rate of such patients. Recent encouraging results from phase I and I/II clinical trials using adjuvant therapy with murine (14G2a) and human/mouse chimeric (ch14.18) monoclonal antibodies (MoAbs) directed against the disialoganglioside GD2 showed more than 50% response rates in these patients, including several long-term and complete remissions.2-5Other than their use in the treatment of neuroblastoma and a few other malignancies, eg, colon carcinoma,6 the therapeutic application of unmodified MoAbs has enjoyed only limited success in the clinical setting, despite their extensively documented unique targeting abilities in vivo and in vitro. Effector functions elicited by MoAbs include complement and antibody-dependent cellular cytotoxicity, the latter mediated primarily by such Fc receptor–bearing effector cells as monocytes, macrophages, and natural killer (NK) cells.7,8 A strategy to increase the therapeutic efficiency of MoAbs is to additionally stimulate effector cells by means of immunomodulators, eg, recombinant interleukin-2 (rIL-2). This cytokine stimulates a broad range of immune cells, including T and B cells, monocytes, macrophages, and NK cells. Furthermore, rIL-2 was also shown to generate unique activated lymphocyte populations of NK-cell phenotype, ie, lymphokine activated killer (LAK) cells9,10in vitro and in vivo, which effectively mediate antibody-dependent cellular cytotoxicity.11 Therefore, systemic IL-2, combined with LAK cells, was applied in clinical trials that produced encouraging antitumor responses in some patients with melanoma12 and renal cell carcinoma.13 In contrast, in neuroblastoma such systemic and nonspecific IL-2 treatments resulted in only modest regressions of metastases.14 The promising data from clinical trials with anti-GD2 MoAbs, per se, and the experience from systemic IL-2 therapies led to a first phase I/Ib trial.15 In this study of the Children's Cancer Group, 31 patients with refractory neuroblastoma received IL-2 and 14.G2a and were monitored for toxicities and responses to therapy. One patient had a partial response with a reduction of a large retroperitoneal mass decreasing in size by 75%. A reduction in bone marrow metastases was detectable in three additional patients.
The tumor-specific delivery of immunomodulators, in contrast to systemic delivery, achieves high cytokine concentrations within the tumor microenvironment that effectively stimulate cellular immune responses against syngeneic malignancies. This approach can be accomplished by either cytokine gene therapy or antibody-cytokine fusion proteins. The first approach uses patient-specific, ex vivo genetic modification of autologous tumor cells or fibroblasts to express various cytokines,16 inducing a local inflammatory response capable of mediating a systemic immune response effective against distant metastases. However, patients who are subjected to such immunotherapeutic approaches are frequently pretreated by standard radio-/chemotherapy protocols followed by bone marrow transplantation, which are known to be highly immune suppressive and adversely affect the T-cell–dependent immune system. This therapy is even more severe in cases involving allograft transplantation, because the prevention of graft-versus-host disease requires treatment with cyclosporin A, a strong T-cell–suppressive agent.17,18 Consequently, immunotherapeutic approaches that exclusively rely on the induction and/or redirection of cytotoxic T-cell responses are of limited value in such clinical situations. This is reflected by preliminary results of a clinical trial with autologous neuroblastoma cells transduced with the IL-2 gene that resulted in a relatively low response rate with one complete and one partial response among 14 stage 4 patients.16 Therefore, alternative approaches may be preferable, including the use of a greater and possibly more effective variety of immune cells, including T cells and NK cells.
We recently showed the feasibility of such an alternative approach to cancer immunotherapy that combines the effective concentration of cytokines in the tumor microenvironment with a technically simplemodus operandi.19 Thus, a recombinant fusion protein consisting of tumor-specific antiganglioside GD2antibody and IL-2 specifically induced a CD8+ T-cell response, which was followed by a long-lasting, protective immunity in a syngeneic mouse model of B78 melanoma cells that were transduced with specific transferases to express high levels of the disialoganglioside GD2.20-22 The ongoing clinical efforts in combining IL-2 with anti-GD2 immunotherapy of neuroblastoma provided the rationale for evaluating the efficacy of a recombinant anti-GD2–IL-2 fusion protein in a pathophysiologically relevant, preclinical model of murine neuroblastoma with experimental metastases to bone marrow and liver.
Here, we extend our preclinical findings on the ch14.18–IL-2 fusion protein in a novel, GD2-positive murine neuroblastoma model in immunocompetent A/J mice featuring experimental metastases to various distant organ sites, including bone marrow, which are pathophysiologically highly relevant for human neuroblastoma. We show for the first time that the ch14.18–IL-2 fusion protein can effectively eradicate established bone marrow and liver metastases more efficiently than equivalent mixtures of antibody and recombinant human (rh) IL-2. Furthermore, we show that the effector mechanism involved is exclusively dependent on NK cells. These data support the hypothesis that an effective concentration of cytokines in the tumor microenvironment stimulates NK cells sufficiently to elicit strong antitumor responses in vivo. Therefore, the future clinical application of this type of therapy seems warranted, particularly after high-dose chemotherapy and peripheral blood stem cell rescue, because the NK-cell system is not deficient in this adjuvant setting.23
MATERIALS AND METHODS
Mice
Syngeneic female A/J and C.B-17 severe combined immunodeficiency (SCID) or C.B-17 SCID/BEIGE mice were obtained at 8 weeks of age from Jackson Laboratory (Bar Harbor, ME) and Taconic Farms (Germantown, NY), respectively. They were housed in the pathogen-free mouse colony at our institution in groups of four mice each. Mice were fed ad libitum on standard mouse laboratory chow. Animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cells
The murine NXS2 hybrid neuroblastoma cell line was created by fusion of the GD2-negative C1300 murine neuroblastoma cell line (A/J background) with murine dorsal root ganglional cells from C57BL/6J mice,24 followed by fluorescence and magnetic-activated cell sorting for high GD2 expression, as previously described.25 This hybrid cell line was shown to be major histocompatibility complex (MHC) class I syngeneic to A/J mice, as shown by its H2Kk-positive/H2Kb-negative phenotype.25 GD2-positive NXS2 hybrid neuroblastoma and NK-resistant P815 cells were maintained in Dulbecco's minimal essential medium. GD2-negative TBJ mouse neuroblastoma and NK-sensitive YAC1 cells were cultured in RPMI 1640 at 5% CO2 and 37°C. All media were supplemented with 2 mmol/L glutamine and 10% fetal calf serum (FCS).
Antibody and Antibody–IL-2 Fusion Protein
Mouse-human chimeric antibody ch14.18, directed against disialoganglioside GD2, was constructed by joining the cDNA for the variable region of the murine antibody with the constant regions of the γ1 heavy chain and the κ light chain, as described previously.26 The ch14.18–IL-2 fusion protein was constructed by fusion of a synthetic sequence coding for human IL-2 to the carboxyl terminal of the human Cγ1 gene, as described.27 The fused gene was introduced into the vector pdHL2, which encodes the dihydrofolate reductase gene. The expression plasmid was introduced into Sp2/0-Ag14 cells and selected in the presence of increasing concentrations of methotrexate (100 nmol/L to 5 μmol/L). The fusion protein was purified on Protein A Sepharose, and its specific IL-2 activity was determined in bioassays, as described previously.19,28 ch14.18–IL-2 (1 μg) was found to be equivalent to 3,000 IU rhIL-2.19 28
Experimental Bone Marrow and Liver Metastases
NXS2 cells were harvested by trypsinization and washed three times by centrifugation. Tumor cells were used for induction of metastases only if their cell viability exceeded 95%, as determined by trypan blue staining. Experimental metastases were induced by tail vein injection of either 1 × 106 or 5 × 104 NXS2 cells, respectively, and mice were sacrificed for evaluation after 21 or 26 to 28 days. The number of metastatic liver foci, the percentage of metastatic liver surface, and the liver weight was determined on fresh specimens. For evaluation of bone marrow metastases, the bone cavities of both femurs and tibiae of each animal were flushed with 3 mL phosphate-buffered saline (PBS; pH 7.4). The cell pellet was used for total RNA isolation and subsequent reverse-transcription polymerase chain reaction (RT-PCR) for the detection of tyrosine hydroxylase.
Established liver and bone marrow metastases were detected by sequential analysis of liver and bone marrow in a group of eight mice 5 days after intravenous injection of 5 × 104 NXS2 cells. Livers were divided in half. One half was fixed in 10% buffered formalin followed by paraffin embedding. Sections of three different levels were stained with hematoxylin eosin (HE) and examined microscopically for micrometastases. The second half of each liver was homogenized and lysed in 2 mL RLT buffer, RNeasy (Qiagen, Chatsworth, CA), for subsequent RNA isolation and RT-PCR for tyrosine hydroxylase.
RNA Isolation, RT, and PCR Amplification
Total cellular RNA was isolated by using a commercially available silica gel membrane binding procedure, RNeasy (Qiagen), followed by the synthesis of cDNA with 1 μg RNA in the presence of moloney murine leukemia virus reverse transcriptase, SuperScriptII (GIBCO-BRL, Grand Island, NY), according to manufacturer's guidelines. A denatured cDNA equivalent of 100 ng was used in a 25 μL PCR reaction mixture, which contained 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 0.2 mmol/L deoxynucleotide triphosphate, 2.5 U of Taq DNA polymerase (Grand Island, NY), and 0.5 μmol/L sense and anti-sense oligonucleotide primers for amplification of mouse tyrosine hydroxylase. For the detection of tyrosine hydroxylase, the PCR was adjusted at low and high sensitivity, as described previously. Briefly, for low sensitivity, amplification was done with sense 5′ TCT CAC TTC TTG AAG GAA CG 3′ and anti-sense 5′ CCC CAT TCT GTT TAC ACA GC 3′ for 36 cycles (15 seconds at 96°C, 30 seconds at 63°C, 90 seconds at 72°C) leading to a 325-bp fragment designated TH1. High sensitivity was achieved by nested amplification of 1.5 μL TH1 after 20 cycles using sense 5′ AGT ACA TCC GTC ATG CCT CC 3′ and anti-sense 5′ GAG ATG CAA GTC CAA TGT CC 3′ for 30 cycles to create a 132-bp fragment designated TH2. TH1 and TH2 PCR fragments were analyzed by polyacrylamide gel electrophoresis. The sensitivity of TH1 and TH2 tyrosine hydroxylase RT-PCR was established in reconstituion experiments at detection thresholds of one NXS2 cell in 102 or 105 bone marrow cells, respectively.25 If amplification revealed neither TH1 nor TH2 signals, the cDNA integrity was tested by amplification of glycerol-aldehyde-phosphate-dehydrogenase (GAPDH) with sense 5′ CAT TGA CCT CAA CTA CAT GG 3′ and anti-sense 5′ CAC ACC CAT CAC AAA CAT GG 3′ leading to a 295-bp fragment. The specificity of all fragments was verified by molecular sequencing. According to high- and low-sensitivity tyrosine hydroxylase RT-PCR results, bone marrow metastasis was designated as stage 0 with no PCR signal, stage 1 with an exclusive TH2 signal, and stage 2 in the presence of both TH1 and TH2 signals.
Cytotoxicity Assays
Effector cells were prepared from mouse spleen cells by hypotonic lysis of red blood cells with ACK lysis buffer (GIBCO-BRL, Gaithersburg, MD) and either used for the cytotoxicity assay or for subsequent separation into subpopulations. Enriched NK-cell populations and pure CD8+ effector cells were prepared by magnetic activated cell sorting, Mini MACS (Miltenyi Biotec, Auburn, CA). Briefly, mouse splenocytes were incubated with either anti-mouse CD8 microbeads (Miltenyi Biotec) or biotinylated pan NK-cell antibody DX5 (Pharmingen, San Diego, CA) and followed by labeling with streptavidin microbeads (Miltenyi Biotec). The magnetic-activated cell sorting was performed according to manufacturer's guidelines. The purity of the cell fractions was determined by fluorescence-activated cell sorter (FACS) analysis. Target cells were incubated in the presence of 0.5 μCi Na251CrO4 (Amersham, Cleveland, OH) for 2 hours at 37°C, washed three times, and seeded in a flat-bottom 96-well plate at a density of 5,000 cells per well. Effector cells were added at various effector-to-target cell ratios in a final volume of 200 μL per well and incubated for 4 or 18 hours. Total release was induced with 5 μL sodium dodecyl sulfate (SDS;10%). The supernatant was collected from each well for determination of 51Cr release. The percentage of target cell lysis was calculated as follows:
The results were expressed as mean value ± standard deviation of at least three experiments.
Immunohistology
Frozen sections of livers were fixed in cold acetone for 10 minutes, followed by removal of endogeneous peroxidase with 0.03% H2O2 for 30 minutes at room temperature. Endogenous biotin was removed from liver tissues by incubation with 0.1% avidin for 10 minutes at room temperature, followed by buffer rinses. Excess avidin was neutralized by treatment with low concentration biotin (0.01%) for 10 minutes at room temperature. Nonspecific binding was blocked with 10% species-specific serum in 1% bovine serum albumin (BSA)/PBS. Biotinylated anti-mouse CD45 MoAb (Pharmingen), biotinylated anti-mouse CD8, and NK-cell–specific rabbit anti-asialo GM1 antiserum (WAKO, Richmond, VA) were diluted to 10 μg/mL in 10% goat serum (1% BSA/PBS, pH 7.4) and overlaid onto serial sections. Slides were incubated in a humid chamber for 30 minutes followed by the application of a biotinylated goat anti-rabbit antibody onto slides with anti-asialo GM1 antiserum preincubation for 10 minutes. Streptavidin-labeled alkaline phosphatase was incubated for 10 minutes followed by substrate development using the VectorR Red substrate kit (Vector Laboratories, Burlingame, CA) and hematoxylin nuclear counterstain. All incubations were followed by three wash steps with 0.05 mol/L Tris with 150 mmol/L NaCl, pH 8.0 on sections that received the alkaline phosphatase-conjugated streptavidin and PBS (pH 7.4) on slides that received the peroxidase-conjugated streptavidin.
Statistics
The statistical significance of differential findings between experimental groups of animals was determined by two-tailed Student'st-test. The nonparametric Wilcoxon test was used to determine the statistical significance of metastatic scores. Findings were regarded as significant if two-tailed P values were <.01.
RESULTS
Effector Mechanisms of the Immune Response Induced by the ch14.18–IL-2 Fusion Protein
In Vivo.
We previously reported the complete growth suppression of experimental liver and bone marrow metastases of neuroblastoma by treatment with ch14.18–IL-2 fusion protein, an effect that could not be achieved with a mixture of ch14.18 and rhIL-2 at equivalent concentrations.25 Again, only mice treated with the fusion protein (10 μg, ×6) revealed no signs of experimental metastases to either bone marrow or liver, as determined by RT-PCR of tyrosine hydroxylase or liver weight and count of macroscopic tumor foci, respectively (Table 1). In addition, we proved the specificity of the ch14.18–IL-2 fusion protein therapy, because its complete treatment effect was abrogated against GD2 target antigen–negative TBJ mouse neuroblastoma cells (Table 1).
Therapeutic Effect of Anti-GD2Antibody–IL-2 Fusion Protein on Experimental Liver and Bone Marrow Metastases in Immunocompetent and Immune-Deficient Mice
| Mouse Strain . | Tumor Cells . | Treatment-150 . | Bone Marrow†‡ . | Liver (no. of foci)-152 . | Liver (weight, mg)-152 . |
|---|---|---|---|---|---|
| A/J | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 3,956 ± 402 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 1, 1 | >250, >250, 195, 170, 115, 58 | 2,490 ± 330 | ||
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 1,020 ± 94 | ||
| A/J | TBJ | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 4,070 ± 167 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >37 | 2,692 ± 1,376 | ||
| ch14.18–IL-2 fusion protein | 2, 2, 2, 2, 1, 1 | 173, 55, 46, 31, 27, 15 | 1,170 ± 535 | ||
| C.B-17 SCID | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 3,365 ± 178 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 2 | >250, >250, 97, 85, 53, 25 | 1,543 ± 355 | ||
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 1, 1 | 0, 0, 0, 0, 0, 0 | 1,028 ± 156 | ||
| C.B-17 SCID/BEIGE | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 5,180 ± 717 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 1, 1 | >250, >250, >250, >250, >250, >250 | 3,007 ± 371 | ||
| ch14.18–IL-2 fusion protein | 2, 2, 1, 1, 1, 1 | >250, >250, >250, >250, >250, 200 | 2,205 ± 684 | ||
| ch14.18–IL-2 fusion protein + NK cells-153 | 0, 0, 1, 1 | 0, 0, 4, 10 | 1,170 ± 135 |
| Mouse Strain . | Tumor Cells . | Treatment-150 . | Bone Marrow†‡ . | Liver (no. of foci)-152 . | Liver (weight, mg)-152 . |
|---|---|---|---|---|---|
| A/J | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 3,956 ± 402 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 1, 1 | >250, >250, 195, 170, 115, 58 | 2,490 ± 330 | ||
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 1,020 ± 94 | ||
| A/J | TBJ | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 4,070 ± 167 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >37 | 2,692 ± 1,376 | ||
| ch14.18–IL-2 fusion protein | 2, 2, 2, 2, 1, 1 | 173, 55, 46, 31, 27, 15 | 1,170 ± 535 | ||
| C.B-17 SCID | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 3,365 ± 178 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 2 | >250, >250, 97, 85, 53, 25 | 1,543 ± 355 | ||
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 1, 1 | 0, 0, 0, 0, 0, 0 | 1,028 ± 156 | ||
| C.B-17 SCID/BEIGE | NXS2 | PBS | 2, 2, 2, 2, 2, 2 | >250, >250, >250, >250, >250, >250 | 5,180 ± 717 |
| ch14.18 + IL-2 | 2, 2, 2, 2, 1, 1 | >250, >250, >250, >250, >250, >250 | 3,007 ± 371 | ||
| ch14.18–IL-2 fusion protein | 2, 2, 1, 1, 1, 1 | >250, >250, >250, >250, >250, 200 | 2,205 ± 684 | ||
| ch14.18–IL-2 fusion protein + NK cells-153 | 0, 0, 1, 1 | 0, 0, 4, 10 | 1,170 ± 135 |
Experimental bone marrow and liver metastases were induced by intravenous injection of 106 NXS2 hybrid neuroblastoma cells.
Treatment was initiated 24 hours after tumor cell inoculation by daily intravenous injections ×6 of either PBS, 10 μg ch14.18 antibody + 30,000 IU rIL-2 or 10 μg ch14.18–IL-2 fusion protein.
Bone marrow metastasis was staged according to tyrosine hydroxylase RT-PCR as described in Materials and Methods.
Differences in bone marrow staging, numbers of metastatic liver foci, and liver weights between fusion protein treatment of A/J, C.B-17 SCID and NK-cell reconstituted C.B-17 SCID/BEIGE mice, and all control groups were statistically significant (P < .001).
NK cells were prepared from A/J splenocytes after stimulation with 1,000 IU/mL IL-2 for 5 days. A purity of 85% for these cells was determined by FACS analysis. Each mouse was reconstituted with 3 × 107 cells by three intravenous injections at day 0, 1 and 2.
The effector mechanisms involved in the treatment effect were delineated by using immunodeficient (Table 1) and in vivo–depleted immunocompetent mice (Table 2). In a first set of experiments, we used T- and B-cell–deficient C.B17 SCID mice and C.B17 SCID/BEIGE mice deficient in both T and B cells, as well as NK cells. The ch14.18–IL-2 fusion protein was completely effective in the T- and B-cell–deficient strain. In contrast, the additional absence of NK cells in the SCID/BEIGE mouse completely abrogated the antitumor effect of the fusion protein, implying a mechanism mediated by NK cells. Two additional lines of evidence further established proof for an NK-cell–mediated mechanism evoked by the ch14.18–IL-2 fusion protein. First, the treatment effect was fully restored when SCID/BEIGE mice were reconstituted with NK cells, generated in vitro by incubation of splenocytes from naı̈ve A/J mice with 1,000 IU/mL rhIL-2 (Table 2). Second, depletion of NK cells in A/J mice by anti-asialo GM1 antiserum abrogated the effect of the ch14.18–IL-2 fusion protein, whereas depletion of CD8+ T cells by anti-CD8 antibody had no effect on the efficacy of the fusion protein treatment (Table 2).
Therapeutic Effect of Anti-GD2Antibody–IL-2 Fusion Protein on Experimental Liver Metastasis of NXS2 Cells in Immunocompetent and In Vivo–Depleted A/J Mice
| Depletion* . | Treatment† . | No. of Foci‡ . | Liver Weight (mg)‡ . |
|---|---|---|---|
| None | PBS | >250, >250, >250, >250, >250, >250 | 4,356 ± 459 |
| ch14.18 + IL-2 | >250, 240, 189, 165, 120, 98 | 2,670 ± 361 | |
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 0, 0 | 1,040 ± 52 | |
| Anti-asialo GM1 | ch14.18–IL-2 fusion protein | >250, >250, >250, >250 | 3,913 ± 464 |
| Anti-CD8 | ch14.18–IL-2 fusion protein | 0, 0, 0, 7 | 1,208 ± 108 |
| Depletion* . | Treatment† . | No. of Foci‡ . | Liver Weight (mg)‡ . |
|---|---|---|---|
| None | PBS | >250, >250, >250, >250, >250, >250 | 4,356 ± 459 |
| ch14.18 + IL-2 | >250, 240, 189, 165, 120, 98 | 2,670 ± 361 | |
| ch14.18–IL-2 fusion protein | 0, 0, 0, 0, 0, 0 | 1,040 ± 52 | |
| Anti-asialo GM1 | ch14.18–IL-2 fusion protein | >250, >250, >250, >250 | 3,913 ± 464 |
| Anti-CD8 | ch14.18–IL-2 fusion protein | 0, 0, 0, 7 | 1,208 ± 108 |
Experimental liver metastases were induced by intravenous injection of 10 6 NXS2 hybrid neuroblastoma cells.
At day 3 and 1 before tumor cell inoculation and then once weekly for 3 weeks animals were injected intraperitoneally with either 350 μg anti-CD8 antibody or 100 μl anti-asialo GM1 antiserum, respectively.
Treatment was initiated 24 hours after tumor cell inoculation by daily intravenous injections for 5 days of either PBS, 10 μg ch14.18 antibody + 30,000 IU rIL-2 or 10 μg ch14.18–IL-2 fusion protein.
Differences in numbers of metastatic foci and liver weights between immunocompetent mice receiving fusion protein treatment and such animals depleted of CD8+ T cells and all control groups were statistically significant (P < .001).
In Vitro.
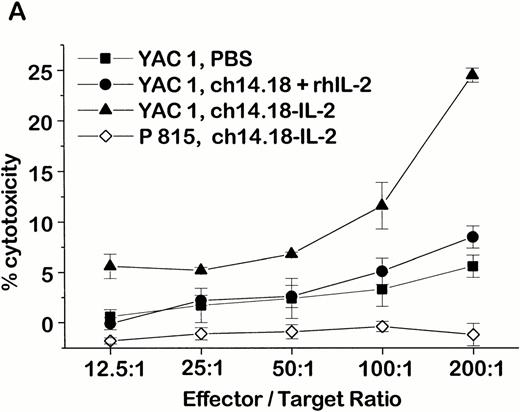
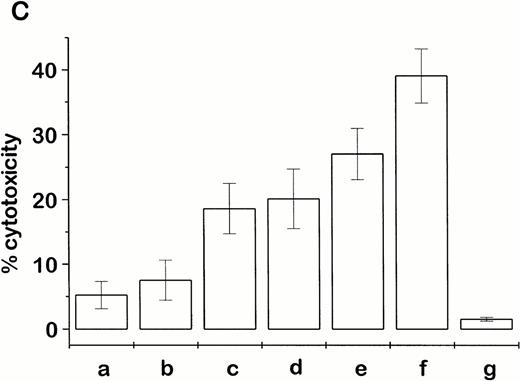
An NK-cell mechanism mediated by the ch14.18–IL-2 fusion protein was also shown in vitro by a strong stimulation of NK-cell–mediated lysis against YAC1 and NXS2 target cells after treatment with ch14.18–IL-2 fusion protein (Fig 1). In this case, tumor-bearing mice were treated with daily intravenous injections (×6) of either 10 μg ch14.18–IL-2 fusion protein, a mixture of 10 μg ch14.18 antibody plus an equivalent amount of rhIL-2 (30,000 IU), or PBS (pH 7.4). When these animals were killed 1 day after completion of the treatment, only splenocytes of mice treated with ch14.18–IL-2 fusion protein showed strong NK activity in a 4-hour chromium release assay (Fig 1A). In contrast, splenocytes from mice treated with PBS or the antibody/IL-2 mixture mediated marginal lysis of NK-sensitive YAC1 cells only at high effector to target cell ratios. Lysis of NK-resistant P815 murine mastocytoma cells by splenocytes of mice that received the ch14-18–IL-2 fusion protein therapy is shown as a control (Fig 1A). This fusion protein–induced stimulation was only inhibited after in vivo depletion of NK cells with anti-asialo GM1 antiserum (Fig 1B). No inhibition of lysis was observed after depletion of CD8+ T cells with anti-CD8 antibody (Fig 1B), which is in agreement with the demonstrated therapeutic effect of the ch14.18–IL-2 fusion protein in vivo (Table 2). After successful tumor therapy with fusion protein, splenocytes from A/J mice produced the strongest lysis of NXS2 cells in the presence of 10 μg/mL ch14.18–IL-2. However, this lysis was less effective in the presence of either equivalent mixtures of ch14.18 antibody and IL-2 or ch14.18 and IL-2 alone (Fig 1C). After magnetic-activated cell sorting of splenocytes from fusion protein–treated mice with T-cell–specific anti-CD8 or NK-cell–specific DX5 antibodies, cytotoxic activity against NXS2 cells was only evident in the NK-cell fraction; only background lysis was shown with pure CD8+ T cells, suggesting a T-cell–independent cytotoxic activity.
Cytotoxic activity of splenocytes from tumor-bearing A/J mice after ch14.18–IL-2 fusion protein therapy. (A) Experimental metastasis was induced by intravenous injection of 1 × 106 NXS2 cells followed by six daily intravenous administrations of either 10 μg ch14.18–IL-2, an equivalent mixture of ch14.18 antibody and rhIL-2, or PBS. Twenty-four hours after completion of the treatment, the cytotoxic activity of splenocytes was tested in a 4-hour chromium release assay against YAC1 (closed symbols) and P815 (open symbols) target cells. (B) Splenocytes of immunocompetent (closed symbols) and in vivo–depleted mice (open symbols) were tested for lysis against YAC1 cells in a 4-hour chromium release assay 24 hours after completion of the treatment with PBS or 10 μg ch14.18–IL-2 fusion protein (daily, ×6, starting 24 hours after induction of experimental metastases). Immunodepletion was started at day −3 and −1 before tumor cell inoculation followed by a once-weekly schedule with intraperitoneal injection of either 350 μg anti-CD8 antibody or 100 μL anti-asialo GM1 antiserum, respectively. (C) Splenocytes of tumor-bearing mice treated with 10 μg ch14.18–IL-2 fusion protein (daily, ×6, starting 24 hours after induction of experimental metastases) were tested for lysis of NXS2 target cells in a 4-hour chromium release assay. Splenocytes were used at an effector-to-target-cell ratio of 100:1 in the presence of (a) PBS; (b) 30,000 IU/mL rhIL-2; (c) 10 μg/mL ch14.18; (d) 30,000 IU/mL rhIL-2+10 μg/mL ch14.18; and (e) 10 μg/mL ch14.18–IL-2. The lysis of NK-cell–enriched (f) and pure CD8+ T-cell (g) subfractions was compared with that of whole splenocytes (e) in the presence of 10 μg/mL ch14.18–IL-2.
Cytotoxic activity of splenocytes from tumor-bearing A/J mice after ch14.18–IL-2 fusion protein therapy. (A) Experimental metastasis was induced by intravenous injection of 1 × 106 NXS2 cells followed by six daily intravenous administrations of either 10 μg ch14.18–IL-2, an equivalent mixture of ch14.18 antibody and rhIL-2, or PBS. Twenty-four hours after completion of the treatment, the cytotoxic activity of splenocytes was tested in a 4-hour chromium release assay against YAC1 (closed symbols) and P815 (open symbols) target cells. (B) Splenocytes of immunocompetent (closed symbols) and in vivo–depleted mice (open symbols) were tested for lysis against YAC1 cells in a 4-hour chromium release assay 24 hours after completion of the treatment with PBS or 10 μg ch14.18–IL-2 fusion protein (daily, ×6, starting 24 hours after induction of experimental metastases). Immunodepletion was started at day −3 and −1 before tumor cell inoculation followed by a once-weekly schedule with intraperitoneal injection of either 350 μg anti-CD8 antibody or 100 μL anti-asialo GM1 antiserum, respectively. (C) Splenocytes of tumor-bearing mice treated with 10 μg ch14.18–IL-2 fusion protein (daily, ×6, starting 24 hours after induction of experimental metastases) were tested for lysis of NXS2 target cells in a 4-hour chromium release assay. Splenocytes were used at an effector-to-target-cell ratio of 100:1 in the presence of (a) PBS; (b) 30,000 IU/mL rhIL-2; (c) 10 μg/mL ch14.18; (d) 30,000 IU/mL rhIL-2+10 μg/mL ch14.18; and (e) 10 μg/mL ch14.18–IL-2. The lysis of NK-cell–enriched (f) and pure CD8+ T-cell (g) subfractions was compared with that of whole splenocytes (e) in the presence of 10 μg/mL ch14.18–IL-2.
Effect of ch14.18-IL-2 Fusion Protein Treatment on Established Bone Marrow and Liver Metastases
To document established bone marrow and liver metastasis, specimens of both organ systems were collected from eight mice each 5 days after injection of 5 × 104 NXS2 cells. The presence of tumor cells was detected by tyrosine hydroxylase RT-PCR and histology. All mice had established liver metastases at that time, as shown by the presence of a TH2 signal, which indicates detection of liver metastases at a sensitivity of one tumor cell in 106 hepatocytes (Fig 2C). Microscopic examination of such livers revealed tumor foci with an average size of 5 to 15 cells, as depicted in Fig 2D. This same analysis of bone marrow on day 5 after tumor cell inoculation indicated established metastases by the presence of a TH2 signal in at least 50% of mice (Fig 2A). Amplification of murine GAPDH was used as a control template for bone marrow samples without a TH2 signal (Fig 2A). It should be noted that the sensitivity of detecting NXS2 cells in naı̈ve bone marrow by tyrosine hydroxylase nested RT-PCR is only one tumor cell in 105bone marrow cells, as previously described.25 However, histological examination of bone marrow specimens also revealed 5 to 15 tumor cell foci, as depicted in Fig 2B. Treatment of such mice with established liver and bone marrow metastases by six daily injections of 40 μg ch14.18–IL-2 fusion protein resulted in complete eradication of bone marrow and liver disease (Table 3).
Demonstration of established bone marrow and liver metastasis after intravenous injection of 5 × 104 NXS2 cells by histology and tyrosine hydroxylase RT-PCR. Mice (n = 8) were killed 5 days after intravenous injection with 5 × 104NXS2 cells. Bone marrow (A and B) and liver (C and D) specimens were analyzed by tyrosine hydroxylase– nested RT-PCR (A and C, top) and histology (B and D). The presence of a TH2 signal indicates NXS2 infiltration into bone marrow (A) or liver (C). GAPDH was amplified with probes lacking a TH2 signal to prove cDNA integrity (A, bottom). A sensitivity of one tumor cell in 106hepatocytes was established with tyrosine hydroxylase–nested RT-PCR of NXS2 cells in liver tissue was established with reciprocal tumor to liver cell ratios of 1:104 to 1:107 (C, lanes 9 to 12). Paraffin-embedded sections of bone marrow and liver specimen were stained with hematoxylin/eosin. Arrows indicate focal tumor cell infiltrates photographed at 1,000 × magnification (oil immersion).
Demonstration of established bone marrow and liver metastasis after intravenous injection of 5 × 104 NXS2 cells by histology and tyrosine hydroxylase RT-PCR. Mice (n = 8) were killed 5 days after intravenous injection with 5 × 104NXS2 cells. Bone marrow (A and B) and liver (C and D) specimens were analyzed by tyrosine hydroxylase– nested RT-PCR (A and C, top) and histology (B and D). The presence of a TH2 signal indicates NXS2 infiltration into bone marrow (A) or liver (C). GAPDH was amplified with probes lacking a TH2 signal to prove cDNA integrity (A, bottom). A sensitivity of one tumor cell in 106hepatocytes was established with tyrosine hydroxylase–nested RT-PCR of NXS2 cells in liver tissue was established with reciprocal tumor to liver cell ratios of 1:104 to 1:107 (C, lanes 9 to 12). Paraffin-embedded sections of bone marrow and liver specimen were stained with hematoxylin/eosin. Arrows indicate focal tumor cell infiltrates photographed at 1,000 × magnification (oil immersion).
Therapeutic Effect of Anti-GD2 Antibody IL-2 Fusion Protein on Established Experimental Metastasis of NXS2 Cells in C.B-17 SCID Mice
| Treatment* . | Metastatic Score† . | Metastatic Score‡ . | Weight (mg) . |
|---|---|---|---|
| Bone Marrow | Liver | Liver | |
| PBS | 2, 2, 2, 2, 2, 2 | 4, 4, 4, 4, 3, 2 | 4,502 ± 1,113 |
| ch14.18 + IL2 | 2, 2, 2, 2 | 4, 4, 4, 3 | 3,145 ± 780 |
| ch14.18–IL22-153 | 0, 0, 0, 0 | 0, 0, 0, 0 | 1,028 ± 37 |
| Treatment* . | Metastatic Score† . | Metastatic Score‡ . | Weight (mg) . |
|---|---|---|---|
| Bone Marrow | Liver | Liver | |
| PBS | 2, 2, 2, 2, 2, 2 | 4, 4, 4, 4, 3, 2 | 4,502 ± 1,113 |
| ch14.18 + IL2 | 2, 2, 2, 2 | 4, 4, 4, 3 | 3,145 ± 780 |
| ch14.18–IL22-153 | 0, 0, 0, 0 | 0, 0, 0, 0 | 1,028 ± 37 |
Experimental bone marrow and liver metastases were induced by intravenous injection of 5 × 104 NXS2 hybrid neuroblastoma cells.
Treatment was initiated at day 5 after tumor cell inoculation by daily intravenous injections ×6 of either PBS, 40 μg ch14.18 antibody + 120,000 IU rhIL-2, or 40 μg ch14.18–IL-2 fusion protein.
Bone marrow metastases were staged according to results obtained by high- and low-sensitivity tyrosine hydroxylase RT-PCR as described in Materials and Methods.
Liver metastases were staged according to the percentage of metastatic liver surface: 0, 0%; 1 <0% to 25%; 2, 25% to 50%; 3, 50% to 75%; 4 ≥75%.
Differences in bone marrow staging, numbers of metastatic liver foci, and liver weights between fusion protein treatment and all control groups were statistically significant (P < .001).
Mechanism of the Antitumor Response Induced by the ch14.18–IL-2 Fusion Protein Against Established NXS2 Neuroblastoma Metastases
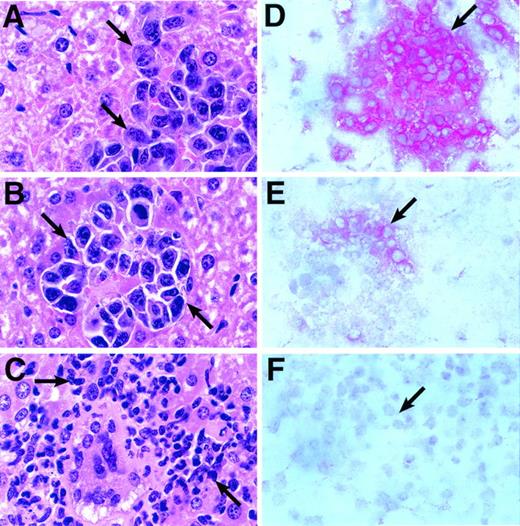
A reduction in dosage from 40 μg (×6) to 10 μg ch14.18–IL-2 (×5) still resulted in a significant reduction of liver weights in fusion protein treated animals with established liver metastases (P < .001; Table 4). Histological examination of livers from such mice revealed an infiltrate of polymorphonuclear cells as well as mononuclear cells (Fig 3C). This is in contrast to livers from mice that showed no inflammatory infiltrates when treated with either PBS (pH 7.4) or a mixture of 10 μg ch14.18 antibody plus an equivalent amount of rhIL-2 (30,000 IU; Fig 3A and B). Immunophenotyping of such cellular infiltrates revealed strong staining with anti-asialo GM1 antiserum, a well-established marker for mouse NK cells (Fig 3E). In contrast, only background staining was observed in adjacent sections with monoclonal anti-CD8 antibody, a marker for cytotoxic T cells (Fig 3F). Staining with anti-mouse CD45 antibody, recognizing a common leukocyte antigen, is shown as a control (Fig 3D). None or only very weak staining was observed with monoclonal anti-CD4 antibody (data not shown). The lack of a CD8+T-cell–mediated systemic immune response was shown when NXS2 tumor challenge of A/J mice, previously successfully treated with ch14.18–IL-2 fusion protein, did not result in a delay of subcutaneous tumor growth (Table 4).
Effect of NK Cell Activation on Anti-GD2Antibody IL-2 Fusion Protein Therapy of Established Experimental Neuroblastoma Metastases
| Activation3-150 . | Treatment3-151 . | Bone Marrow . | Liver . | Liver . | Challenge3-152 . |
|---|---|---|---|---|---|
| Metastatic score3-153 | Metastatic score¶ | Weight [mg] | Subcutaneous tumor [μL] | ||
| None | PBS | 2, 2, 2, 2, 2, 1 | 4, 4, 4, 3, 3, 1 | 2,620 ± 1,101 | 129 ± 19 |
| ch14.18 + IL-2 | 2, 1, 1, 1, 1, 1 | 4, 4, 4, 2, 1, 1 | 2,231 ± 1,173 | 119 ± 21 | |
| ch14.18–IL-23-155 | 2, 2, 1, 1, 1, 1, 1, 1 | 2, 2, 1, 1, 1, 1 | 1,170 ± 535 | 141 ± 15 | |
| Poly IC | PBS | 2, 2, 2, 2, 2, 1 | 4, 4, 2, 2, 1, 1 | 1,623 ± 726 | |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 1 | 1, 1, 1, 1, 1, 0 | 998 ± 70 | ||
| ch14.18–IL-23-155 | 2, 2, 1, 1, 1, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 986 ± 89 | ||
| mIFN-γ | ch14.18–IL-23-155 | 0, 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0, 0 | 1,077 ± 120 |
| Activation3-150 . | Treatment3-151 . | Bone Marrow . | Liver . | Liver . | Challenge3-152 . |
|---|---|---|---|---|---|
| Metastatic score3-153 | Metastatic score¶ | Weight [mg] | Subcutaneous tumor [μL] | ||
| None | PBS | 2, 2, 2, 2, 2, 1 | 4, 4, 4, 3, 3, 1 | 2,620 ± 1,101 | 129 ± 19 |
| ch14.18 + IL-2 | 2, 1, 1, 1, 1, 1 | 4, 4, 4, 2, 1, 1 | 2,231 ± 1,173 | 119 ± 21 | |
| ch14.18–IL-23-155 | 2, 2, 1, 1, 1, 1, 1, 1 | 2, 2, 1, 1, 1, 1 | 1,170 ± 535 | 141 ± 15 | |
| Poly IC | PBS | 2, 2, 2, 2, 2, 1 | 4, 4, 2, 2, 1, 1 | 1,623 ± 726 | |
| ch14.18 + IL-2 | 2, 2, 2, 2, 2, 1 | 1, 1, 1, 1, 1, 0 | 998 ± 70 | ||
| ch14.18–IL-23-155 | 2, 2, 1, 1, 1, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 986 ± 89 | ||
| mIFN-γ | ch14.18–IL-23-155 | 0, 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0, 0 | 1,077 ± 120 |
Experimental bone marrow and liver metastases were induced by intravenous injection of 5 × 104 NXS2 hybrid neuroblastoma cells.
Animals received either 3 daily injections of 100 μg polyriboinosinic:polyribocytidylic acid (poly IC) intraperitoneal or rmIFN-γ 300,000 IU subcutaneously during 6 days with a micro-osmotic pump (ALZET, Alza Corp, Palo Alto, CA), starting on day 4 after tumor cell inoculation.
Treatment was initiated on day 5 after tumor cell inoculation by daily intravenous injections ×5 of either PBS, 10 μg ch14.18 antibody + 30,000 IU rhIL-2 or 10 μg ch14.18–IL-2 fusion protein.
Mice were challenged by subcutaneous injection of 1 × 106 NXS2 cells 3 days after completion of treatment. Volumes of subcutaneous tumors were calculated by measuring width/2 × width × length at day 11 after subcutaneous inoculation and expressed as cubic millimeters ± standard error.
Bone marrow metastasis was staged according to results obtained by high- and low-sensitivity tyrosine hydroxylase RT-PCR as described in Materials and Methods.
¶Liver metastases were staged according to the percentage of metastatic liver surface: 0, 0%; 1, <0% to 25%; 2, 25% to 50%; 3, 50% to 75%; 4, >75%.
Differences in liver staging, or liver weights between fusion protein treatment and PBS control groups were statistically significant (P < .001).
Histological and immunohistochemical analyses of tumor-bearing mice after fusion protein therapy. Five days after intravenous inoculation with 5 × 104 NXS2 cells, mice with established liver metastases were treated by daily (×5) intravenous injections of either 10 μg ch14.18–IL-2 fusion protein, an equivalent mixture of 10 μg ch14.18 and 30,000 IU rhIL-2, or PBS (pH 7.4). Twenty-four hours after completion of treatment, paraffin-embedded sections of livers from either PBS (A), IL-2/antibody mixture (B), and fusion protein (C) treated animals were stained with hematoxylin/eosin. Tumor foci or infiltrates are depicted at a magnification of 1,000×. Arrows delineate tumor foci (A and B) or inflammatory cells (C). Frozen liver sections of mice treated with fusion protein were stained with monoclonal anti-leukocyte CD45 antibody (D), anti-asialo GM1 antiserum (E), and monoclonal anti-CD8 antibody (F). Red cells indicate positive staining for each marker.
Histological and immunohistochemical analyses of tumor-bearing mice after fusion protein therapy. Five days after intravenous inoculation with 5 × 104 NXS2 cells, mice with established liver metastases were treated by daily (×5) intravenous injections of either 10 μg ch14.18–IL-2 fusion protein, an equivalent mixture of 10 μg ch14.18 and 30,000 IU rhIL-2, or PBS (pH 7.4). Twenty-four hours after completion of treatment, paraffin-embedded sections of livers from either PBS (A), IL-2/antibody mixture (B), and fusion protein (C) treated animals were stained with hematoxylin/eosin. Tumor foci or infiltrates are depicted at a magnification of 1,000×. Arrows delineate tumor foci (A and B) or inflammatory cells (C). Frozen liver sections of mice treated with fusion protein were stained with monoclonal anti-leukocyte CD45 antibody (D), anti-asialo GM1 antiserum (E), and monoclonal anti-CD8 antibody (F). Red cells indicate positive staining for each marker.
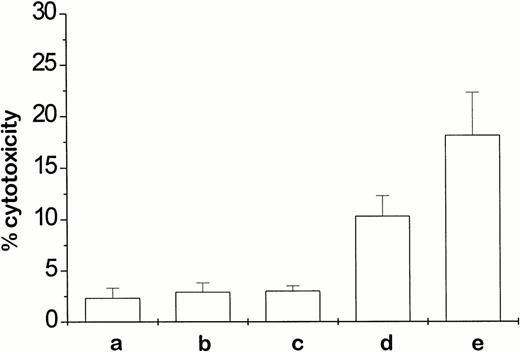
Additional activation of NK cells with polyriboinosinic:polyribocytidylic acid (poly IC; 100 μg intraperitoneally, ×3, days 4 to 6) or recombinant mouse interferon-γ (rmIFNγ; 300,000 IU subcutaneously/6 days, ALZET micro-osmotic pump [Alza Corp, Palo Alto, CA]) resulted in an increased antitumor response if applied in combination with the ch14.18–IL-2 fusion protein (Table 4). In fact, the combination with rmIFN-γ led to a complete eradication of liver and bone marrow metastases (Table 4). Splenocytes from these animals, collected 24 hours after completion of treatment, produced the highest in vitro cytotoxic effect against NXS2 target cells. In comparison, splenocytes from mice treated with either the fusion protein or rmIFN-γ alone were far less effective (Fig 4). The question was addressed of whether the mIFN-γ–induced increase in expression of MHC class I antigens is followed by a switch from NK-cell to T-cell effector activity. In fact, FACS analyses showed a sevenfold increase of H2Kk expression after incubation of NXS2 cells with 100 IU mIFN-γ for 48 hours in vitro. Furthermore, the subcutaneous application of 300,000 IU mIFN-γ by way of micro-osmotic pump over 6 days led to a threefold increase in H2Kk expression in subcutaneous tumors that were induced by subcutaneous injection of 5 × 106 NXS2 cells 3 days before mIFN-γ application (data not shown). However, in spite of this increase in MHC class I antigens, splenocytes from mice with established metastases that were successfully treated with a combination therapy of ch14.18–IL-2 fusion protein and mIFN-γ did not kill NXS2 target cells in an MHC class I–restricted fashion (Fig 4). On the contrary, the addition of anti-MHC class I antibodies to the assay actually increased the cytolytic effect of splenocytes obtained from animals 24 hours after the completion of the ch14.18–IL-2 and mIFN-γ combination treatment (Fig 4). This increase cannot be attributed to antibody-dependent cellular cytotoxicity mediated by the addition of H2Kk MHC class I antibodies, because lysis of NXS2 cells with splenocytes from PBS-treated control animals was not increased in the presence of these antibodies (data not shown). These data suggest an inhibitory effect of MHC class I antigens in this system, which is, incidentally, a typical feature of NK-cell–mediated responses.
In vitro lysis of NXS2 neuroblastoma cells by splenocytes from tumor-bearing A/J mice after treatment with fusion protein. Five days after intravenous inoculation with 5 × 104 NXS2 cells, mice with established liver metastases were treated by daily (×5) intravenous injections with either 10 μg ch14.18–IL-2 fusion protein or PBS (pH 7.4), in the presence or absence of additional rmIFN-γ (300,000 IU/6 days subcutaneous via ALZET osmotic pump). Cytotoxic activity was determined 24 hours after completion of treatment in an 18-hour chromium release assay against NXS2 target cells using splenocytes at an effector-to-target-cell ratio of 100:1. Splenocytes were obtained from mice previously treated with either PBS (a), rmIFN-γ (b), fusion protein (c), fusion protein + rmIFN-γ (d), or additional anti-H2Kk antibody (25 μg/mL) in vitro (e).
In vitro lysis of NXS2 neuroblastoma cells by splenocytes from tumor-bearing A/J mice after treatment with fusion protein. Five days after intravenous inoculation with 5 × 104 NXS2 cells, mice with established liver metastases were treated by daily (×5) intravenous injections with either 10 μg ch14.18–IL-2 fusion protein or PBS (pH 7.4), in the presence or absence of additional rmIFN-γ (300,000 IU/6 days subcutaneous via ALZET osmotic pump). Cytotoxic activity was determined 24 hours after completion of treatment in an 18-hour chromium release assay against NXS2 target cells using splenocytes at an effector-to-target-cell ratio of 100:1. Splenocytes were obtained from mice previously treated with either PBS (a), rmIFN-γ (b), fusion protein (c), fusion protein + rmIFN-γ (d), or additional anti-H2Kk antibody (25 μg/mL) in vitro (e).
DISCUSSION
The induction of an effective cellular immune response against syngeneic tumors by a local increase of inflammatory cytokines in the tumor microenvironment is a promising approach in hematology/oncology. In contrast to patient-specific ex vivo transduction of an individual's tumor cells or fibroblasts by cytokine genes, our approach is an attempt to induce a cellular antitumor immune response by using a fusion protein to direct cytokines to the tumor microenvironment. Such recombinant antibody-cytokine fusion proteins combine the unique targeting ability of antibodies with the inflammatory activity of cytokines and can be applied by a simplemodusoperandi in a patient-independent manner.
Here, we show the superiority of a recombinant anti-GD2ch14.18–IL-2 fusion protein over a mixture of equivalent amounts of this cytokine with ch14.18 antibody in effectively eradicating established experimental neuroblastoma metastases to bone marrow and liver. This effect was achieved by NK cells in a novel, immune competent syngeneic model for murine neuroblastoma that naturally expresses GD2. This tumor model has many pathophysiological similarities with human neuroblastoma and expresses the tumor marker tyrosine hydroxylase. In fact, two thirds of neuroblastoma patients initially present with bone marrow metastases.29 Most of these patients suffer from minimal residual disease to the bone marrow after conventional therapy protocols and high-dose chemotherapy followed by peripheral stem cell rescue. This was substantiated by highly sensitive detection systems such as anti-GD2immunohistochemistry or tyrosine hydroxylase RT-PCR.30-33
Here, we present the first evidence for a mechanism by which the ch14.18–IL-2 fusion protein induces NK-cell–mediated suppression of tumor growth and eradication of established bone marrow and liver metastases in a syngeneic model for murine neuroblastoma. This is in contrast to a previously described mechanism by which this same fusion protein induced a T-cell–mediated immune response, independent of NK cells, in a syngeneic model for murine melanoma.20-22 To assess whether low expression of MHC class I antigens might favor NK cells over T cells in this neuroblastoma tumor model, the expression of these antigens was increased by the addition of mIFN-γ. Although mIFN-γ strongly upregulated MHC class I expression in vitro and in vivo, splenocytes from mice successfully treated with a combination therapy of ch14.18–IL-2 fusion protein and mIFN-γ were incapable of MHC class I–restricted killing of NXS2 target cells. Thus, a switch from an NK- to a T-cell–mediated mechanism did not occur. In fact, quite the opposite was observed as the addition of anti-MHC class I antibodies increased the cytotolytic effect of splenocytes obtained from mice 24 hours after completion of the ch14.18–IL-2/mIFN-γ combination treatment. This is a pattern for NK-cell–mediated killing of tumor cells, beause NK-cell activity can be downregulated by properly stimulated lectin type-C inhibitory NK-cell receptors of the LY49 family that are specific for MHC class I molecules.34 35 In our experiments this downregulation of NK-cell activity was counteracted by the addition of MHC class I blocking antibodies. Therefore, the increase achieved in antitumor activity of the ch14.18–IL-2 fusion protein by the further application of mIFN-γ in vivo can be attributed to an additional stimulation of NK cells. This contention is supported by the finding that splenocytes from mice treated with the ch14.18–IL-2/mIFN-γ combination therapy achieved the highest lysis of YAC1 cells, as compared with mIFN-γ and ch14.18–IL-2 controls (data not shown).
The exclusive response by NK cells in our neuroblastoma model was underlined by the effect of subcutaneous tumor cell challenge on fusion protein–treated mice. Specifically, when such mice were challenged by subcutaneous injection of NXS2 cells 3 days after completion of the treatment, they developed subcutaneous tumors similar to untreated mice or mice receiving the equivalent mixture of antibody and IL-2 (Table4). The absence of a T-cell–mediated immune response in this particular model and its concomitant replacement by NK cells might be caused by factors secreted by the tumor cells that suppress T cells but stimulate NK cells. In fact, we observed that NXS2 cells produce transforming growth factor-β1 (TGF-β1) and IL-10 in vitro and in vivo (data not shown), both immunomodulators associated with T-cell anergy.36-41 However, IL-10 is a factor that was also found to stimulate NK cells, because they showed an increase in [3H]thymidine uptake and killing of NK-sensitive target cells after incubation with IL-10.42 However, it was also shown that IL-10 can inhibit tumor metastasis by an NK-cell–dependent mechanism.43 Consequently, it is possible that the presence of TGF-β1 and IL-10 in our model may favor NK-cell–dependent antitumor mechanisms by causing NK-cell stimulation and concomitant T-cell anergy.
The availability of effective adjuvant treatment for neuroblastoma in the postchemotherapy and transplant phase remains as a major challenge in pediatric hematology/oncology. In this regard, the effectiveness of the ch14.18–IL-2 fusion protein is striking, especially in view of the effector mechanism involved. Thus, we could clearly show in vivo and in vitro that the ch14.18–IL-2 fusion protein stimulates a cellular antitumor response exclusively mediated by NK cells. It is of considerable interest that NK cells, in contrast to T cells, were not found to be deficient in patients with solid and hematological malignancies, even after high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation.23 This finding suggests that neuroblastoma patients who are deficient in T cells following high-dose chemotherapy still have NK effector cells that, if properly stimulated, can effectively elicit an antitumor response. When tumor growth and progression of patients can be held to a minimum, they may eventually recover their T-cell–dependent immune system. Once this is achieved, T-cell–dependent effector mechanisms could become more effective for tumor cell killing. However, because most human neuroblastoma cells or cell lines show a low expression of MHC class I molecules,44 45 few, if any, effective T-cell–dependent antitumor responses, followed by a protective immunity with a T-cell memory, were observed thus far in neuroblastoma. Because stimulated NK cells proved to be very effective in our animal model in the absence of a memory immune response, multiple treatments with the ch14.18–IL-2 fusion protein may well be required to achieve optimal antitumor responses in neuroblastoma patients.
In summary, we show here that NK cells stimulated by the ch14.18–IL-2 fusion protein can effectively suppress tumor dissemination and growth and effectively eradicate established bone marrow metastasis in a syngeneic model of neuroblastoma in A/J mice. The mechanism responsible for this antitumor effect proved to be exclusively dependent on NK cells. Taken together, our preclinical data described here suggest that the application of the ch14.18–IL-2 fusion protein in an adjuvant setting may lead to further improvement in the treatment of neuroblastoma patients with minimal residual disease.
ACKNOWLEDGMENT
We thank Carrie Dolman for her excellent technical assistance and also express our appreciation to Lynne Kottel for the preparation of this manuscript. We extend special thanks to Petra Kleindienst for her dedicated help with FACS and RT-PCR analyses.
Supported by the National Institutes of Health Outstanding Investigator's Award Grant No. CA-42508 (R.A.R.). H.N.L. was supported by a training grant of the Deutsche Forschungsgemeinschaft.
Address reprint requests to Ralph A. Reisfeld, PhD, The Scripps Research Institute, Department of Immunology, 10550 N Torrey Pines Rd, IMM13, La Jolla, CA, 92037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal