Abstract
Lymphocyte recirculation facilitates the detection and elimination of pathogens and the dissemination of immunologic memory. It is generally assumed that all small lymphocytes in the blood are actively recirculating, yet there is little quantitative data directly comparing the migration of this population with actively recirculating, lymph-derived lymphocytes. In this study blood lymphocytes were labeled with fluorescein isothiocyanate (FITC), and lymph lymphocytes were labeled with CM-DiI, reinfused intravenously, and monitored in blood and lymph. After equilibration the concentration of blood lymphocytes was several times higher in blood than in lymph, whereas lymph lymphocytes displayed the opposite behavior. This suggested that blood lymphocytes did not recirculate as efficiently as lymph lymphocytes, so we examined the following blood lymphocyte subsets in greater detail: B cells, CD4+, CD8+, and γδ T cells. Within 4 hours postinjection the percentage of FITC+CD8+ and CD4+ lymphocytes fell in the blood and remained significantly lower than the injected sample. In contrast, the concentration of FITC+ γδ T cells did not change, and the percentage of FITC+ B cells increased. These data suggest that subpopulations of B and perhaps γδ T lymphocytes in the blood do not recirculate efficiently through lymph nodes.
THE EXPERIMENTS of Gowans1,2showed conclusively that lymphocytes recirculate from blood to lymph. This landmark discovery triggered intense interest in the underlying mechanisms and immunological relevance of this phenomenon. The majority of lymphocyte recirculation experiments since performed have used rats or mice, and these systems have provided the framework for our current understanding of the molecular basis of lymphocyte-endothelial interaction and lymphocyte homing. On the other hand, research using larger species such as sheep has permitted the study of the flux of lymphocytes through individual lymph nodes and discrete anatomic compartments and has permitted a very detailed analysis of the physiology of lymphocyte recirculation. The picture that has emerged using all of these systems is that the migration of lymphocytes is not entirely random but rather is regulated by the interaction of adhesion molecules on lymphocytes and vascular endothelium. Superimposed on tissue-specific homing of lymphocyte pools is the phenomenon of subset-specific homing; venous blood, afferent lymph, and efferent lymph all contain different proportions of lymphocyte subsets, such as B cells and CD4+, CD8+, and γδ T cells.3-5 Many studies using sheep have documented the tendency of certain lymphocyte subsets to recirculate through afferent versus efferent lymph or to migrate through mesenteric rather than subcutaneous lymph nodes.6-8 The phenomenon of lymphocyte homing does not adequately explain the different proportions of lymphocyte subsets in blood and lymph, leading to speculation that certain lymphocyte subsets in the blood may not possess the ability to migrate through lymph nodes into efferent lymph.7 Other studies in which lymph-derived lymphocytes were labeled and reinfused intravenously showed that these actively recirculating cells did not distribute randomly between the blood and lymph compartments. Rather, within about 1 day their concentration became approximately twofold higher in lymph than in blood, and this behavior was stable for more than 2 months.9,10 One interpretation of this finding is that certain subpopulations of blood lymphocytes recirculate poorly through lymph nodes. This would have the effect of lowering the percentage of labeled lymph lymphocytes in the blood compartment.11 It is important to understand the behavior of such subpopulations, because their migration through lymphoid and nonlymphoid tissue, and their role in immunity, could be unique from lymphocytes that actively recirculate through lymph nodes.
The blood is a compartment that receives lymphocytes from the thoracic duct (which receives lymphocytes from the lymph nodes and Peyer's patches), other lymphatics from the head and neck, the spleen, bone marrow, thymus, and other sources. Lymphocytes are exchanged between the blood and these tissues many times per day, and the relationship between blood lymphocytes and each of the different organs seems to be quite unique. For example, different molecular mechanisms are involved in the regulation of lymphocyte extravasation and migration into lymph nodes versus the spleen. The exit routes are unique as well; lymphocytes do not recirculate (travel from blood to lymph) through the spleen as they do through lymph nodes, but rather they migrate directly back to the blood.12 Also, the blood pool of lymphocytes is in equilibrium with large reservoirs of lymphocytes that marginate in the microvasculature of nonlymphoid organs, such as the lung.13,14 It is likely that the cell adhesion mechanisms in the microvessels of this tissue are unique.15 Because the behavior of lymphocytes is different in each of these tissues, it should not be assumed that the blood is a homogeneous source of recirculating lymphocytes.
Lymphocyte recirculation experiments in the sheep have generally used lymph-derived cells, because efferent lymph is a virtually pure source of lymphocytes with a known capacity to recirculate.16,17Fewer studies have examined the blood to lymph recirculation of blood lymphocytes,18 19 particularly in a way that would reveal any differences in the recirculatory ability of lymphocyte subsets obtained from blood versus lymph. The most direct way to do this would be to compare the recirculation of lymph lymphocytes from multiple lymphoid compartments with that of blood lymphocytes obtained from the same animal. If the latter population failed to recirculate as efficiently as the former, this would provide direct evidence for a unique blood lymphocyte population with distinctive recirculatory patterns. In this report the two lymphocyte populations were labeled with different fluorescent dyes, and their recirculation from blood to lymph was monitored. Also, the concentration of directly labeled lymphocytes was great enough that the recirculation of different subsets of blood lymphocytes could also be examined. When the results are considered in the context of the available literature on the recirculation of lymph lymphocytes, it seems likely that blood γδ T cells and B cells can be further subdivided into subsets that recirculate efficiently and others that recirculate poorly through lymph nodes. These findings may help to explain several of the seemingly contradictory reports on the recirculation of lymphocyte subsets.
MATERIALS AND METHODS
Animals
Fifteen female sheep, approximately 6 to 8 months old and 25 to 35 kg body weight, were used for these studies. They were fed hay and pellets twice daily, and had free access to water. All handling and experimental procedures were performed in accordance with the Canadian Council on Animal Care, the Animals for Research Act of Ontario, and the Animal Care Committee at Sunnybrook Health Science Centre.
Protocol 1. Comparison of the Recirculation of Blood Lymphocytes and Lymph Lymphocytes
Surgery and lymphocyte labeling.
All surgical procedures were performed under sterile conditions. Animals were anesthetized with pentothal sodium (15 to 25 mg/kg intravenously; Boehringer Ingelheim, Burlington, Ontario, Canada). They were intubated with an endotracheal tube, and anesthesia was maintained with 2% halothane (Fluothane; Ayerst Laboratories, New York, NY) in O2. A catheter with a three-way stopcock was surgically positioned in the jugular vein. Also, surgical access was gained to an efferent subcutaneous lymphatic vessel (two animals) or gut efferent vessel (three animals), and a chronic fistula was established. Several thorough descriptions are available of the precise anatomical location of these lymph nodes and the surgical methods used.17 20Essentially, 1 to 2 cm of the lymphatic vessel was exposed and ligated, then a small incision was made in the wall. Polyvinyl chloride tubing (SV45; Dural Plastics and Engineering, Dural, Australia) with an outside diameter ranging from 0.6 to 1.2 mm was inserted into the lymph vessel and sutured firmly in place. The wound was carefully closed, and lymph was diverted into a sterile 250-mL or 500-mL bottle affixed to a holder on the skin of the sheep. Heparin (300 to 500 IU; Hepalean; Organon Teknika, Toronto, Ontario, Canada) and penicillin G potassium (7,000 to 10,000 IU; Marsam, Toronto, Ontario, Canada) were added to the bottle every time a collection was made. Animals were allowed to recover until at least the following day before experiments were performed. All animals were given 0.005 mg/kg intramuscular buprenorphine HCl analgesic (Temgesic; Reckitt and Colman, Hull, UK) immediately after waking from surgery and as needed thereafter.
To gain access to subcutaneous afferent lymph, “pseudoafferent” lymph vessels were generated in two animals.17 Studies of peripheral lymph (draining directly from tissues) are hindered because the tiny afferent lymphatic vessels are prone to clotting. However, if a lymph node is surgically removed, within 4 to 6 weeks the numerous afferent lymphatic vessels formerly entering the node will anastomose with the larger, single efferent lymphatic vessel, thereby permitting the collection of relatively large volumes of peripheral lymph. Cytospins of the lymph were made and counterstained with Leishman's stain to confirm the presence of dendritic cells/macrophages, which are not normally seen in efferent lymph.
Cell labeling and collection of blood and lymph samples.
The methodologies used for labeling and tracking blood lymphocytes with fluorescein isothiocyanate (FITC) and lymph lymphocytes with CM-DiI have been published elsewhere.21,22 These protocols were shown to have minimal impact on the ability of the cells to recirculate. Also, the viability of lymphocytes after labeling was assessed by the exclusion of 2% trypan blue dye and found to be 80% to 96% when labeling lymph lymphocytes with CM-DiI22 and greater than 95% when labeling blood lymphocytes with FITC.21 The labeling procedure for lymph lymphocytes was similar to established protocols with other carbocyanine dyes, but the blood lymphocyte labeling technique was unique in that all of the cellular components in a sample of whole blood were labeled with FITC and reinjected into the animal. The time required for blood labeling was approximately 3 hours, and the temporary depletion was well tolerated by the animals. The blood volume of the sheep is 75.1 ± 4.6 mL/kg,23 so the 300-mL sample represented about 11% to 16% of their blood volume. Other investigators have reported that losses of 25% or more cause no apparent distress to sheep.24 This blood labeling technique circumvented the use of lymphocyte isolation procedures, which could potentially alter the ability of the cells to recirculate. Dead, damaged, or overlabeled cells are rapidly cleared following intravenous injection and never reach lymph.21 To briefly describe the procedure, 300 mL blood was obtained, washed two times with Hanks' Balanced Salt Solution (HBSS) to remove free protein, and labeled with 600 mL FITC for 30 minutes at 4°C. The cells were washed twice to remove free FITC, resuspended in Ringer's lactate to a total volume of 300 mL, then reinfused intravenously. Upon reinfusion, the FITC+lymphocytes were distinguished from other labeled cells using flow cytometry. Typically, 1 × 109 lymph lymphocytes and 0.2 to 1.2 × 109 blood lymphocytes were labeled and reinfused.
Control experiment.
These experiments showed clear differences in the recirculation of blood lymphocytes and lymph lymphocytes, but it was necessary to rule out the possibility that this was a consequence of the unique labeling conditions used for blood lymphocytes. To determine whether the recirculation of lymph lymphocytes would be hindered when subjected to the blood-labeling protocol, the following experiment was performed. A sample of blood was depleted of leukocytes by repeated centrifugation and aspiration of the buffy coat. After the blood was over 90% depleted of leukocytes as determined using a Coulter cell counter (Coulter Immunology, Hialeah, FL), 1 × 109lymph lymphocytes were added to the red blood cells (RBCs), and this sample was labeled with FITC using the standard blood lymphocyte labeling protocol. The cells were infused and their recirculation monitored. If the labeling procedure damaged the cells or altered their recirculation, it was expected that they would be eliminated from the blood and their concentrations in lymph would not approach the values predicted by previous investigations.10
Data analysis.
Lymph was obtained in sequential collections usually ranging from 6 to 12 hours, and a blood sample was taken every time the lymph collection bottle was replaced. Erythrocytes were lysed using Tris:NH4Cl solution, then the blood and lymph samples were washed 2 times in HBSS. After fixation in 1% paraformaldehyde for 1 hour the samples were washed in HBSS and stored for subsequent analysis. The concentration of labeled lymphocytes was determined using a Coulter Epics Elite flow cytometer (Coulter Immunology) by gating on small lymphocytes and further gating on FITC+ cells. At least 1 × 105 lymphocytes were counted per sample, and the number of FITC+ or CM-DiI+cells detected varied from 100 to 1,000, depending on the nature of the sample and the duration of the experiment (ie, the concentration of labeled cells decreased over time). The sensitivity and alignment of the instrument were monitored daily using Coulter Immunocheck fluorescent beads. For each time point the ratio of FITC+and CM-DiI+ cells in blood and lymph was calculated. After the initial equilibration period of approximately 1 day this ratio was consistent throughout most experiments; therefore, the data from all samples taken after 40 hours were pooled and expressed as an average for each animal. Between three and eight paired blood and lymph samples were obtained per animal over a period of 3 to10 days, depending on the patency of the venous and lymphatic catheters. Table 1 provides the duration of each experiment (days) and the number of samples obtained (N).
Blood/Lymph Ratio of FITC+ Blood Lymphocytes Versus CM-Dil+ Lymph Lymphocytes
| Experiment . | Lymph Source . | Days-150 . | N-151 . | Ratio Blood/Lymph . | |
|---|---|---|---|---|---|
| Blood Lymphos . | Lymph Lymphos . | ||||
| 1 | Subcutaneous pseudoafferent | 10.7 | 5 | 2.92 ± 0.03 | ND |
| 2 | Subcutaneous pseudoafferent | 6.5 | 3 | 2.51 ± 0.17 | ND |
| 3 | Subcutaneous efferent | 8.4 | 8 | 1.96 ± 0.13 | ND |
| 4 | Subcutaneous efferent | 2.8 | 3 | 2.90 ± 0.18 | 0.64 ± 0.09 |
| 5 | Mesenteric efferent | 4.0 | 3 | 6.55 ± 1.19 | 0.52 ± 0.02 |
| 6 | Mesenteric efferent | 6.6 | 5 | 3.87 ± 0.11 | 0.27 ± 0.02 |
| 7 | Mesenteric efferent | 5.5 | 3 | 3.80 ± 0.92 | 0.60 ± 0.20 |
| 3.50 ± 0.57-152 | 0.51 ± 0.08-152 | ||||
| Experiment . | Lymph Source . | Days-150 . | N-151 . | Ratio Blood/Lymph . | |
|---|---|---|---|---|---|
| Blood Lymphos . | Lymph Lymphos . | ||||
| 1 | Subcutaneous pseudoafferent | 10.7 | 5 | 2.92 ± 0.03 | ND |
| 2 | Subcutaneous pseudoafferent | 6.5 | 3 | 2.51 ± 0.17 | ND |
| 3 | Subcutaneous efferent | 8.4 | 8 | 1.96 ± 0.13 | ND |
| 4 | Subcutaneous efferent | 2.8 | 3 | 2.90 ± 0.18 | 0.64 ± 0.09 |
| 5 | Mesenteric efferent | 4.0 | 3 | 6.55 ± 1.19 | 0.52 ± 0.02 |
| 6 | Mesenteric efferent | 6.6 | 5 | 3.87 ± 0.11 | 0.27 ± 0.02 |
| 7 | Mesenteric efferent | 5.5 | 3 | 3.80 ± 0.92 | 0.60 ± 0.20 |
| 3.50 ± 0.57-152 | 0.51 ± 0.08-152 | ||||
Abbreviation: ND, not determined.
Experiments were continued until the lymphatic cannulae were occluded or dislodged.
Indicates the number of paired blood and lymph samples obtained during each experiment.
Mean ± SEM.
The percentage of labeled cells in lymph collected over several hours was compared with a corresponding blood sample taken at the end of the same sampling period. After lymphocytes equilibrate between blood and lymph their concentration decreases relatively slowly, so this difference in the sampling methods would not have introduced a significant error in the calculations. Young and Hay10reported that the turnover rate of small lymphocytes (time required for the concentration of PKH-labeled cells to halve) in the sheep is 16.5 days, consistent with our observations that the change in the percentage of labeled cells in lymph over a typical collection period was minor.
Protocol 2. Analysis of the Recirculation of Blood Lymphocyte Subsets
Animals and surgery.
Eight animals were used for this part of the study. As above, a venous catheter was established in the jugular vein and one or more subcutaneous (prescapular, prefemoral, or popliteal) efferent lymphatic vessels was cannulated. Some experiments were incomplete because lymphatic cannulae became dislodged or clotted, and in other experiments it was not possible to phenotype the injected sample of cells because the fluorescence intensity of the FITC+ cells was too intense to permit immunophenotyping with a second color antibody. Therefore, the tables and figures depicting the pooled data for these experiments indicate in parentheses the number of samples obtained.
Blood labeling, reinfusion, and sampling.
A 300-mL sample of blood was labeled with FITC and injected intravenously, as described above. Following this, 60-mL samples of blood were drawn 4 hours, 3 days, and 11 days later. These time points were chosen so that the short-term distribution of blood lymphocytes could be compared with that of more extended time points. The erythrocytes were lysed using Tris:NH4Cl solution, then the remaining leukocytes were washed 2 times in HBSS containing 1% autologous lymph plasma by centrifugation at 600g for 10 minutes at 4°C.
Immunophenotypic analysis.
The cells were immunostained in microtitre wells using standard procedures described elsewhere.25 They were phenotyped using monoclonal antibodies (MoAbs) against the following surface antigens: CD4,5 CD8,26 CD5,27γδ T-cell receptor,28 and a surface marker on ovine B cells (provided by Wayne Hein, Basel Institute for Immunology, Basel, Switzerland). It was confirmed that the anti–B-cell MoAb stains the same percentage of blood lymphocytes as a commercially available antibody (VPM-8; Serotec, Mississauga, Ontario, Canada) against Ig light chain (32.90% v 33.16%, respectively). The primary antibodies were tissue culture supernatants, and most were used undiluted. A phycoerythrin-conjugated goat anti-mouse IgG (Cedarlane, Hornby, Ontario, Canada) was used for detection of the primary antibodies. Controls included unstained cells, cells incubated with nonspecific mouse IgG followed by the secondary antibody, and cells incubated with the secondary antibody only.
For these studies a FACscan flow cytometer (Becton Dickinson, San Jose, CA) was used. The phenotypic profile of labeled and unlabeled cells in each sample was determined by gating on small lymphocytes and further gating on the FITC+ lymphocytes. Because of the relatively low concentration of labeled lymphocytes, particularly in the lymph, it was necessary to count between 1 × 105 and 1 × 106 total lymphocytes to analyze at least 1,000 FITC+ cells per sample. For some samples taken on day 11 it was possible to count only 400 to 800 FITC+ cells per 106 total lymphocytes.
Analysis of samples.
A method similar to that used by other investigators7 25was used to compare the migratory efficiency of the different lymphocyte subsets. The percentages of the various lymphocyte subsets were determined and then used to calculate ratios. For example, in one experiment the intravenously injected sample contained 18.94% CD4+ and 30.24% B lymphocytes, so the ratio of CD4/B cells was 18.94/30.24 = 0.63. The average CD4/B ratio of injected cells in all experiments was 0.68 ± 0.18. The same ratio for labeled cells that had actively recirculated to lymph 3 days later was 5.39 ± 1.74. The eightfold enrichment of CD4+ cells was statistically significant, indicating that this subset was extracted more efficiently from the blood than B cells.
Statistics
To determine whether FITC+ or CM-DiI+ cells selectively distributed between the blood and lymph, a ratio of their percentages in each compartment was calculated. If blood lymphocytes and lymph lymphocytes reached the same equilibrium between blood and lymph, their concentrations in each compartment should have been approximately equal. To determine whether the ratios were significantly different a Student's paired t-test was performed. A value ofP < .05 was considered significant. All results were expressed as the mean ± SEM. For multiple comparisons of data ANOVA was performed, and if a globally significant difference (P < .05) was found it was followed by the Student-Newman-Keuls (SNK) test to make comparisons between different paired samples.29
RESULTS
Kinetics of Recirculation and Concentration of Lymphocytes in Blood and Lymph
Figure 1 shows the flow cytometric analysis of FITC+ (blood) and CM-DiI+ (lymph) lymphocytes in blood and efferent lymph following their intravenous infusion. The FITC+ cells remained higher in blood than in lymph at all times, whereas the CM-DiI+ lymphocytes disappeared rapidly from blood and reached higher concentrations in lymph. The difference in the distribution of the two lymphocyte populations was most striking in experiments where both populations were tracked in the same animal because it was obvious that each population was most concentrated in its respective compartment of origin (Fig 2). The pooled data for all seven experiments are shown in Table 1. After infusing FITC+ lymphocytes intravenously, the concentration of labeled blood lymphocytes averaged 3.50 ± 0.57 times higher in the blood than in the three lymph compartments examined: subcutaneous pseudoafferent and efferent lymph, and mesenteric efferent lymph. This difference was particularly large between the blood and mesenteric efferent lymph (Table 1). As expected, lymph lymphocytes were more concentrated in their respective lymph compartment than in blood; the blood/lymph ratio of CM-DiI+ lymphocytes was on average 0.51 ± 0.08. The ratio of FITC+ lymphocytes in blood/lymph was significantly different from that of CM-DiI+ lymphocytes (P < .05).

Simultaneous detection of FITC+ and CM-DiI+ cells using flow cytometry. In every blood sample the percentage of FITC+ blood lymphocytes was higher than that of CM-DiI+ lymph lymphocytes, whereas lymph samples consistently showed the opposite trend.

Distribution of FITC+ blood lymphocytes and CM-DiI+ lymph lymphocytes in the blood and lymph compartments. Top panel; in this and all seven experiments performed, the concentration of blood lymphocytes remained consistently higher in blood (▪) than in lymph (○). Middle panel; in contrast, lymph lymphocytes rapidly disappeared from the blood and reached a higher concentration in lymph from about 1 day onward. Bottom panel; to rule out the possibility that the blood labeling protocol hindered lymphocyte recirculation, in one experiment the leukocytes were depleted from a blood sample and replaced with lymph lymphocytes, then the blood was labeled with FITC and reinfused as usual. The lymph lymphocytes reached a higher concentration in lymph than in blood; therefore the blood labeling protocol does not appear to affect the ability of lymphocytes to leave the blood and enter lymph.

Distribution of FITC+ blood lymphocytes and CM-DiI+ lymph lymphocytes in the blood and lymph compartments. Top panel; in this and all seven experiments performed, the concentration of blood lymphocytes remained consistently higher in blood (▪) than in lymph (○). Middle panel; in contrast, lymph lymphocytes rapidly disappeared from the blood and reached a higher concentration in lymph from about 1 day onward. Bottom panel; to rule out the possibility that the blood labeling protocol hindered lymphocyte recirculation, in one experiment the leukocytes were depleted from a blood sample and replaced with lymph lymphocytes, then the blood was labeled with FITC and reinfused as usual. The lymph lymphocytes reached a higher concentration in lymph than in blood; therefore the blood labeling protocol does not appear to affect the ability of lymphocytes to leave the blood and enter lymph.
In all experiments the blood lymphocytes reached their maximum concentration in the lymph at 20 to 30 hours. This was similar to the peak blood to lymph transit time reported for lymph lymphocytes.9
Effect of the Blood Labeling Protocol on Lymphocyte Recirculation
In one experiment the leukocytes in a sample of blood were depleted and replaced with lymph lymphocytes before labeling with FITC (Fig 2, bottom panel). As would be expected of lymph-derived cells, the FITC+ lymphocytes achieved a higher concentration in lymph than in blood after about 1 day. The average blood/lymph ratio in this experiment was 0.54 ± 0.02 (as determined from six paired blood and lymph samples taken between 44.5 and 133.5 hours after the injection of labeled cells). This corresponded closely to the average blood/lymph ratio of 0.51 ± 0.08 for lymph lymphocytes labeled with CM-DiI (Table 1). Because lymph lymphocytes subjected to the blood lymphocyte labeling protocol recirculated normally, it seems highly unlikely that this methodology affected the migratory behavior of blood lymphocytes.
The Redistribution of FITC+ Cells in the Blood
The phenotypic profile of the labeled, injected lymphocytes was somewhat different from that of unlabeled blood lymphocytes (Fig 3). The percentage of CD5+or CD4+ lymphocytes tended to be higher in the injected population, and B lymphocytes tended to be lower, but the only statistically significant difference was between injected CD4+ cells versus unlabeled CD4+ cells 4 hours postinjection. In some experiments it appears that some B cells were lost during labeling, perhaps because of adhesion to the polypropylene containers used for cell labeling. Because this depletion was not consistently observed, and by trypan blue exclusion the viability of labeled cells was greater than 95%, it was felt that the depletion was not caused by the selective damage of certain lymphocyte subsets. However, the changes in the distribution of each FITC+subset were examined in two ways to determine whether similar conclusions were reached: (1) the concentration of labeled cells in the blood was compared with the original infused population and (2) in each sample the concentration of labeled and unlabeled cells of a given subset was compared.
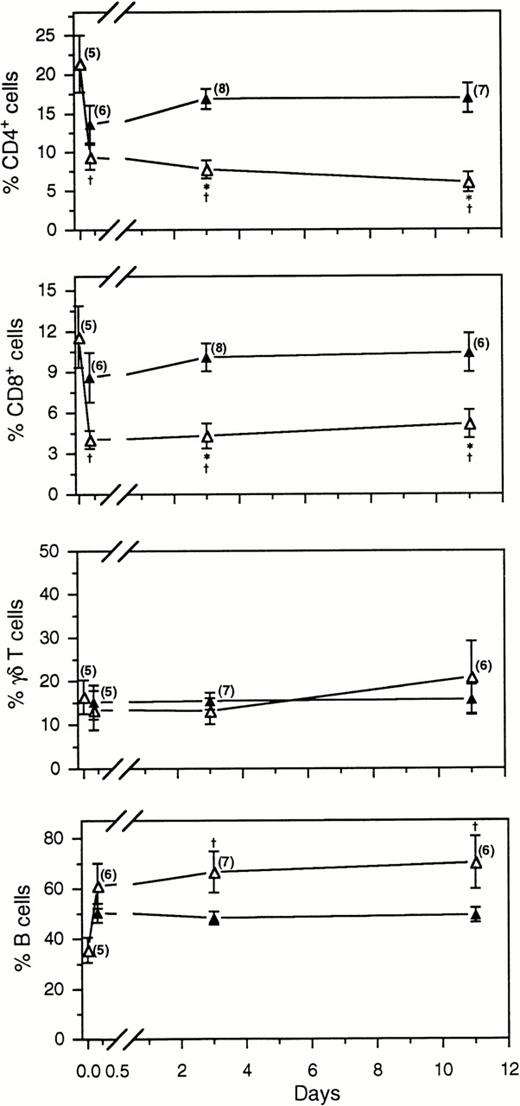
Comparison of the percentage of labeled (▵) and unlabeled lymphocytes (▴) in the blood following the intravenous injection of FITC+ blood lymphocytes. The number of samples per time point is indicated in parentheses. *, indicates a significant difference (P < .05) between the concentration of labeled and unlabeled cells at the same time point; †, indicates a significant difference between the concentration of FITC+ subsets in the original injected dose of labeled cells (the first ▵ on each graph) and in subsequent blood samples.

Comparison of the percentage of labeled (▵) and unlabeled lymphocytes (▴) in the blood following the intravenous injection of FITC+ blood lymphocytes. The number of samples per time point is indicated in parentheses. *, indicates a significant difference (P < .05) between the concentration of labeled and unlabeled cells at the same time point; †, indicates a significant difference between the concentration of FITC+ subsets in the original injected dose of labeled cells (the first ▵ on each graph) and in subsequent blood samples.
Within 4 hours after the injection of labeled lymphocytes into the jugular vein, the phenotypic profile of certain subsets already differed from that of the injected dose (Fig 3). The percentage of labeled CD4+ cells remained significantly lower than in the injected sample at all time points examined. Further, by days 3 or 11 the concentration of FITC+ CD4+ lymphocytes was less than half that of unlabeled CD4+ cells. For instance, on day 3 the percentage of FITC+ CD4+ cells was 7.73 ± 1.16%, yet the percentage of unlabeled CD4+lymphocytes was 16.89 ± 1.28%. The same trend was observed for CD8+ cells; by day 3 the respective concentrations of labeled and unlabeled lymphocytes were 4.31 ± 0.92% versus 10.08 ± 1.03%.
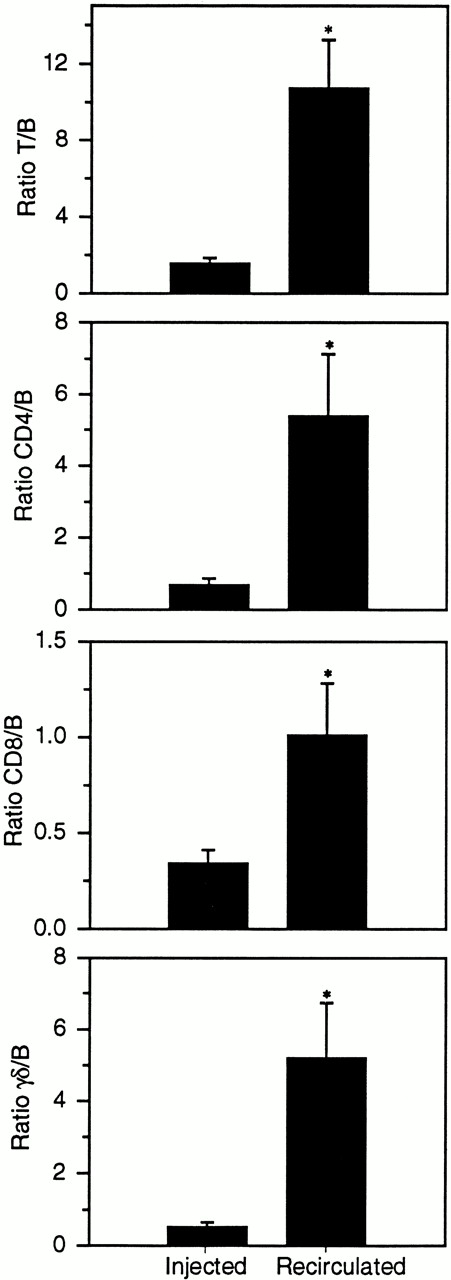
Comparison of the ratios of T and B cells in the intravenously infused sample and recirculated in lymph 3 days later. All T-cell subsets were enriched in efferent subcutaneous lymph compared with the original injected sample. *, denotes a statistically significant difference (P < .05) between the ratios of cells initially injected into the venous circulation and those that left the blood and migrated into lymph.

Comparison of the ratios of T and B cells in the intravenously infused sample and recirculated in lymph 3 days later. All T-cell subsets were enriched in efferent subcutaneous lymph compared with the original injected sample. *, denotes a statistically significant difference (P < .05) between the ratios of cells initially injected into the venous circulation and those that left the blood and migrated into lymph.
No significant differences were detected between labeled and unlabeled γδ T cells in the blood over the course of the experiments, whereas the proportion of FITC+ B cells became significantly higher than the injected dose by days 3 or 11 (Fig 3). Labeled B cells comprised up to 91% of the total FITC+ blood lymphocyte population (average 66.66 ± 8.16%), whereas the percentage of unlabeled B cells did not exceed 57% (average 48.27 ± 2.58%). During these experiments the phenotypic profile of the unlabeled blood lymphocytes was quite stable; no significant differences were observed in any of the subsets analyzed on days 1, 3, and 11 (Fig 3). Therefore, the redistribution patterns of the labeled cells described above cannot be attributed to changes in the entire blood pool.
The Recirculation of Blood B and T Cells
The migration of B and T cells to efferent subcutaneous lymph was compared by calculating phenotypic ratios of the intravenously infused FITC+ lymphocytes and of FITC+ lymphocytes recirculating in lymph 3 and 11 days later. An example calculation using the ratio of CD4/B cells was described in Materials and Methods. As mentioned, a statistically significant change in the ratios of the infused and recirculated populations would indicate a difference in the homing of the two subsets to efferent lymph.
Three days after the injection of labeled cells the recirculated population was significantly enriched for T cells (Fig4). All T-cell subsets entered lymph in substantially higher concentrations than B cell; the T/B ratio was only 1.55 ± 0.30 in the infused sample yet 10.75 ± 2.76 in the recirculated population, an average increase of almost sevenfold (Table 2). Comparing individual T-cell subsets, the greatest change was seen in the ratio of γδ/B cells. The recirculated ratio was on average 10.02 times higher than the infused ratio. By 11 days postinjection of labeled cells, the T/B ratio was on average 4.03 times higher in the recirculated population (Table 2). However, a statistical comparison of the three sample groups (Injected, 3 Days Recirculated, and 11 Days Recirculated) indicated that the only significant difference was between the first two groups.
Comparison of the Ratios of Lymphocyte Subsets Infused Intravenously and Recirculated in Lymph
| Ratio . | Injected (n)† . | 3 Days . | 11 Days . | ||
|---|---|---|---|---|---|
| Recirculated . | R/I‡ . | Recirculated . | R/I . | ||
| T versus B cells | |||||
| T/B | 1.55 ± 0.30 (5) | 10.75 ± 2.76 (6)* | 6.94 | 6.24 ± 1.34 (4) | 4.03 |
| CD4/B | 0.69 ± 0.17 (5) | 5.40 ± 1.91 (6)* | 7.83 | 2.33 ± 0.29 (4) | 3.37 |
| CD8/B | 0.34 ± 0.07 (5) | 1.01 ± 0.30 (6)* | 2.97 | 0.70 ± 0.22 (4) | 2.04 |
| γδ/B | 0.52 ± 0.14 (5) | 5.21 ± 1.53 (5)* | 10.02 | 3.20 ± 1.26 (4) | 6.15 |
| T subsets | |||||
| CD4/CD8 | 2.09 ± 0.37 (5) | 4.89 ± 1.40 (7) | 2.34 | 4.24 ± 1.00 (4) | 2.03 |
| CD4/γδ | 1.71 ± 0.47 (5) | 1.25 ± 0.38 (6) | 0.73 | 1.44 ± 0.50 (4) | 0.84 |
| CD8/γδ | 1.18 ± 0.61 (5) | 0.37 ± 0.13 (6) | 0.31 | 0.43 ± 0.14 (4) | 0.36 |
| Ratio . | Injected (n)† . | 3 Days . | 11 Days . | ||
|---|---|---|---|---|---|
| Recirculated . | R/I‡ . | Recirculated . | R/I . | ||
| T versus B cells | |||||
| T/B | 1.55 ± 0.30 (5) | 10.75 ± 2.76 (6)* | 6.94 | 6.24 ± 1.34 (4) | 4.03 |
| CD4/B | 0.69 ± 0.17 (5) | 5.40 ± 1.91 (6)* | 7.83 | 2.33 ± 0.29 (4) | 3.37 |
| CD8/B | 0.34 ± 0.07 (5) | 1.01 ± 0.30 (6)* | 2.97 | 0.70 ± 0.22 (4) | 2.04 |
| γδ/B | 0.52 ± 0.14 (5) | 5.21 ± 1.53 (5)* | 10.02 | 3.20 ± 1.26 (4) | 6.15 |
| T subsets | |||||
| CD4/CD8 | 2.09 ± 0.37 (5) | 4.89 ± 1.40 (7) | 2.34 | 4.24 ± 1.00 (4) | 2.03 |
| CD4/γδ | 1.71 ± 0.47 (5) | 1.25 ± 0.38 (6) | 0.73 | 1.44 ± 0.50 (4) | 0.84 |
| CD8/γδ | 1.18 ± 0.61 (5) | 0.37 ± 0.13 (6) | 0.31 | 0.43 ± 0.14 (4) | 0.36 |
*Indicates a significant difference (P < .05) from the injected sample.
The number of samples (n) for each time point is indicated in parentheses.
R/I represents the average ratio of the recirculated population divided by the average ratio of the infused population.
The Recirculation of Blood T-Cell Subsets
The recirculation of each of the blood T-cell subsets was also compared, but no significant differences could be detected. Although the CD4/CD8 ratio was on average 2.34 times higher in the recirculated population than in the injected population (Table 2), this was quite variable, and in some animals CD4+ lymphocytes were only slightly enriched in lymph. The CD8/γδ ratio tended to be lower in the recirculated population, suggesting preferential extraction of γδ T cells from the blood. Similar trends were seen in the lymph on day 11 so it is possible that with a larger number of experiments statistically significant differences would have been attained.
DISCUSSION
This study extends the observations of previous investigators by directly comparing the recirculation of blood- and lymph-derived lymphocytes and showing that they do not recirculate to the same extent through any of the lymph compartments examined. It might be argued that this difference was caused solely by the differences in the phenotypic profile of the starting populations. However, in sheep the migratory kinetics of efferent subcutaneous lymph-derived T and B cells are similar.30 The migratory kinetics of various lymph-born T-cell subsets are also comparable; when CD4+, CD8+, and γδ T19+ cells are infused into blood they all reach peak levels in subcutaneous lymph 20 to 30 hours later.7 These findings render it unlikely that differences in the recirculatory kinetics of different lymphocyte subsets could dramatically influence the distribution of lymphocytes between blood and lymph. In addition, it is improbable that the blood labeling protocol itself could account for the unique migratory behavior of blood lymphocytes, because lymph cells subjected to the procedure displayed normal behavior and returned preferentially to efferent lymph. FITC has been used extensively as a cell tracking dye, and lymph cells labeled with this marker migrate as well as cells labeled with other dyes.22 FITC-labeled ovine lymphocytes also respond essentially the same as unlabeled cells in other assays of lymphocyte function.31 Therefore, it is highly unlikely that the differences observed in the migratory behavior of blood and lymph lymphocytes are caused by the cell labeling procedure.
It is apparent from this and previous studies6 that the assortment of CD4+ and γδ T cells in blood and lymph is a complex phenomenon that is not easily explained by simple lymphocyte recirculation models. In this study, efferent lymph contained roughly three times more CD4+ than γδ T cells (48.98 ± 3.18% v 15.25 ± 2.87%, respectively), yet the concentrations of these two subsets in the blood were approximately equal (16.89 ± 1.62% v 15.38 ± 2.34%). The simplest explanation for these findings is that CD4+ lymphocytes are more efficiently extracted from the blood, resulting in a higher proportion in lymph.6 However, this explanation is inconsistent with carefully executed studies in which lymph-derived CD4+ and γδ T19+ lymphocytes were fluorescently labeled and tracked in vivo,7 because both populations were found to recirculate equally well.7 Thus a more complex explanation has been proposed: the proportions of actively recirculating CD4+ and γδ T19+ lymphocytes are indeed the same in blood and lymph, but an additional poorly recirculating population of γδ T cells exists in the blood.7 In the present study it was possible to directly test this concept by labeling and tracking blood lymphocytes rather than lymph lymphocytes used in previous investigations. It was predicted that blood-derived γδ T cells would recirculate less efficiently than CD4+ T cells. Surprisingly, there was no significant difference in the recirculation of these subsets to efferent subcutaneous lymph, so the differences in the distribution of CD4+ and γδ T cells in blood and lymph cannot be explained solely by the presence of a poorly recirculating γδ T-cell population.
To explain the above findings it is also necessary to consider differences in the homing of CD4+ and γδ T cells. Previous studies examining the homing of CD4+ lymphocytes showed that subpopulations of these cells preferentially migrate either to efferent subcutaneous or efferent mesenteric lymph.32 In contrast, γδ T cells tend to preferentially migrate only to efferent subcutaneous lymph.32 If blood subsets behave similarly, this could impact on their concentrations in various compartments. For example, if one subpopulation of blood γδ T cells preferentially migrates to efferent subcutaneous lymph and another subpopulation remains in the blood, this might explain the relatively high concentration of γδ T cells in these two compartments. Conversely, all blood CD4+ lymphocytes may recirculate efficiently through subcutaneous and mesenteric lymph nodes, and they may be less restricted in their homing patterns. This speculation is supported by one experiment in which the traffic of blood lymphocytes though efferent mesenteric lymph was monitored. The CD4/γδ ratio in the recirculated population was approximately 10-fold higher than in the intravenously injected sample, suggesting that CD4+blood lymphocytes recirculate through mesenteric lymphoid tissue much more efficiently than γδ T cells.
To summarize the above observations, if only 3 compartments are considered (blood, efferent subcutaneous lymph, and efferent mesenteric lymph), it appears that CD4+ T cells are efficiently exchanged between the blood-mesenteric and blood-subcutaneous lymph compartments. In contrast, it is possible that only a portion of γδ T cells recirculate efficiently between blood-subcutaneous lymph, and another subpopulation tends to remain in the blood. It should be cautioned that this model ignores the input of other lymphoid organs to the blood lymphocyte pool, but it seems to fit quite well with the available data. Future experiments will be required to confirm whether blood γδ T cells recirculate inefficiently through mesenteric lymph nodes, the spleen, and other lymphoid tissue.
The proportions of B and T cells in blood versus efferent subcutaneous lymph also cannot be explained by differences in their homing preference or recirculation rates. Previous investigators noted that the T/B ratio is almost threefold higher in lymph than blood, leading to the inference that T cells recirculate much more efficiently than B cells in the sheep.6 However, when lymph lymphocytes were fluorescently labeled and their traffic from blood to lymph directly monitored, there was no substantial difference in the magnitude or kinetics of migration of B and T cells.30 In the present study, blood lymphocytes rather than lymph lymphocytes were used, and the results suggest that blood B cells do not recirculate as efficiently as blood T cells. Because lymph-derived T and B cells in ovine efferent subcutaneous lymph possess the same ability to recirculate yet blood-derived T and B cells do not, it seems likely that there are different subsets of blood B cells with unique migratory abilities.
The differences in the recirculation of B and T cells appeared to diminish over time. By 11 days the average T/B ratio of the recirculated population (4.03) was lower than on day 3 (6.94; Table 2). Although these differences were not statistically significant, they are of interest because others have found that lymphocyte homing preferences may not be stable over the long term.11 In this investigation the duration of experiments was limited to 11 days because of the loss of fluorescence intensity of FITC-labeled cells, but it would be informative to follow individual blood lymphocyte subsets over longer periods with more stable lipophilic dyes.
Overall, these experiments suggest that the poorly recirculating blood lymphocytes are mainly subpopulations of B cells and perhaps γδ T cells, although it cannot be excluded that smaller populations of CD4+ and CD8+ T cells also have this character. There are several possible explanations for differences in the recirculatory ability of various blood lymphocyte subpopulations; the state of cellular activation or antigen exposure,33,34 site of immunization,35 proinflammatory mediators in circulation,36 and numerous other factors can influence lymphocyte trafficking and homing specificity. Given the multiple influences on lymphocyte migratory behavior, systematic and thorough investigations will be required to further characterize the poorly recirculating blood lymphocyte populations. Also, it remains to be determined whether these cells preferentially migrate through lymphoid tissues other than lymph nodes.
Confronted with data that show that the delivery of lymphocytes to the blood by the lymphatic system far exceeded the number in the blood at any point in time, Gowans was able to directly demonstrate the recirculation of lymphocytes.1 2 In sheep, at least four to five times the blood pool is delivered to the blood by lymph each day. The continuous redistribution and reassortment between compartments makes it difficult to make universal conclusions when data are obtained from only one compartment such as the blood. Animal models in which the lymph compartment is accessible and readily sampled continue to provide useful data in this regard, although parallel studies need to be done in man. Future experiments will be required to determine whether functional differences can be attributed to these different blood lymphocyte pools and to further examine the relationship between these pools during systemic infections, in relation to age, and in a variety of pathological situations.
ACKNOWLEDGMENT
The authors thank Cheryl Smith for her skilled analysis of samples using the flow cytometer. Monoclonal antibodies were generously provided by Dr Alan Young and Dr Wayne Hein.
Supported by MRC Canada, and W.N.A. was the recipient of a MRC Studentship.
Address reprint requests to John B. Hay, PhD, Department of Immunology, 5242 Medical Sciences Building, University of Toronto, Toronto, Ontario, Canada M5S 1A8.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal