Abstract
To examine the potential clinical usefulness of the hexadentate phenolic aminocarboxylate iron chelatorN,N-bis(2-hydroxybenzyl)ethylenediamine-N,N-diacetic acid (HBED) for the chronic treatment of transfusional iron overload, we compared the iron excretion induced by subcutaneous (SC) injection of HBED and deferoxamine (DFO), the reference chelator, in rodents and primates. In the non–iron-overloaded, bile-duct–cannulated rat, a single SC injection of HBED, 150 μmol/kg, resulted in a net iron excretion that was more than threefold greater than that after the same dose of DFO. In the iron-loaded Cebus apella monkey, a single SC injection of HBED, 150 μmol/kg, produced a net iron excretion that was more than twice that observed after the same dose of SC DFO. In patients with transfusional iron overload, SC injections of HBED may provide a much needed alternative to the use of prolonged parenteral infusions of DFO.
IN PATIENTS WITH transfusional iron overload, the magnitude of the body iron burden is the principal determinant of the severity of iron toxicity and clinical outcome.1,2 During the past 30 years, iron-chelating therapy with deferoxamine B mesylate (DFO, Fig 1) has been shown to be a generally safe and efficacious means of controlling body iron that can prolong survival and prevent or ameliorate organ dysfunction.3-7 Unfortunately, treatment with DFO is cumbersome, inefficient, expensive, and unpleasant. Because DFO is poorly absorbed from the gastrointestinal tract and rapidly eliminated from the circulation, prolonged parenteral infusion is needed.8-10 Moreover, DFO is inefficient as an iron chelator; typically only 5% or less of the drug administered binds iron.8 11 Effective therapy usually requires subcutaneous (SC) or intravenous administration by a portable infusion pump for 9 to 12 hours daily. Not surprisingly, almost all patients have difficulty in complying with such a demanding regimen.
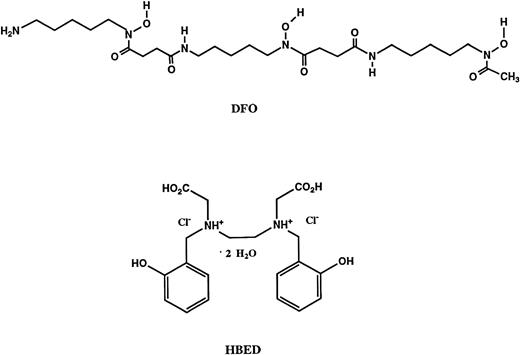
Structures of the iron chelators chosen for evaluation: deferoxamine B (DFO) andN,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid dihydrochloride dihydrate (HBED).
Structures of the iron chelators chosen for evaluation: deferoxamine B (DFO) andN,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid dihydrochloride dihydrate (HBED).
DFO is a bacterial siderophore that is commercially produced by large-scale fermentation of a strain of Streptomyces pilosus,12 a method of manufacture that contributes to the high cost of the drug and to the allergic reaction that some patients experience, possibly caused by cytokines or other fermentation products not removed during purification. Almost all patients develop discomfort or pain at the site of DFO infusion; moreover, some people are severely allergic to this compound.13-15 A recent study indicated that, although DFO does not stimulate human basophils to release histamine, the drug nevertheless induces the nonimmunologic activation of the dermal mast cells.16 The volunteers in that study were exposed to DFO for the first time: all developed a dose-dependent wheal and flare reaction after the intradermal injection of DFO. The investigators even suggested that DFO could be used as a positive control in the allergy clinic in intradermal skin tests. Although allergy with anaphylactic reactions to DFO is rare,17,18 a variety of neurotoxic19 and other adverse effects20-23 of DFO have now been recognized, especially with intensive therapy. Thus, although the experience with DFO has shown that adequate control of body iron can avert complications of transfusional iron overload, problems of toxicity in some patients, the high cost of production, the inefficiency of chelation, local reactions, and the difficulties with compliance associated with prolonged parenteral administration have prompted an ongoing search for safe, inexpensive alternatives to DFO.
Most efforts to develop substitutes for DFO for iron-chelating therapy have concentrated on bidentate or tridentate iron-chelating agents that remain active after oral administration.24,25 Because of concerns that these partial ligands might exacerbate iron toxicity and the realization that some patients may have difficulty in taking an oral formulation three or more times daily, we have examined the possibility of the administration of a hexadentate iron chelator by SC injection. The polyanionic amineN,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid26 (HBED; Fig 1) is a synthetic hexadentate ligand that, like DFO, forms a 1:1 complex with iron with high affinity and selectivity. HBED and its dimethyl ester prodrug (DMHBED) have both been thoroughly investigated in rodents.27-29 Both of the drugs looked very promising in this model when administered orally, and not unexpectedly, these findings generated a great deal of excitement. The availability of an orally active iron chelator seemed imminent. Unfortunately, the rodent findings were not mirrored in higher animals. In previous studies in the iron overloaded Cebus apella model, an excellent predictor of how a chelator will perform when administered to humans, we showed that while the iron clearing efficiency of SC-administered DFO30 given at a dose of 150 μmol/kg was 5.5% ± 0.9%, the efficiencies of orally (PO) administered HBED or DMHBED,31 also dosed at 150 μmol/kg, were significantly lower: 0.5% ± 0.5% and 1.5% ± 0.6%, respectively. Not surprisingly, HBED was also a disappointment when given orally to patients; 32-33 the limited iron excretion that resulted is insufficient for use of this agent in the treatment of transfusional iron overload. The result of oral administration of DMHBED has not yet been evaluated clinically, but the primate findings suggest that the iron excretion to be expected, although greater than that with HBED, will still be only about one third of that produced by DFO.
We reconsidered the potential therapeutic usefulness of HBED given parenterally after recalling that intraperitoneal injection of the drug in the hypertranfused rat produced an iron excretion that was twofold to threefold greater than that following injection of DFO.34 Accordingly, in addition to the preceding studies, we also performed preliminary investigations in the primates into the iron clearing properties of SC-administered HBED and DMHBED. In these studies, HBED and DMHBED were given to the monkeys SC at a dose of 150 μmol/kg. Whereas the efficiency of SC DMHBED was only 2.4% ± 1.1% (range, 1.0% to 3.4%), the efficiency of SC-administered HBED was 12.6% ± 3.9% (range, 9.1% to 16.8%), an efficiency more than twice that of DFO given SC and approximately six times that of DMHBED administered SC at an equimolar dose. On the basis of this initial finding, we have now thoroughly explored the iron-clearing properties of SC-administered HBED in the Cebus monkey model. In this report, we have compared the iron excretion induced DFO with that induced by HBED, each administered SC to rats and monkeys to assess the possibility that SC HBED might provide an alternative to DFO infusions in patients with transfusional iron overload.
MATERIALS AND METHODS
Materials.
Deferoxamine B in the form of the methanesulfonate salt, Desferal, was obtained from Ciba-Geigy Ltd, (Basel, Switzerland). HBED dihydrochloride dihydrate was obtained from Strem Chemical Co (Newburyport, MA). Cremophor RH-40 was obtained from BASF (Parsippany, NJ). Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). C apella monkeys were obtained from World Wide Primates (Miami, FL). All reagents and standard iron solutions were obtained from Aldrich Chemical Co (Milwaukee, WI). Nalgene metabolic cages, rat jackets, and fluid swivels were purchased from Harvard Bioscience (South Natick, MA). Intramedic polyethylene tubing was obtained from Fisher Scientific (Pittsburgh, PA). Atomic absorption measurements were made on a Perkin-Elmer model 5100 PC (Norwalk, CT). Ultrapure salts were obtained from Johnson Matthey Electronics (Royston, UK). Imferon, an iron dextran solution, was obtained from Fisons (Bedford, MA). All hematological and biochemical studies30 were performed by Allied Clinical Laboratories (Gainesville, FL).
Cannulation of bile duct in rats.
Male Sprague-Dawley rats averaging 400 g were housed in Nalgene plastic metabolic cages during the experimental period and given free access to water. The animals were anesthetized using sodium pentobarbital (55 mg/kg) given intraperitoneally. The bile duct was cannulated, using 22-gauge polyethylene tubing, about 1 cm from the duodenum. The cannula was inserted about 2 cm into the duct, and once bile flow was established, the cannula was tied snugly in place. A skin tunneling needle was inserted from the shoulder area around to the abdominal incision. The cannula was threaded through the needle until it emerged from the shoulder opening. The cannula was then passed from the rat to the swivel inside a metal torque-transmitting tether, which was attached to a rodent jacket around the animal's chest. The cannula was directed from the rat to a Gilson micro fraction collector (Middleton, WI) by a fluid swivel mounted above the metabolic cage. This system allowed the animal to move freely in the cage while continuous bile samples were being collected. Bile samples were collected at 3-hour intervals for 24 hours. Urine samples were taken every 24 hours. Sample collection and handling were as previously described.35
Iron loading of C apella monkeys.
After intramuscular anesthesia with ketamine, an intravenous infusion was started in a leg vein. The iron dextran was added to approximately 90 mL of sterile normal saline and administered to the animals by slow infusion at a dose of 200 to 300 mg of iron per kilogram of body weight over 45 to 60 minutes. Two to three infusions, separated by between 10 and 14 days, were necessary to provide about 500 mg of iron per kilogram of body weight. After administration of iron dextran, the serum transferrin iron saturation rose to between 70% and 80%. The serum half-life of iron dextran in humans is 2.5 to 3.0 days.36 We waited at least 20 half-lives, 60 days, before using any of the animals in experiments evaluating iron-chelating agents.
Iron-balance studies in C apella monkeys.
Seven days before the administration of the drug, the animals were placed in metabolic cages37 and started on a low-iron liquid diet.30 The monkeys were maintained on the low-iron liquid diet for the duration of the experiment. They were given food according to their body weight, and intake was very carefully monitored.
Three days before drug administration, day 2 to day 0, baseline iron intake and output values were measured. This same measurement was made for day +1 to day +3. The total amount of iron intake was compared with the total amount of iron excreted.
Primate fecal and urine samples.
Fecal and urine samples were collected at 24-hour intervals. The collections began 4 days before the administration of the test drug and continued for an additional 5 days after the drug was given. Fecal samples were assayed for the presence of occult blood, weighed, and mixed with distilled deionized water before autoclaving for 30 minutes. The mixture was then freeze-dried, and a known portion of the powder was mixed with low-iron nitric acid and refluxed for 24 hours. Once any particulate matter in the digested samples was removed by centrifugation, iron concentrations were determined by flame atomic absorption. Monkey urine samples were acidified and reconstituted to initial volume after sterilization, if necessary.
Drug preparation and administration.
DFO was administered to the rats at a dose of 150 μmol/kg in 40% Cremophor RH-40. HBED was administered SC and PO to the rats at a dose of 150 μmol/kg in a phosphate buffer.
In the primates, DFO was administered SC in sterile water for injection at a dose of 150 μmol/kg, whereas HBED was first dissolved in a phosphate buffer and given SC in 40% Cremophor RH-40 at a dose of 75 or 150 μmol/kg. In addition, to more closely mimic clinical applications in patients, HBED was also given to the monkeys SC at a dose of 150 μmol/kg in a phosphate buffer; no Cremophor vehicle was used.
Calculation of iron chelator efficiency.
The efficiency of each chelator was calculated on the basis of a 1:1 ligand-iron complex. In the monkeys the numbers were generated by averaging the iron output for 4 days before the administration of the drug, subtracting these numbers from the 2-day iron clearance after the administration of the drug, and then dividing by the theoretical output; the result is expressed as a percent. The efficiencies in the rodent model were calculated by subtracting the iron excretion of control animals from the iron excretion of treated animals. This number was then divided by the theoretical output; the result is expressed as a percent.
Statistical analysis.
Data are presented as the mean ± the standard error of the mean. For comparisons of the means of two groups, the two-samplet-test (without the assumption of equality of variances) was used for analyzing the rodent data, whereas the primate data were analyzed using a paired t-test. All tests were two-tailed, and a significance level of P < .05 was used.
RESULTS
Chelator-induced iron excretion in rats.
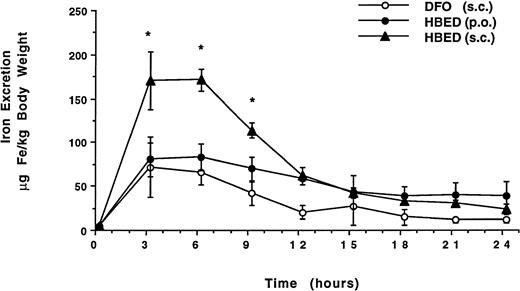
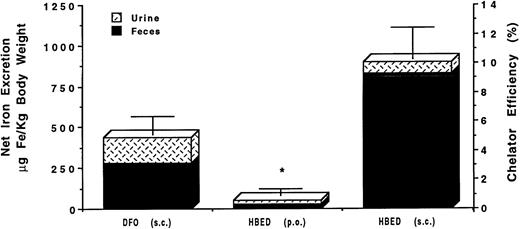
These studies were conducted in non–iron-overloaded, bile-duct–cannulated rats. The chelators were administered at a dose of 150 μmol/kg body weight. Groups of rats were given DFO by SC injection (n = 6) or HBED PO by gavage (n = 4) or SC injection (n = 3). Figure 2 shows the time course of the mean biliary iron excretion induced by the chelators in each group of rats, expressed as μg Fe/kg body weight. The peak amounts of iron excreted with SC HBED were more than twofold greater than the peak iron excretion after either SC DFO or HBED given by gavage (P < .05 at 3 hours and P < .01 at 6 hours). The iron excretion induced by the chelators in each group of rats is shown in Fig 3 expressed as the net mean amount of iron excreted in the urine and in the bile (μg Fe/kg body weight) and as the efficiency of iron chelation (net iron excretion/total iron-binding capacity of chelator administered, expressed as a percent).
Time course of mean biliary iron excretion in normal rats after administration of DFO by SC injection and after administration of HBED by gavage or by SC injection. Both chelators were given at a dose of 150 μmol/kg body weight. The peak amounts of iron excreted with SC HBED (*) were more than twofold greater than the peak iron excretion after either SC DFO or HBED given by gavage (P < .05 at 3 hours and P < .01 at 6 hours).
Time course of mean biliary iron excretion in normal rats after administration of DFO by SC injection and after administration of HBED by gavage or by SC injection. Both chelators were given at a dose of 150 μmol/kg body weight. The peak amounts of iron excreted with SC HBED (*) were more than twofold greater than the peak iron excretion after either SC DFO or HBED given by gavage (P < .05 at 3 hours and P < .01 at 6 hours).
Mean net iron excretion in normal rats after administration of DFO by SC injection and after administration of HBED by gavage or by SC injection. Excretion is shown as μg Fe/kg body weight on the scale of the left vertical axis and as efficiency of chelation (net iron excretion/total iron-binding capacity of chelator administered, expressed as a percent) on the right vertical axis. Both chelators were given at a dose of 150 μmol/kg body weight.
Mean net iron excretion in normal rats after administration of DFO by SC injection and after administration of HBED by gavage or by SC injection. Excretion is shown as μg Fe/kg body weight on the scale of the left vertical axis and as efficiency of chelation (net iron excretion/total iron-binding capacity of chelator administered, expressed as a percent) on the right vertical axis. Both chelators were given at a dose of 150 μmol/kg body weight.
Subcutaneous DFO induced the excretion of 209 ± 59 μg Fe/kg body weight and was found to have an efficiency of 2.5% ± 0.7% (range, 1.7% to 3.7%), with about 74% of the chelator-induced iron excreted through the bile and about 26% through the urine. Compared to SC DFO, HBED given PO by gavage resulted in a twofold greater iron excretion of 436 ± 176 μg Fe/kg body weight (P < .02); the efficiency was 5.2% ± 2.1% (range, 3.5% to 8.3%), with about 97% of the chelator-induced iron excretion through the bile and about 3% through the urine. HBED given to the rodents by SC injection was more than three times as effective in inducing iron excretion as DFO administered SC (P < .001), inducing the excretion of 679 ± 8 μg Fe/kg body weight with an overall efficiency of 8.1% ± 0.1% (range, 7.9% to 8.2%). About 83% of the iron was excreted in the bile and 17% in the urine. No untoward side effects were noted in the rodents.
Chelator-induced iron excretion in C apella monkeys.
The studies were conducted with C apella monkeys who had previously been administered intravenous iron dextran to provide about 500 mg of iron per kilogram body weight, as described in Materials and Methods. Groups of monkeys (6 in each group) were given SC injections of either DFO or HBED. DFO in aqueous solution was given SC at a dose of 150 μmol/kg and induced the excretion of 435 ± 115 μg Fe/kg body weight and was found to have an efficiency of 5.1% ± 1.3% (range, 3.3% to 6.6%), with about 65% of the chelator-induced iron excretion in the stool and about 35% in the urine. In our initial experiments, the HBED was dissolved in a phosphate buffer and administered in 40% Cremophor RH-40 (a polyethoxylated castor oil used as a vehicle for compounds with poor aqueous solubility), because of the low water solubility of the HBED dihydrochloride dihydrate. HBED-Cremophor was administered by SC injection at doses of 75 or 150 μmol/kg. At the dose of 75 μmol/kg, HBED-Cremophor induced the clearance of 793 ± 410 μg/kg of iron and had an efficiency of 18.4% ± 9.1% (range, 7.4% to 27.8%). Most of the iron (92%) was excreted in the feces, whereas 8% was excreted in the urine. At a dose of 150 μmol/kg, HBED-Cremophor induced the clearance of 1,349 ± 475 μg/kg, an efficiency of 16.1% ± 5.6% (range, 9.3% to 23.0%). Once again, the majority of the iron, 90%, was excreted in the feces; 10% was found in the urine.
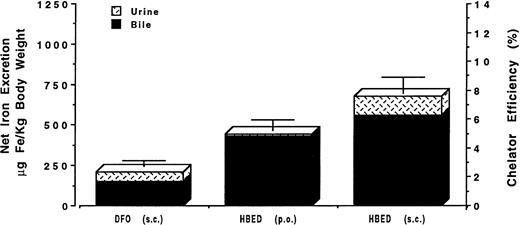
Finally, to more closely mimic potential clinical applications, HBED has also been given SC to the same group of monkeys used in the preceding experiments at a dose of 150 μmol/kg in a phosphate buffer; no Cremophor vehicle was used. Once again, subcutaneous HBED induced the excretion of about twice as much iron as DFO, 899 ± 193 μg Fe/kg body weight (P < .001), and was found to have an efficiency of 10.7% ± 2.3% (range, 8.3% to 13.8%), with about 92% of the chelator-induced iron excretion in the stool and about 8% in the urine. At the dose of 150 μmol/kg, no significant difference (P > .2) was found between the mean net iron excretion induced by HBED prepared in phosphate buffer or in Cremophor. Figure 4 shows the mean iron excretion induced by DFO given SC in an aqueous solution and HBED administered SC in a buffer at a dose of 150 μmol/kg body weight. The data are expressed as the mean net amount of iron excreted in the urine and in the feces (μg Fe/kg body weight) and as the efficiency of iron chelation. For convenience, Fig 4 also shows, marked with an asterisk, the results of our previously published study of the oral administration of HBED in a phosphate buffer to a group ofC apella monkeys with a similar magnitude of iron overload.31 Oral HBED induced the excretion of only 50 ± 44 μg Fe/kg body weight and was found to have an efficiency of 0.5% ± 0.5% (range, 0.1% to 1.1%), with about 56% of the chelator-induced iron excretion in the stool and about 44% in the urine. No adverse effects of chelator administration were noted in the monkeys; all hematologic and biochemical tests remained within normal ranges.
Mean net iron excretion in C apella monkeys with iron overload (see text) after administration of DFO by SC injection and of HBED by SC injection. Excretion is shown as μg Fe/kg body weight on the scale of the left vertical axis and as efficiency of chelation (net iron excretion/total iron-binding capacity of chelator administered, expressed as a percent) on the right vertical axis. Both chelators were given at a dose of 150 μmol/kg body weight. For convenience, the result of our previously published study31of the oral administration of HBED in phosphate buffer to C apella monkeys with a similar magnitude of iron overload is also shown; this previous result is indicated by an asterisk.
Mean net iron excretion in C apella monkeys with iron overload (see text) after administration of DFO by SC injection and of HBED by SC injection. Excretion is shown as μg Fe/kg body weight on the scale of the left vertical axis and as efficiency of chelation (net iron excretion/total iron-binding capacity of chelator administered, expressed as a percent) on the right vertical axis. Both chelators were given at a dose of 150 μmol/kg body weight. For convenience, the result of our previously published study31of the oral administration of HBED in phosphate buffer to C apella monkeys with a similar magnitude of iron overload is also shown; this previous result is indicated by an asterisk.
Primate iron balance studies.
The results of these studies clearly indicate that both DFO and HBED can hold the monkeys in a negative iron balance (Table1). Iron balance was calculated by comparing the amount of iron absorbed by the untreated animals over a 3-day period (day 2 to day 0) with the amount of iron absorbed by treated animals over a 3-day period (day +1 to day +3). Net iron balance = dietary iron intake (urinary + fecal iron excretion); animals in a negative iron balance are excreting more iron than they are absorbing. Monkeys treated with 150 μmol/kg of DFO on the average excreted 278 ± 185 μg/kg more iron than they absorbed (P < .0001), whereas animals treated with 75 μmol/kg of HBED excreted 606 ± 406 μg/kg more iron than they absorbed (P < .005). Finally, animals given HBED SC at the same molar quantity as DFO excreted 1,141 ± 456 μg/kg more than they absorbed when the compound was dosed with the Cremophor vehicle (P < .001) and 689 ± 158 μg/kg more than they absorbed (P < .001) when the drug was given without the Cremophor vehicle. As was observed with the urinary and fecal iron clearance data, the animals given HBED SC at a dose of 150 μmol/kg had a negative iron balance that was significantly greater than that seen with DFO, 4.1 times greater when the compound was dosed with the Cremophor vehicle (P < .001), and 2.5 times greater when the drug was given without the Cremophor vehicle (P < .01). At the dose of 150 μmol/kg, no significant difference (P > .1) was found between the iron balance observed with HBED prepared in a phosphate buffer versus HBED given in the Cremophor vehicle.
Net Iron Balance in CebusMonkeys
| . | Dosage . | Route . | Vehicle . | Predrug (μg/kg) . | Postdrug (μg/kg) . | Significance of t-test . |
|---|---|---|---|---|---|---|
| DFO | 150 μmol/kg | SC | dH2O | 169 ± 132 | −278 ± 185 | P < .001 |
| HBED | 75 μmol/kg | SC | 40% Cremophor | 173 ± 7 | −606 ± 406 | P < .005 |
| HBED | 150 μmol/kg | SC | 40% Cremophor | 175 ± 70 | −1,141 ± 456 | P < .001 |
| HBED | 150 μmol/kg | SC | buffer | 212 ± 105 | −689 ± 158 | P < .001 |
| . | Dosage . | Route . | Vehicle . | Predrug (μg/kg) . | Postdrug (μg/kg) . | Significance of t-test . |
|---|---|---|---|---|---|---|
| DFO | 150 μmol/kg | SC | dH2O | 169 ± 132 | −278 ± 185 | P < .001 |
| HBED | 75 μmol/kg | SC | 40% Cremophor | 173 ± 7 | −606 ± 406 | P < .005 |
| HBED | 150 μmol/kg | SC | 40% Cremophor | 175 ± 70 | −1,141 ± 456 | P < .001 |
| HBED | 150 μmol/kg | SC | buffer | 212 ± 105 | −689 ± 158 | P < .001 |
The amount of iron absorbed by the untreated animals over a 3-day period is compared with the amount of iron excreted by the treated animals over a 3-day period. Net iron balance = dietary iron intake − (urinary iron + fecal iron). Animals in a negative iron balance are excreting more iron than they are absorbing.
DISCUSSION
Previous efforts to develop alternatives to DFO for iron-chelating therapy have focused almost exclusively on identifying agents that remain active after oral administration. DFO (Mr 657) is a hexadentate ligand (Fig 1); one molecule of DFO binds a single atom of iron and the chelate (feroxamine) is virtually inert biologically. Because absorption across the gastrointestinal tract is generally better for compounds of lower molecular weight (Mr < 350),38,39 most of the efforts to develop orally active agents have been devoted to smaller chelators (Mr 100 to 250) that are bidentate (2,3 dihydroxybenzoic acid, hydroxypyridones) or tridentate (pyridoxal isonicotinoyl hydrazone, desferrithiocin). However, recent theoretical considerations, laboratory studies in vitro and in vivo, and clinical experience have raised concerns that, in some circumstances, these incomplete ligands might exacerbate iron-related tissue damage. To occupy the six coordination sites of a single atom of iron, one molecule of a hexadentate (eg, DFO or HBED), two molecules of a tridentate, or three molecules of a bidentate chelator are needed. At low concentrations of bidentate or tridentate chelators relative to the available iron, partially liganded forms of iron (bound to only one or two molecules of bidentate or tridentate ligands) appear in which the unoccupied coordination sites are either open or are occupied by another readily dissociable ligand such as water. Although iron is poorly soluble under physiological conditions, bidentate or tridentate chelators can greatly increase the solubility of iron, in part through the production of partially liganded chelates. Theoretically, these partially liganded forms of iron may catalyze the formation of hydroxyl radicals or other reactive oxygen species, in which rapid and nonspecific reactivity may be particularly injurious.40Laboratory studies have confirmed that bidentate or tridentate chelators may increase both the availability and reactivity of iron for participation in free radical-mediated lipid peroxidation,41,42 which accumulating evidence implicates in the pathogenesis of hepatic fibrosis and other tissue damage.43,44 In addition, studies in an animal model of human iron overload45 have found that the combination of iron overload and treatment with a bidentate hydroxypyridone chelator (1,2-diethyl-3-hydroxypyridin-4-one; CP94) produced a worsening of hepatic fibrosis and increased cardiac iron accumulation.46A closely related orally active bidentate hydroxypyridone, deferiprone (1,2-dimethyl-3-hydroxypyridin-4-one; CP20; L1), is now in clinical trials.47 Recently, evidence of exacerbation of hepatic fibrosis was found in a group of patients who have had long-term treatment with deferiprone.48 Overall, these theoretical concerns and the related clinical and laboratory findings have created caution about the sole reliance on the development of orally active bidentate and tridentate iron-chelating agents as potential alternatives to parenteral DFO.
HBED (Mr 388) is a phenolic aminocarboxylate chelator that was first synthesized by Martell et al some three decades ago26 and, like DFO, forms a 1:1 hexadentate complex with ferric iron. In rats, the LD50 (PO or intraperitoneally) is in excess of 800 mg/kg,49 and the compound was originally chosen for further development as an iron-chelating agent after studies in rodents suggested that it was well absorbed from the gastrointestinal tract and remained active as an iron chelator after oral administration.34,50 The results of the studies in rats without iron overload that are reported in this paper confirm that an oral dose of HBED of 150 μmol/kg is about twice as effective as the same dose of DFO given by SC injection. Although rodents provide a valuable first-line animal screen, allowing for a rapid and inexpensive way to identify and discard chelators that are ineffective in vivo, there is no strict correspondence between the effectiveness of a chelator in rodents and that in humans. Therefore, for the secondary screening of iron-chelating agents intended for clinical use, we have developed and validated an iron loaded C apella monkey model. In previous studies with this model, we found that the iron excretion induced by PO administration of HBED was only about 10% of that resulting from the SC administration of an equivalent dose of DFO31 (Fig 4). Iron balance studies with PO administration of HBED to human volunteers32 33 have now confirmed the lack of activity predicted from the primate model. The iron excretion produced after oral administration of HBED to patients is insufficient for therapeutic use in transfusional iron overload. Nonetheless, no adverse effects of oral administration of HBED were noted in these trials.
The potential therapeutic usefulness of HBED given parenterally was evaluated in the monkeys after recalling that intraperitoneal injection of the drug in the hypertranfused rat produced an iron excretion that was significantly greater than that after injection of DFO.34 Accordingly, we compared the iron excretion induced by SC injection of HBED in rats and monkeys with that induced by SC injection of DFO. The net iron excretion after SC HBED in the rat was more than threefold greater than that after DFO (Fig 3). Most importantly, in the primate model with iron overload, a single SC injection of HBED produced iron excretion that was at least twice that observed after SC DFO in the same group of monkeys (Fig 4). The use of a Cremophor vehicle did not significantly increase HBED-induced iron excretion.
In addition to enhanced iron excretion, parenteral HBED has some possible advantages over DFO as a candidate iron-chelating agent for the chronic treatment of iron overload. For patients allergic to DFO, HBED, a member of a different family of chelators, would be unlikely to provoke a similar response. Because HBED is a synthetic product, problems of local reactions to fermentation products not removed during purification would be avoided. Finally, HBED, like DFO, protects against iron-mediated free radical generation.42 The results reported here strongly suggest that SC injection of HBED could potentially provide patients with a clinically effective form of iron-chelating therapy.
In quantitative terms, most patients with thalassemia major require between 200 and 300 mL/kg body weight per year of blood, an amount equivalent to about 250 to 400 μg Fe/kg body weight per day. In theC apella monkey with iron overload, the iron excretion observed after a single SC injection of DFO, 435 ± 115 μg Fe/kg body weight, is consistent with the established ability of the daily use of DFO to control body iron. Giving DFO by prolonged SC infusion would be expected to increase iron excretion, but the clinical significance of the monkey data may be put into perspective by considering recent reports indicating that twice-daily SC injections of DFO may be as effective as the same dose of the drug administered by prolonged SC infusion.51,52 The results of the primate studies presented here suggested that, in patients with iron overload, a single SC injection of HBED might produce an iron excretion even greater than that induced by the same dose of DFO. Although caution is needed in extrapolating from the primate model to patients with iron overload, the higher iron excretion observed after a single SC injection of HBED in a phosphate buffer, 899 ± 193 μg Fe/kg, suggests that a regimen in which SC HBED was used every other day might be effective in maintaining iron balance. The possibility of using higher doses of HBED administered less frequently, eg, once or twice weekly, also needs examination. Overall, these findings suggest that preclinical evaluation of parenteral HBED should be completed promptly, followed by iron balance studies in human volunteers. Subcutaneous injection of HBED may provide patients with transfusional iron overload an alternative to the use of prolonged parenteral infusions of DFO for iron-chelating therapy.
Supported in part by research grants from the National Institutes of Health, Bethesda, MD (DK49108, AI35827, and HL57607).
Address reprint requests to Raymond J. Bergeron, PhD, Box 100485 JHMHC, Department of Medicinal Chemistry, University of Florida, Gainesville, FL 32610.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal