Abstract
Interferon-alpha (IFNα) mediates its biological effects through activation of the JAK-STAT signaling pathway and it has been shown to be one of most effective therapeutic agents for a number of hematological malignancies, including cutaneous T-cell lymphoma (CTCL). Nevertheless, its efficacy is limited by the development of clinical resistance but the reasons for resistance in CTCL are unknown. Here, we report the development of an IFNα-resistant CTCL cell line (HUT78R), characterized by its ability to proliferate in high concentration of recombinant IFNα, which can be used as a model system to study IFN resistance. The levels of IFN receptor expression and binding affinity were found to be comparable between the parental sensitive (HUT78S) and resistant (HUT78R) cells. However, IFNα stimulation failed to induce interferon-stimulated gene factor 3 (ISGF3) complex formation in HUT78R cells. In addition, the expression of the IFN-inducible 2-5 OAS gene was significantly reduced in HUT78R cells, suggesting the presence of a defect in the Jak-STAT signaling pathway. Our results showed that the IFNα-activated form of a latent transcriptional factor STAT1 was not found in HUT78R cells, whereas activated STAT2 and STAT3 were clearly detectable. By Western blotting and reverse transcriptase-polymerase chain reaction (RT-PCR) analyses, we found that HUT78R cells do not express any STAT1 protein or mRNA, suggesting the possibility of a null mutation in the STAT1 gene. Resistance to the growth inhibitory effect of IFNα in CTCL cells may result from lack of STAT1 expression.
INTERFERONS (IFN) ARE A group of cytokines whose biological activities include antiviral, antiproliferative, and immunomodulatory effects.1,2 There are two classes of IFNs, Type-I and II, and IFNα belongs to the Type-I family. All the Type-I IFNs bind to the Type-I IFN receptor (IFNR) that is expressed in a number of neoplastic cell lines, as well as in normal monocytes and lymphocytes.2,3 IFNα mediates its signal transduction by binding to its receptor, which rapidly become tyrosine phosphorylated.4,5 Phosphorylation of the receptor is regulated by two Janus kinases, Tyk2 and Jak1, which become activated upon stimulation of IFNα.6,7 The activated Tyk2 and Jak1 then phosphorylate three STAT proteins.4,8Activated STAT1 and 2 associate with a 48 kD protein (p48) to form the interferon-stimulated gene factor-3 (ISGF-3) complex, which binds specifically to the IFNα-stimulated response element (ISRE), resulting in gene transcription.9-11 In addition, IFNα treatment induces STAT3 phosphorylation, and activated STAT3 can bind to the interferon responsive element (IRE) sequence, forming a protein-DNA complex distinct from the ISGF3-ISRE complex.12
IFNα has emerged as an important therapeutic cytokine that exerts potent antineoplastic effects involving regulation of cell cycle, suppression of oncogenes, and modulation of cell adhesion and angiogenesis.13 It has been shown to be effective against hematological malignancies including hairy cell leukemia (HCL), chronic myelogenous leukemia (CML), and cutaneous T-cell lymphoma (CTCL).13,14 Nevertheless, development of resistance to IFNα therapy has often been observed in patients15-17 and the molecular basis of clinical resistance is not known. We have developed an IFN-resistant tumor cell variant (HUT78R) from a human CTCL cell line (HUT78). In characterizing the IFNα-sensitive (HUT78S) and resistant cells, we noted that IFN-inducible 2′,5′-oligoadenylate synthetase (2,5-OAS)18 gene expression and ISGF3 complex formation was significantly reduced in HUT78R cells. Further analysis showed that there was no STAT1 protein expressed in HUT78R cells. On the other hand, IFNα treatment induced both STAT2 and STAT3 phosphorylation in the HUT78R cells, indicating that the signaling defect is solely due to absence of STAT1. RT-PCR analysis also failed to detect any STAT1 transcript in HUT78R cells, suggesting the possibility of a null mutation in the STAT1 gene.
MATERIALS AND METHODS
Tumor cell lines.
HUT78 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were maintained in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. The IFNα-resistant (HUT78R) cells were generated by culturing the HUT78 cells in increasing concentrations of IFNα-2a (recombinant IFNα-2a was kindly provided by Hoffman-LaRoche Inc, Nutley, NJ) beginning with 100 U/mL. The cells were passaged once a week and the viability was determined by trypan blue exclusion. The concentration of IFNα-2a was increased incrementally until it reached 1 × 106 U/mL over a period of several months. Both parental and IFN-resistant HUT78 cells were plated on 0.5% noble agar containing 20% FCS, penicillin (100 U/mL), and streptomycin (100 ng/mL) and incubated for an additional 14 days. Individual colonies were picked and seeded in the 96-well microtitier plates in 200 μL of RPMI-1640 with 20% FCS. Two IFNα-resistant (HUT78R1 and R2) and two sensitive (HUT78S1 and HUT78S2) clones were isolated. The sensitivity of the cells to IFNα treatment was determined by colorimetric cell proliferation assay (CellTiter96TM, AQueous Plate, Promega, Madison, WI). The resistant phenotype of HUT78R cells was confirmed after removing the cells from IFNα treatment for as long as 1 year.
IFNα-2a binding assay.
Recombinant IFNα-2a was labeled with [125I] diiodinated Bolton-Hunter reagent (New England Nuclear, Boston, MA) following manufacturer's instructions. The labeled protein was purified by chromatography using a BioGel P-6DG column (BioRad, Richmond, CA). The cells (1.5 × 106) were incubated with 5 pmol/L of [125I] IFNα-2a in one mL of RPMI medium/1% bovine serum albumin (BSA), with increasing amounts of unlabeled IFNα-2a for 2 hours at 4°C. The mixture was then centrifuged in dibutyl phthalate oil (Sigma, St Louis, MO) briefly to pellet the cells and counted in a gamma counter. The levels of IFNα-2a binding to the HUT78 variants were determined by Scatchard analysis.
Slot blot analysis of IFNR and 2,5-OAS mRNA.
Total poly A+ RNA was isolated from HUT78R and HUT78S cell lines with the Ribosep kit (Collaborative Research, Bedford, MA) and diluted with 6.25 mol/L formaldehyde/10× SSPE (1.8 mol/L NaCl, 0.1 mol/L sodium phosphate, pH 7.7, and 0.1 mol/L Na2EDTA) at 65°C for 5 minutes. The treated RNA (10 μg) was transferred onto nylon filters, UV-cross-linked, and probed with human IFNR19 or 2,5-OAS (kindly provided by Dr Bryan Williams) cDNA probe. The autoradiograph was scanned on a densitometer and the levels of either IFNR or 2,5-OAS mRNA were normalized to the levels of β-actin mRNA.
Electromobility shift assay (EMSA).
The HUT78 cells were treated with or without IFNα-2a (10,000 U/mL) for 15 minutes. Briefly, cells were washed with ice-cold phosphate-buffered saline (PBS) and resuspended in lysis buffer (20 mmol/L HEPES, 1 mmol/L vanadate, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethylsulfonic acid, 1 mmol/L dithiothreitol). Nuclear extracts were obtained by agitating the cell lysates in hypertonic buffer (350 mmol/L NaCl, 10 mmol/L HEPES [pH 8.0], 25% glycerol, 1 mmol/L EDTA, 10 μg/mL aprotinin, 5 mmol/L spermidine, 1 mmol/L PMSF) for 1 hour at 4°C and centrifuging at 1,500 × g for 15 minutes at 4°C. The nuclear extracts were aliquoted and stored at −20°C for future use. The sequence of the ISRE probe is derived from the 5′ translation initiation site of the 2,5-OAS gene (TGGACTGCTGTTGGTTTCGTTTCCTCAGAAGGGAGGAG), containing the consensus ISRE sequence in bold.18 The sequence of the second ISRE probe contains the first 26 nucleotides of the first ISRE probe (TTGGACTGCTGTTGGTTTCGTTTCCT). The oligo probes were synthesized at the Northwestern University Biotech DNA Synthesis Facility (Chicago, IL) and 5′-end labeled with [32P]. The nuclear extract (10 μg) was incubated with the [32P]-labeled ISRE probe (50,000 cpm) for 15 minutes at 30°C in binding buffer (40 mmol/L KCl, 20 mmol/L HEPES, pH 7.9, 1 mmol/L MgCl2, 0.1 mmol/L EDTA, 0.5 mmol/L DTT, 0.1% NP-40, 10% glycerol) in a total volume of 20 μL. The binding mixtures were subjected to electrophoresis followed by autoradiography.
Immunoprecipitation and immunoblotting.
The HUT78R or HUT78S cells (2 × 107 cells) were stimulated with IFNα-2a (10,000 U/mL) for 5 minutes and then lysed with lysis buffer (50 mmol/L HEPES, pH 7.3, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, pH 7.4, 10 mmol/L NaF, 10 μmol/L Na-pyrophosphate, 200 μmol/L Na-orthovanadate, 0.5% Triton-X) on ice in a total volume of one mL. The whole cell lysates were incubated with agarose-protein G beads (Pharmacia, Uppsala, Sweden; 40 μL) and with either nonimmune serum, anti-STAT1, anti-STAT2, anti-STAT3, anti-Tyk2, or anti-Jak1 polyclonal antibody (Santa Cruz Biotech, CA) for 5 hours at 4°C with constant agitation. The immunoprecipitates were washed extensively in lysis buffer containing 0.1% Triton-X and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% acrylamide gel). The proteins were then transferred to Immobilon membranes and blotted with antiphosphotyrosine antibody (1 μg/mL; UBI). Bands of protein phosphorylation were detected by enhanced chemiluminescence (ECL kit; Amersham, Arlington Heights, IL). The same blots were stripped and reprobed with either anti-STAT1, anti-STAT2, anti-STAT3, anti-Tyk2, or anti-Jak1 antibody to determine the levels of STAT proteins loaded in each lane.
RT-PCR for STAT1 transcript detection.
The primers were designed based on the STAT1 cDNA sequence10 (GenBank Accession # M97935), with the OLIGO 4.0 software (National Biosciences, Plymouth, MN). Primer 1 (5′AAGGTGGCAGGATGTCTCGTG-3′) spans the STAT1 translational initiation site and is complementary to nucleotides 186-207. Primer 2 (5′TGGTCTCGTGTTCTTCTGTTCTG-3′) covers the junction of exon 5 and 6 and is complementary to bases 728-749. The expected PCR product corresponds to a 564 bp fragment that represents the first five exons of the STAT1 coding sequence. Total RNA was extracted with Trizol reagent (GIBCO), treated with DNase I (Promega, Madison, WI) and diluted in DEPC-treated water (1 μg/mL). RT-PCR reactions were performed with the Access RT-PCR kit (Promega). The reverse transcription was performed at 48°C for 45 minutes after a 2 minute 94°C AMV RT inactivation and RNA/cDNA/primer denaturation step. The parameters for the PCR are: denaturation at 94°C for 2 minutes, 40 cycles of 94°C for 30 seconds, annealing for 1 minute, 2 minute extension at 68°C, and a final 7 minute extension at 68°C. The actin mRNA was amplified with primers that are commercially available (Clonetech, San Diego, CA).
RESULTS
Development of IFNα-resistant HUT78 cells.
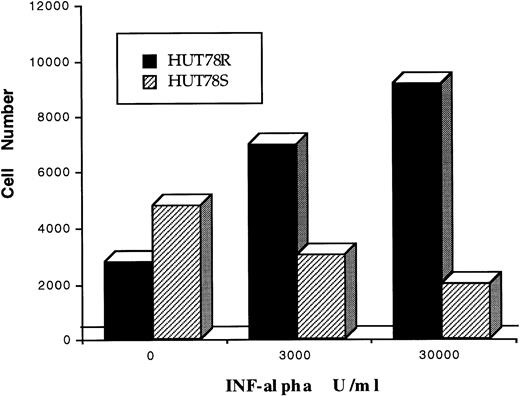
HUT78 cells were originally derived from a CTCL patient with Sezary syndrome.20 IFN-resistant HUT78R cells were developed by culturing the HUT78 cells over several months in increasing concentrations of IFNα beginning with 100 U/mL. The cells were passaged once a week and examined for their viability by trypan blue exclusion. When cell viability exceeded 85%, a higher concentration of IFN was added to the medium. Cells growing in 1 × 106U/mL of IFNα were plated on soft agar for subcloning, and two clones (HUT78R1, HUT78R2) were isolated and compared with two clones of the IFN-sensitive HUT78 cells (HUT78S1 and HUT78S2). To confirm the resistant phenotype, HUT78R cells were cultured in IFNα-free medium for up to 1 year and were shown to retain resistance to IFNα as determined by the MTS cell proliferation assay. The LD50(the concentration of IFNα required for 50% growth inhibition in 4 days) of HUT78S cells was determined to be between 2500 U/mL to 3000 U/mL. As shown in Figure 1, IFNα (3000 U/mL to 30,000 U/mL) exerted cytostatic effects on HUT78S cells (□). In contrast, HUT78R cells (▪) grew at a faster rate in the presence of IFNα, in a dose-dependent manner.
Antiproliferative effect of IFNα on HUT78 variants. The anti-proliferative effect of IFNα on HUT78 cells was determined by colorimetric cell proliferation assay. Cells were serum-starved overnight (in 1% BSA with RPMI) and seeded in a 96-well plate (3000 cell/well). Cells were treated with three different concentrations of IFNα (0, 3,000, and 30,000 U/mL) for 4 days and cell proliferation was determined. Numbers of HUT78S and HUT78R cells are represented by –– and —, respectively. Results in figure 1 represent one of three independent experiments.
Antiproliferative effect of IFNα on HUT78 variants. The anti-proliferative effect of IFNα on HUT78 cells was determined by colorimetric cell proliferation assay. Cells were serum-starved overnight (in 1% BSA with RPMI) and seeded in a 96-well plate (3000 cell/well). Cells were treated with three different concentrations of IFNα (0, 3,000, and 30,000 U/mL) for 4 days and cell proliferation was determined. Numbers of HUT78S and HUT78R cells are represented by –– and —, respectively. Results in figure 1 represent one of three independent experiments.
HUT78R cells express comparable levels of IFNR.
We first sought to determine if HUT78R cells have reduced number of IFNR or express receptors with lower ligand affinity by binding assay. The Scatchard analyses showed no significant differences in dissociation constants (Kd) or receptor numbers between the HUT78 variants (Table 1). At the transcriptional level, Northern blot showed the presence of a single 2.5 Kb band in all variants (data not shown) and slot blot analysis showed no significant differences in the levels of IFNR mRNA between HUT78R and HUT78S cells before or after 48 hours of IFNα treatment (Fig2).
IFNα Receptor Binding in HUT78 Variants
| Cell Lines . | Kd (×10−10 M) . | Receptor Number/Cell . |
|---|---|---|
| HUT78 S1 | 5.8 ± 1.5 | 7500 ± 2100 |
| HUT78 S2 | 5.5 ± 2.0 | 7760 ± 2300 |
| HUT78 R1 | 6.0 ± 2.3 | 8010 ± 2500 |
| HUT78 R2 | 6.2 ± 2.3 | 8190 ± 2400 |
| Cell Lines . | Kd (×10−10 M) . | Receptor Number/Cell . |
|---|---|---|
| HUT78 S1 | 5.8 ± 1.5 | 7500 ± 2100 |
| HUT78 S2 | 5.5 ± 2.0 | 7760 ± 2300 |
| HUT78 R1 | 6.0 ± 2.3 | 8010 ± 2500 |
| HUT78 R2 | 6.2 ± 2.3 | 8190 ± 2400 |
Recombinant IFNα-2a was labeled with [125I] and purified by chromatography. The cells (1.5 × 106) were incubated with 5 pmol/L of [125I] IFNα-2a in 1 mL of RPMI medium/1% bovine serum albumin (BSA), with increasing amounts of unlabeled IFNα-2a for 2 hours at 4°C. The mixture was then centrifuged in dibutyl phthalate oil briefly to pellet the cells and counted in a gamma counter. The levels of IFNα binding to the HUT78 variants were determined by Scatchard analysis.
Levels of IFNR mRNA expression in HUT78 variants. The levels of IFNR mRNA were assessed by slot blot analysis. Total poly A+ RNA was isolated from HUT78R and HUT78S cells treated with (+) or without (−) IFNα and diluted with 6.25 mol/L formaldehyde/10X SSPE at 65°C. The treated RNA (10 μg) was transferred to nylon filters, UV-cross-linked and probed with human IFNR cDNA probe labeled with 32[P], followed by autoradiography.
Levels of IFNR mRNA expression in HUT78 variants. The levels of IFNR mRNA were assessed by slot blot analysis. Total poly A+ RNA was isolated from HUT78R and HUT78S cells treated with (+) or without (−) IFNα and diluted with 6.25 mol/L formaldehyde/10X SSPE at 65°C. The treated RNA (10 μg) was transferred to nylon filters, UV-cross-linked and probed with human IFNR cDNA probe labeled with 32[P], followed by autoradiography.
IFNα fails to induce ISGF-3 formation in HUT78R cells.
Activation of transcriptional factors by IFNα in the HUT78 variants was assessed by performing EMSA assays with two oligo probes containing the consensus ISRE sequence found in the promoter region of the 2,5-OAS gene. Our results showed that IFNα treatment failed to induce the ISGF-3 complex formation in HUT78R cells (Fig3). In addition, slot blot analysis showed that IFNα stimulation induced a 15-fold to 20-fold increase in 2,5-OAS mRNA level in the HUT78S cells, compared to only fourfold to sixfold increase in HUT78R cells (Fig4). However, expression of the metallothionein-II and HLA-B7 genes, which is not mediated by ISGF-3 binding to the ISRE sequence,21 was found to be similar between HUT78R and HUT78S cells (data not shown). These results support the notion that activation of the JAK-STAT pathway is defective in HUT78R cells.
IFNα treatment fails to induce ISGF3 formation in HUT78R cells. IFNα–induced-ISGF3 complex formation was analyzed by gel shift assays using oligo probes. ISRE probe I: TTGGACTGCTGTTGGTTTCGTTTCCTC (left panel) and a longer version of the ISRE probe II: TTGGACTGCTGTTGGTTTCGTTTCCTCAGAAGGGAGGAG (right panel). HUT78R and HUT78S cells were treated with (+) or without (−) IFNα (10,000 U/mL) for 5 minutes. Nuclear extracts were prepared and incubated with 32[P]-labeled probes for 15 minutes at 30°C and the binding mixture was subjected to electrophoresis followed by autoradiography. The arrows indicate the location of ISGF-3 complexes.
IFNα treatment fails to induce ISGF3 formation in HUT78R cells. IFNα–induced-ISGF3 complex formation was analyzed by gel shift assays using oligo probes. ISRE probe I: TTGGACTGCTGTTGGTTTCGTTTCCTC (left panel) and a longer version of the ISRE probe II: TTGGACTGCTGTTGGTTTCGTTTCCTCAGAAGGGAGGAG (right panel). HUT78R and HUT78S cells were treated with (+) or without (−) IFNα (10,000 U/mL) for 5 minutes. Nuclear extracts were prepared and incubated with 32[P]-labeled probes for 15 minutes at 30°C and the binding mixture was subjected to electrophoresis followed by autoradiography. The arrows indicate the location of ISGF-3 complexes.
IFNα-induced 2,5-OAS gene expression is reduced in HUT78R cells. The levels of 2,5-OAS mRNA were determined in two clones of the resistant (HUT78R1 and HUT78R2) and sensitive (HUT78S1 and HUT78S2) cells treated with (+) or without (−) IFNα by slot blot analysis with a 32[P]-labeled cDNA probe for the human 2,5-OAS gene.
IFNα-induced 2,5-OAS gene expression is reduced in HUT78R cells. The levels of 2,5-OAS mRNA were determined in two clones of the resistant (HUT78R1 and HUT78R2) and sensitive (HUT78S1 and HUT78S2) cells treated with (+) or without (−) IFNα by slot blot analysis with a 32[P]-labeled cDNA probe for the human 2,5-OAS gene.
IFNα induces Tyk2 and Jak1 activation in both HUT78S and HUT78R cells.
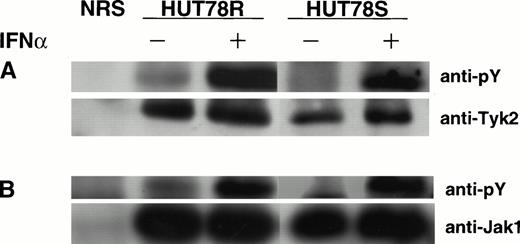
To determine if IFNα stimulation induces activation of Janus kinases associated with the IFNR, immunoprecipitation and Western blot were performed. Our results showed that IFNα induced comparable levels of Tyk2 and Jak1 phosphorylation in both HUT78S and HUT78R cells (Fig 5A and B), indicating that the defect in IFN signaling is not related to the Janus kinases.
IFNα-induced Janus kinases activation HUT78 cells. The cells were treated with (+) or without (−) IFNα (10,000 U/mL for 5 minutes) and whole cell extracts were prepared for immunoprecipitation with anti-Tyk2 (Fig 5A) or anti-Jak1 (Fig 5B) antibody. The immunocomplex was then analyzed by Western blot using anti-phosphotyrosine (anti-pY) antibody (upper panels). The same blot was stripped and reprobed with anti-Tyk2 or Jak1 antibody to determine the levels of protein in each lane (lower panels).
IFNα-induced Janus kinases activation HUT78 cells. The cells were treated with (+) or without (−) IFNα (10,000 U/mL for 5 minutes) and whole cell extracts were prepared for immunoprecipitation with anti-Tyk2 (Fig 5A) or anti-Jak1 (Fig 5B) antibody. The immunocomplex was then analyzed by Western blot using anti-phosphotyrosine (anti-pY) antibody (upper panels). The same blot was stripped and reprobed with anti-Tyk2 or Jak1 antibody to determine the levels of protein in each lane (lower panels).
HUT78R cells do not express STAT1.
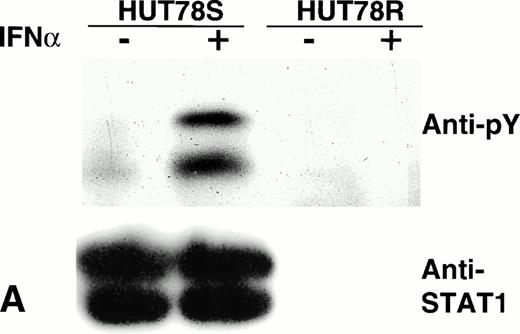
To assess STAT protein activation induced by IFNα treatment in both HUT78R and HUT78S cells, we immunoprecipitated STAT1, -2, or -3 and performed Western blots of the immunoprecipitates using antiphosphotyrosine antibody. We found that IFNα treatment-induced STAT1 phosphorylation in the HUT78S cells but not in HUT78R cells (Fig6A, upper panel). When the same blot was stripped and reprobed with anti-STAT1 antibody, we failed to detect any STAT1 protein present in the lysates of HUT78R cells (Fig 6A, lower panel), which explains the lack of phosphorylation signal.
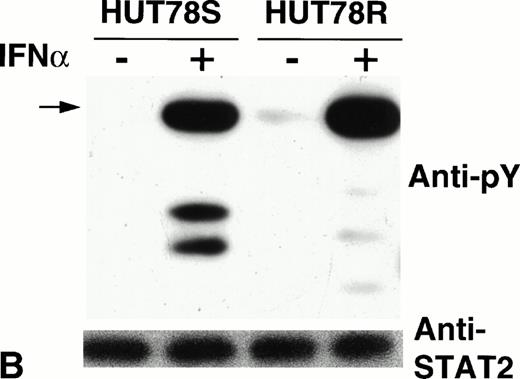
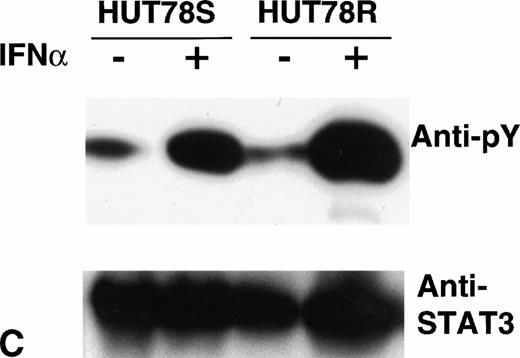
Phosphorylation of STAT proteins in HUT78 cells. The cells were treated with (+) or without (−) IFNα (10,000 U/mL for 5 minutes) and whole cell extracts were prepared for immunoprecipitation with anti-STAT antibody. The immunocomplex was then analyzed by Western blot using anti-phosphotyrosine (anti-pY) antibody (upper panel). The same blot was stripped and reprobed with anti-STAT antibody to determine the levels of protein in each lane (lower panel). STAT1 immunoprecipitation is shown in Figure 6A, STAT2 in Figure 6B, and STAT3 in Figure 6C. The arrow in Figure 6B indicates the location of STAT2 and activated STAT1 (doublet) was coprecipitated with STAT2 in HUT78S cells only (Fig 6B, upper panel).
Phosphorylation of STAT proteins in HUT78 cells. The cells were treated with (+) or without (−) IFNα (10,000 U/mL for 5 minutes) and whole cell extracts were prepared for immunoprecipitation with anti-STAT antibody. The immunocomplex was then analyzed by Western blot using anti-phosphotyrosine (anti-pY) antibody (upper panel). The same blot was stripped and reprobed with anti-STAT antibody to determine the levels of protein in each lane (lower panel). STAT1 immunoprecipitation is shown in Figure 6A, STAT2 in Figure 6B, and STAT3 in Figure 6C. The arrow in Figure 6B indicates the location of STAT2 and activated STAT1 (doublet) was coprecipitated with STAT2 in HUT78S cells only (Fig 6B, upper panel).
On the other hand, STAT2 phosphorylation was comparable in both HUT78R and HUT78S cells (Fig 6B, upper panel), indicating that signaling from ligand binding through Tyk2/Jak1 kinase activation is not affected. IFNα stimulation also augmented the level of STAT3 phosphorylation in both cell lines (Fig 6C, upper panel). Similar levels of STAT proteins loaded in each lane were confirmed by reprobing the same blot with the respective anti-STAT antibody, as shown in the lower panels of Figure6B and C.
HUT78R cells do not express STAT1 transcript.
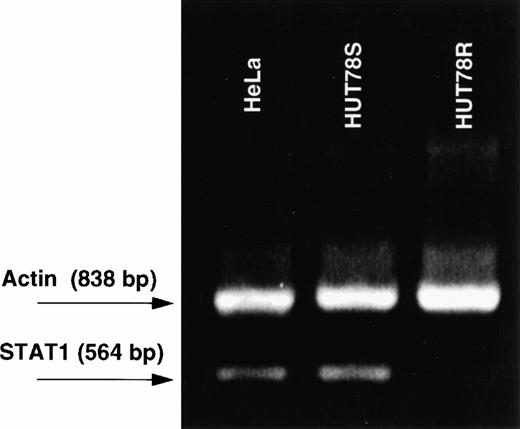
To determine whether inhibition of STAT1 protein expression in HUT78R cells is caused by translational or transcriptional defect(s), RT-PCR analysis was employed to detect the presence of STAT1 transcript in HUT78 cells. The primers were designed to amplify a 564 bp that represents the first five exons of the STAT1 coding sequence. As expected, we detected a PCR product of 564 bp fragment from HUT78S cells and HeLa cells, (which is known to express STAT1 protein)10 but no amplification was detected in HUT78R cells (Fig 7).
RT-PCR of STAT1 transcript. Three cell lines were subject to RT-PCR, including HUT78R, HUT78S, and HeLa cells. Primer 1 (5′AAGGTGGCAGGATGTCTCGTG-3′) spans the STAT1 translational initiation site and is complementary to nucleotides 186-207. Primer 2 (5′TGGTCTCGTGTTCTTCTGTTCTG-3′) covers the junction of exon 5 and 6 and is complementary to bases 728-749. The actin mRNA was amplified using primers that are commercially available.
RT-PCR of STAT1 transcript. Three cell lines were subject to RT-PCR, including HUT78R, HUT78S, and HeLa cells. Primer 1 (5′AAGGTGGCAGGATGTCTCGTG-3′) spans the STAT1 translational initiation site and is complementary to nucleotides 186-207. Primer 2 (5′TGGTCTCGTGTTCTTCTGTTCTG-3′) covers the junction of exon 5 and 6 and is complementary to bases 728-749. The actin mRNA was amplified using primers that are commercially available.
DISCUSSION
In this report we describe the development and characterization of an IFNα-resistant human CTCL cell line (HUT78R) that may be used as a model system for study of clinical resistance to IFNα therapy. We first sought to determine if IFNα-binding and INFR expression are altered in HUT78R cells. Our results showed that the levels of IFNR mRNA were similar between HUT78S and HUT78R cells. In addition, Scatchard analysis of the IFNα-binding assay showed that dissociation constants of IFNR between HUT78R and HUT78S cells were comparable. Next, we examined if IFNα-induced transcriptional activation was affected in HUT78R cells. With oligo probes containing the ISRE consensus sequence in gel shift assays, we found that IFNα stimulation failed to induce ISGF3 complex formation in HUT78R cells. In addition, IFNα-induced 2,5-OAS gene expression was inhibited significantly in HUT78R cells, compared with HUT78S cells. The fourfold to sixfold increase in 2,5-OAS gene expression observed in HUT78R cells (in contrast to 20-fold increase in HUT78S cells) may be due to activation of IFN response sequence (IRS)21 found in the 2,5-OAS promoter region. The IFNα-induced gene expression of histocompatibility locus antigen (HLA)-B7 and metallothionein-II gene, which both contain IRS element, was found to be comparable between HUT78R and HUT78S cells (unpublished results). Taken together, these results suggest a defect(s) in the activation of JAK-STAT signaling pathway in HUT78R cells, resulting in inhibition of ISGF3 formation (ie, ISRE binding), 2,5-OAS gene induction and resistance to the growth inhibitory effect of IFNα.
To identify and characterize such a defect(s), we first determined if type-I IFNR-associated Janus kinases Tyk2 and Jak1 were activated on IFNα stimulation in the resistant cells. Our results showed that IFNα treatment induced in comparable levels of Tyk2 and Jak1 tyrosine phosphorylation, suggesting that the IFN signaling defect in HUT78R cells is probably not a result of Tyk2 or Jak1 dysfunction. We then sought to determine the status of STAT protein activation by IFNα treatment in HUT78 cells. We failed to detect any STAT1 activation in HUT78R cells. In contrast, IFNα-induced similar levels of STAT2 and STAT3 activation in HUT78R cells, compared with HUT78S cells. This result suggests that the signaling defect in HUT78R cells is associated only with STAT1. Immunoprecipitation and Western analyses failed to detect any STAT1 (α and β) protein expressed in HUT78R cells, explaining the lack of detection of STAT1 phosphorylation on IFNα treatment.
A number of IFN-(γ or α) resistant human tumor cell lines have been reported previously.22-28 One was shown to lack expression of one IFNR component27 and the U3A cell line (U3A), derived from high-frequency mutagenesis of the 2fTGH cells,28 was shown to lack STAT1 expression. U3A cells were unresponsive to the growth inhibitory effects of IFNγ and IFNα. It was shown that transfection of STAT1α cDNA into the U3A cells can restore the cells' sensitivity to the growth inhibitory effect of IFNs.29 More recently, STAT1 was shown to regulate the expression of cyclin-dependent kinase (cdk2) inhibitor p21 at the transcriptional level,30 indicating that STAT1 negatively regulates the cell cycle progression. Taken together, STAT1 appears to play a significant role in mediating cytokine signaling and regulation of cell cycle.
IFNα is the only known cytokine that activates STAT2, and STAT2 phosphorylation has been shown to be a prerequisite for STAT1 activation.31,32 However, lack of STAT1 activation did not affect STAT2 phosphorylation induced by IFNα in HUT78R cells, as previously shown in U3A cells.33 Similarly, IFNα stimulation-induced STAT3 activation in both HUT78R and HUT78S cells. Hence, absence of STAT1 in the HUT78R cells did not have any significant effect on STAT3 activation, as shown previously in STAT1−/− embryonic stem cells.34 Tyk2 activation was shown to be obligatory to activation of Jak1, STAT1, and STAT2 in IFNα-mediated signaling.35 Our result showed that IFNα treatment-induced Tyk2 and Jak1 phosphorylation in both HUT78R and HUT78S cells, showing the independence of STAT2 and STAT3 activation to STAT1.
In contrast to the antiproliferative effect of IFNα on HUT78S cells, increasing concentrations of IFNα caused a marked stimulation of growth in HUT78R cells (Fig 1). It is conceivable that lack of STAT1 may alter the balance of STAT complex formation, resulting in inappropriate signaling and gene transcription. Whether IFNα-induced activation of STAT3 and/or STAT2 in the absence of STAT1 is associated with this accelerated growth of HUT78R cells, is a topic which warrants future investigation. We also noted that STAT3 was constitutively activated in both HUT78R and HUT78S cells (Fig 6C). Others have reported constitutive activation of STAT3 in anaplastic large T-cell lymphoma (ALTL), Sezary syndrome,36leukemia,37 and to contribute to oncogenesis by Src oncoprotein.38 Therefore, constitutive activation of STAT3 in HUT78 cells may be associated with their neoplastic characteristics.
The lack of STAT1 protein expression in HUT78R cells raises the possibility of a null mutation in the STAT1 gene. Our RT-PCR analysis failed to detect any STAT1 transcript in HUT78R cells, indicating that the putative mutation results in inhibition of transcription or expression of highly unstable mRNA. Comparative Southern blot analysis of genomic DNA obtained from HUT78S and HUT78R cells revealed distinct patterns of hybridization with a STAT1 cDNA probe (unpublished results). This result supports the notion that HUT78R cells harbor a STAT1 gene mutation(s) that results in lack of STAT1 expression. Additional experiments are needed to define the nature of this mutation in HUT78R cells. Because acquisition of clinical resistance to IFNα is commonly observed in CTCL, it will be of great significance to determine if tumor cells isolated from patients with IFNα resistance lack STAT1 or have other defects in the IFNα-signaling pathway. Understanding the mechanism of clinical resistance to IFNα at a molecular level may lead to development of innovative treatment strategies or alternative therapies.
ACKNOWLEDGMENT
We thank Dr Amy Paller for her support.
Supported by a Leukemia Research Foundation grant (W.H.S.) and the NCI Lurie Cancer Center Support Grant No. CA 60553-03.
Address correspondence to Wenn H. Sun, MD, PhD, Division of Dermatology, Department of Pediatrics, Northwestern University Medical School; Children's Memorial Hospital, 2300 Children's Plaza/#203, Chicago, IL 60614.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Levels of IFNR mRNA expression in HUT78 variants. The levels of IFNR mRNA were assessed by slot blot analysis. Total poly A+ RNA was isolated from HUT78R and HUT78S cells treated with (+) or without (−) IFNα and diluted with 6.25 mol/L formaldehyde/10X SSPE at 65°C. The treated RNA (10 μg) was transferred to nylon filters, UV-cross-linked and probed with human IFNR cDNA probe labeled with 32[P], followed by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.570/3/m_blod4024002.jpeg?Expires=1769128329&Signature=SiQQXZ-gCenFkVpnrZywTL1PWlJcFzbfIEvX~4mXJMalo8fMZaaRNNR57I1QW7W-sbgTgH3jdGDhYadaELhGxeqEy4jVlSlxVLiYidtRL~sWJkE24UWA0FovLikxfOaai5RtPn0jljbEa4nFtYigVkCc7L9fV-oavnelk1-YLI6KXV0X9phlrm0o6aU9783fvI-0jak~mQycK7SVYcSJ9e216Pbi1fIXk2X-ljd7~m6nLt7UxUD8BNUMnm3trIZLsxPRC5WKAsQzn60gGuNaf4GtHVmnJxCEOTZj5LVjgxuxIA0VdaMXmCu8oqjDXey7ORPSAD-r61QJQx-NdFB7EA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. IFNα treatment fails to induce ISGF3 formation in HUT78R cells. IFNα–induced-ISGF3 complex formation was analyzed by gel shift assays using oligo probes. ISRE probe I: TTGGACTGCTGTTGGTTTCGTTTCCTC (left panel) and a longer version of the ISRE probe II: TTGGACTGCTGTTGGTTTCGTTTCCTCAGAAGGGAGGAG (right panel). HUT78R and HUT78S cells were treated with (+) or without (−) IFNα (10,000 U/mL) for 5 minutes. Nuclear extracts were prepared and incubated with 32[P]-labeled probes for 15 minutes at 30°C and the binding mixture was subjected to electrophoresis followed by autoradiography. The arrows indicate the location of ISGF-3 complexes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.570/3/m_blod4024003.jpeg?Expires=1769128329&Signature=0TfFwbOKOgV3MNQunw7MCrJtGFCaREKQjGNSFr-1prG0a15ia-Xa42JfdMb4kIiuvdSO1hPBeUNIB5XwOJt-BMeYH5dFsRY9zvLljdR88~A9yQKPknFThcve0QeBJ-IwiH7l6E8UPLH2eBm1YkNMFlAXu8b2JJS74OwO2LFjdThiis6D8kPlLK4llZKsyo4Jk2-OKK2YZKN43gpUTe1xJ7-vj--zwjunLx4GFltvTx4vUBQgVAR70iXa1BIw4sx5vg2VUPQH6~jjw4Ft0r5TgQLUc-4PKk21Vcdh65Ebxv5CW4f8E0lX-Y212zh1VL99d9ZCWkFYOzyOdWSpXQbW7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. IFNα-induced 2,5-OAS gene expression is reduced in HUT78R cells. The levels of 2,5-OAS mRNA were determined in two clones of the resistant (HUT78R1 and HUT78R2) and sensitive (HUT78S1 and HUT78S2) cells treated with (+) or without (−) IFNα by slot blot analysis with a 32[P]-labeled cDNA probe for the human 2,5-OAS gene.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.570/3/m_blod4024004.jpeg?Expires=1769128329&Signature=4zSpiecGnNDZEBagyWhegLiZ2kXNSsvH87nCLu-cIR3xd56oUgIrvkK~NVsGxAtwFVrYIYVbichrF71WsPIL~wU~cWJe9BqcLUpujeyfj4Yu4VjaWu2IaoUv4-DrFZntJZrheJTYsOu8slhhUf91VvdpXP0Bx1ng5-CeAhzBHgrKsb4GfC9YAFTjsIdsMyR9fNHueqcXBP~-bAO7Ng16H3unA2bOTDVlode70UwvukvlTZS6Hj6Tgl8wdR1tXBDuDvN0ZsOEBSzsIzquMHZKZJn6hT~4r3tA7Bd3cnlnRxHTv11IAjyuSlJrcA-R2O6cp3nC5JM0R06UQ2lrsTaQlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal