Abstract
The tetrapeptide Acetyl-N-Ser-Asp-Lys-Pro (AcSDKP or Goralatide), a physiological regulator of hematopoiesis, inhibits the entry into the S-phase of murine and human hematopoietic stem cells. It has been shown to reduce the damage to specific compartments in the bone marrow resulting from treatment with chemotherapeutic agents, ionizing radiations, hyperthermy, or phototherapy. The present study was performed to assess the therapeutic potential of AcSDKP in vivo in reducing both the toxicity and the hematopoietic damage induced by fractionated administration of doxorubicin (DOX), a widely used anticancer drug. Here we showed that AcSDKP could reduce DOX-induced mortality in mice and could protect particularly the long-term reconstituting cells (LTRCs) in addition to colony forming units-spleen, high proliferative potential colony-forming cells, and colony-forming units–granulocyte-macrophage (CFU-GM) from DOX toxicity. The protection against DOX-induced mortality in mice was improved when AcSDKP was administered for 3 days, at a dose of 2.4 μg/d, by continuous subcutaneous (SC) infusion or fractionated SC injections starting 48 hours before DOX treatment. Moreover, the recovery of the CFU-GM population in the AcSDKP-DOX–treated mice was optimized by the subsequent administration of granulocyte colony-stimulating factor (G-CSF). The coadministration of AcSDKP with DOX may improve its therapeutic index by reducing both acute hematotoxicity on late stem cells and progenitors and long-term toxicity on LTRCs. Optimization of these treatments combined with G-CSF may provide an additional approach to facilitate hematopoietic recovery after cancer chemotherapy.
MYELOSUPPRESSION IS a major limiting factor in anticancer chemotherapy. Repeated or high-dose cycles of chemotherapy or radiotherapy may be responsible for severe stem cell depletion leading to important long-term hematopoietic sequelae and marrow exhaustion.1 Growth factors may reduce the short-term side-effects2 but uncertainties remain about long-term hematopoietic damage.3,4 Promising results were obtained in the prevention of short-term myelotoxicity by the use of negative hematopoietic regulatory factors. It has been proposed that the use of exogenous inhibitors, which may also prevent quiescent primitive hematopoietic cells from entering S-phase after chemotherapy or radiotherapy, could protect this cell population from subsequent doses of cytotoxic agents.5
In recent years, a number of molecules have been reported to exert a suppressive effect on hematopoietic stem cell proliferation. These include transforming growth factor-β (TGF-β), macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor α (TNF-α), the pentapeptide pyro-Glu-Glu-Asp-Cys-Lys (pEEDCK), and the tetrapeptide Acetyl-N-Ser-Asp-Lys-Pro (AcSDKP or Goralatide). All of these factors are potential myeloprotectors because they were shown to inhibit the proliferation of hematopoietic cells in vivo or in vitro.6
AcSDKP was isolated and purified from fetal calf bone marrow and subsequently chemically synthesized.7,8 It is constitutively produced in vivo and biosynthesized in vitro by bone marrow cells in murine long-term cultures.9 It has also been suggested that macrophages produce AcSDKP, whereas bone marrow stromal cells degrade it.10 It was reported that AcSDKP may be derived from thymosin β4, which contains the complete N-terminal AcSDKP amino acid sequence.11 Catabolism studies have shown that AcSDKP is a physiological substrate of the N-terminal catalytic site of angiotensin-I–converting enzyme (ACE), which cleaves the tetrapeptide by a dipeptidyl carboxypeptidase.12-14 It has recently been suggested that ACE has a role in the regulation of hematopoiesis.15
Several in vitro inhibitory effects of AcSDKP have been described. The reversible inhibition of the proliferation of murine and human early progenitors, colony-forming units–granulocyte-macrophage (CFU-GM), and burst forming units-erythroid (BFU-E) was observed in the presence of the tetrapeptide.16-19 AcSDKP has also been shown to reduce in vitro the proliferation of more primitive hematopoietic cells such as the murine and human high proliferative potential colony-forming cells (HPP-CFCs) as well as human long-term culture initiating cells (LTC-ICs).16,20,21 Moreover, it inhibits the proliferative response of purified human CD34+ cells to a combination of seven growth factors.21 This inhibitory effect was dose-dependent being maximal at 10−12 mol/L for murine and at 10−9 mol/L for human hematopoietic cells.16,18 The ability of AcSDKP to maintain the primitive hematopoietic cells in quiescence is probably responsible for the in vitro protection of human and murine progenitors from the toxicities of 3′azido-3′deoxythymidine,22mafosfamide,23 phototherapy,24 and hyperthermy.17
In vivo, the administration of AcSDKP prevents the recruitment of colony-forming units-spleen (CFU-S) into S-phase in mice submitted to cytosine-arabinoside treatment.7,25 It was reported that this activity was specific for cells in G0 or in early G1.26 The protection of stem cell and progenitor compartments was observed when AcSDKP administration was combined with cytosine arabinoside,25cyclophosphamide,25 5-fluorouracil,27 and irradiation.28
All these biological properties of AcSDKP suggest possible therapeutic applications for this molecule, in vivo, as an efficient hemoprotective agent during repeated and intensive chemotherapeutic and radiotherapeutic treatments and, in vitro, as an adjuvant to purging methods.
The present study had two objectives. The first was to investigate whether the administration of AcSDKP in vivo could protect hematopoietic stem cells in mice given lethal doses of doxorubicin (DOX), a major chemotherapeutic drug. The ability of AcSDKP to improve the survival of DOX-treated mice and to enhance the recovery of the differentiated progenitors as well as of the primitive hematopoietic stem cells (long-term repopulating cells [LTRCs]) was examined. In addition, dose, mode, and timing of AcSDKP administration relative to the administration of DOX were investigated to develop a protective regimen with improved capability. The second objective was to determine whether the coadministration of AcSDKP with a growth factor, granulocyte colony-stimulating factor (G-CSF), used routinely to reduce short-term hematological effects of chemotherapy, could improve myeloprotection.
Our data showed a protective effect of AcSDKP against DOX-induced deaths and hematotoxicity, particularly on the most primitive stem cells, the LTRCs. The effects on CFU-GM were optimized by using this inhibitor of stem cell proliferation in combination with G-CSF, suggesting a new approach to improve marrow protection during chemotherapy.
MATERIALS AND METHODS
Animals.
Eight- to 12-week old BALB/c mice (Janvier CERJ, Le Genest-St-lsle, France), housed under specific pathogen-free conditions, were used in accordance with French legislation.
Drugs.
DOX was purchased from Pharmacia (Saint Quentin en Yvelines, France). Synthetic AcSDKP was kindly provided by Ipsen-Biotech (Paris, France). Recombinant G-CSF was purchased from Rhone Poulenc Rorer (Neuilly sur Seine, France).
In vivo treatments.
As shown in Fig 1, AcSDKP (in saline) was administered subcutaneously (SC), 24 hours or 48 hours before the DOX treatment, either by injection or in a continuous infusion regimen. A similar dose of AcSDKP (7.2 μg/mouse = 360 μg/kg) was given according to several schedules: one SC injection 48 hours before DOX treatment, three injections (48 hours, 24 hours, and 1 hour before DOX), six injections (twice a day at 9:00 am and 7:00pm starting 48 hours before DOX) or nine injections (at 8:00am, 4:00 pm, and 12:00 pm each day, starting 48 hours before DOX). The six-injections modality was used to assess a potential dose-response effect (total doses 0.072, 0.72, 7.2, 72, 720 μg/mouse). In the case of a continuous infusion, the total dose of AcSDKP (7.2 μg/mouse) was delivered at a constant delivery rate of 100 ng/h for 3 days using minipumps (Alzet osmotic minipump type 1003 D 3 days, Charles Rivers, France) implanted along the dorsal lateral flank. The pumps were implanted either 24 hours or 48 hours before the beginning of DOX treatment and were removed 3 days later. Control animals received saline either SC or through minipumps.
Treatment schedules. (A) Assessment of the survival. AcSDKP was administred SC, either as a continuous infusion delivered by minipumps (dark ellipses) or as six injections (dark rectangles) beginning either 24 or 48 hours before the first DOX injection. Unless stated otherwise, the total dose of AcSDKP was 7.2 μg/mouse (approximately 360 μg/kg). DOX was given IP at a dose of 2.65 mg/kg/injection twice on the first day and once on the second, to yield a total dose of 7.95 mg/kg. (B) Evaluation of the recovery of progenitors, HPP-CFC and CFU-S. AcSDKP was administered SC as a continuous infusion delivered by minipump beginning 48 hours before the first injection of DOX. The total AcSDKP dose was always 7.2 μg/mouse. (C) Assessment of LTRC survival. AcSDKP was delivered by minipump 48 hours before the first DOX injection. On day 7, male mouse bone marrow was grafted into irradiated female recipients. (D) Combination of AcSDKP with G-CSF. AcSDKP was administered as six injections beginning 48 hours before DOX treatment. G-CSF was injected IP once a day at either 100, 300, or 500 ng/injection/mouse (approximately 5, 15, or 25 μg/kg) for 4 days, beginning on day 3.
Treatment schedules. (A) Assessment of the survival. AcSDKP was administred SC, either as a continuous infusion delivered by minipumps (dark ellipses) or as six injections (dark rectangles) beginning either 24 or 48 hours before the first DOX injection. Unless stated otherwise, the total dose of AcSDKP was 7.2 μg/mouse (approximately 360 μg/kg). DOX was given IP at a dose of 2.65 mg/kg/injection twice on the first day and once on the second, to yield a total dose of 7.95 mg/kg. (B) Evaluation of the recovery of progenitors, HPP-CFC and CFU-S. AcSDKP was administered SC as a continuous infusion delivered by minipump beginning 48 hours before the first injection of DOX. The total AcSDKP dose was always 7.2 μg/mouse. (C) Assessment of LTRC survival. AcSDKP was delivered by minipump 48 hours before the first DOX injection. On day 7, male mouse bone marrow was grafted into irradiated female recipients. (D) Combination of AcSDKP with G-CSF. AcSDKP was administered as six injections beginning 48 hours before DOX treatment. G-CSF was injected IP once a day at either 100, 300, or 500 ng/injection/mouse (approximately 5, 15, or 25 μg/kg) for 4 days, beginning on day 3.
DOX was injected intraperitoneally (IP) according to a three-injection protocol: twice a day on day 1 (at 10:00 am and 5:00pm) and once on day 2 (at 10:00 am) at doses of 2.65 mg/kg/injection (total dose of 7.95 mg/kg).
G-CSF was injected IP (100, 300, or 500 ng/injection = 5, 15, or 25 μg/kg) once a day from day 3 to day 6 after the beginning of DOX treatment.
Survival experiments.
AcSDKP or saline was administered to mice (30 animals/group) either by an SC continuous perfusion or by SC injection. DOX was injected IP according to the modalities described previously. In all experiments, mortality was recorded daily.
Hematologic toxicity experiments.
The kinetics of recovery of three different cellular hematopoietic compartments were followed after lethal DOX treatment using two different protocols. In one protocol, AcSDKP was administered alone, either by injection or as a continuous pump infusion before DOX. Four or five mice from each group were killed on days 3, 4, 7, 11, 14, and 18. Bone marrow cells (BMCs) from tibias and femurs were collected and the kinetics of marrow hematopoietic progenitor recovery were evaluated. In another protocol, AcSDKP administration (6 SC injections starting 48 hours before DOX) was followed by a subsequent administration of G-CSF daily from day 3 to day 6 after DOX. BMCs from tibias and femurs were collected on day 7.
CFU-GM assay.
CFU-GM were assayed as described by Worton.29 BMCs (5 × 104) of treated mice in 1 mL of α minimum essential medium (αMEM) containing 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine (GIBCO, Cergy-Pontoise, France), 20% fetal calf serum (Eurobio, Les Ulis, France), 0.5 units of murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Valbiotec, Paris, France), and 0.8% methyl-cellulose (Tebu, Le Perray-en-Yvelines, France) were plated in 35-mm culture dishes. Quadruplicate cultures were incubated for 7 days at 37°C in a humidified 5% CO2 atmosphere. CFU-GM colonies consisting of 50 or more cells were scored using an inverted microscope.
Colony-forming unit-spleen (CFU-S) assay.
CFU-S were studied using the spleen colony assay.30 BMCs of treated animals were injected intravenously (IV) into eight irradiated recipient mice (9 Gy from a 60Co source) at appropriate concentrations to obtain about 12 macroscopic surface colonies per spleen. Recipients were killed 12 days later and spleens removed and fixed in Bouin's solution. Macroscopic spleen nodules were scored 24 hours after fixation.
HPP-CFC assay.
HPP-CFCs were monitored using a bilayer semisolid agar assay.20 31 Two milliliters of complete medium (Dulbecco's medium containing 20% horse serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin) supplemented with 10% conditioned medium from the WEHI 3B myelomonocytic leukemic cell line, 10% conditioned medium from the L929 fibroblast cell line, and 0.5% melted agar (Bactoagar; Difco, Detroit, Michigan) were aliquoted into 55-mm diameter non-tissue–culture grade plastic petri dishes as the underlayer. Two milliliters of complete medium supplemented with 0.3% melted agar and containing 3 × 104 BMC/mL were then aliquoted over the prepared underlayers. Quadruplicate cultures were incubated for 14 days at 37°C in a fully humidified atmosphere with 5% CO2. Twelve hours before the end of the culture, 1 mL of a colorless 1 mg/mL 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT; Sigma, Saint Quentin Fallavier, France) solution in saline was added, allowing the staining of viable cells by INT processing into a red derivative that precipitates inside cells. HPP-CFC macroscopic colonies defined as those in excess of 2 mm were scored.
Long-term repopulating ability of BMCs.
In an attempt to investigate whether AcSDKP treatment was able to protect the LTRCs, the repopulating ability of BMCs from AcSDKP-DOX treated mice (DOX-AcSDKP-BMCs) was assessed32 and compared with that of BMCs from DOX-treated mice (DOX-BMCs). DOX-AcSDKP-BMCs and DOX-BMCs were obtained from seven donor male mice on day 7 after the beginning of DOX administration and injected IV at concentrations of 104, 4 × 104, 6 × 104, 105, or 106 into lethally irradiated female recipient mice (n > 10). The irradiation dose was 9.5 Gy from a60Co source. The mortality of recipient mice was followed for up to 5 months.
Y-chromosome polymerase chain reaction (PCR) analysis.
To determine whether the hematological reconstitution of recipient mice was endogenous or exogenous,33 genomic DNA from peripheral blood cells (PBCs) of mice surviving at 5 months posttransplantation was analyzed by PCR, amplifying a fragment of the Y chromosome. Heparinized blood (50 μL) was centrifuged three times for 15 seconds in 500 μL of Tris buffer (10 mmol/L, pH 8) containing 1 mmol/L EDTA to lyse the red blood cells. Leucocyte membranes were broken by the addition of 100 μL of Tris-HCl buffer (50 mmol/L) containing 1 mol/L MgCl2, 50 mmol/L KCl, 0.5% Tween 20 and 10 mg/mL of protease K at 56°C for 45 minutes and then at 95°C for 10 minutes. Ten microliters of the mixture was used in 50 μL of PCR mix, including 140 ng of each: forward 5′-TGGGACTGGTGACAATTGTC-3′ and reverse 5′-GAGTCAGGTGTGCAGCTCTA-3′, Y-chromosome–specific primers, 0.5 U of Thermophylus aquaticus DNA polymerase (ATGC) and 5 μL of 10x PCR buffer (Perkin-Ellmer Cetus; St Quentin en Yvelines, France). Samples were amplified for 35 cycles. Each cycle included denaturation at 94°C for 1 minute, annealing at 55°C for 2 minutes and extension at 72°C for 3 minutes. Actin cDNA fragments were amplified as a positive control; the forward and reverse actin primers were 5′-GTACCACAGGCATTGTGATG-3′ and 5′-GCAACATAGCACAGCTTCTC-3′, respectively. Amplified cDNA (10 μL) was run on agarose gel and poststained with ethidium bromide. Gel photographs were scanned on a ScanMaker E6 (Microtek; International Computer, Paris, France) and analyzed using the NIH Image 1.54 software.
Statistical analysis.
Results from survival experiments were analyzed using Fisher's exact test on day 30 or 45. Student's t-test, Wilcoxon's, or Kruskal Wallis rank test were used to compare the results of the clonogenic assays.
RESULTS
The effect of AcSDKP on the survival of mice given lethal doses of DOX: Importance of mode, timing, and schedule of AcSDKP administration.
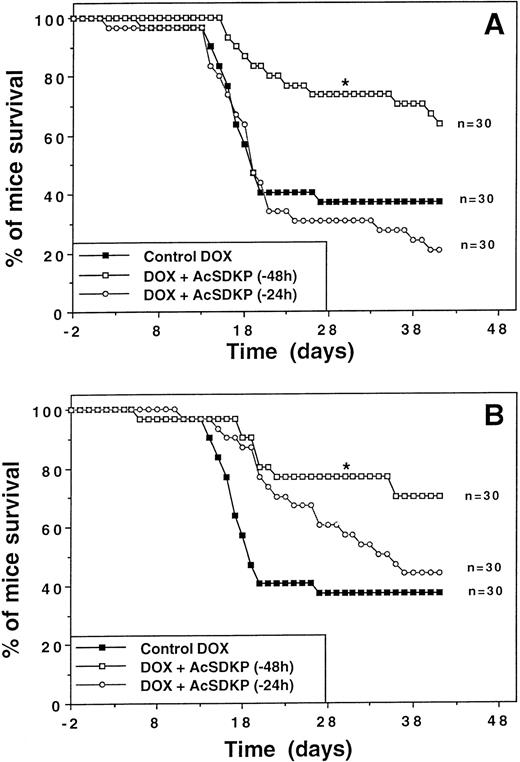
The effect of AcSDKP on DOX-induced mortality in mice was first evaluated when AcSDKP was administered using a 3-day continuous infusion starting either 24 or 48 hours before the first injection of DOX. As shown in Fig 2A, DOX administered alone at a dose of 2.65 mg/kg/injection induced a 65% lethality in mice (LD65) with a median survival time (MST) of around 18 days. A continuous AcSDKP infusion (7.2 μg/mouse) starting 48 hours before the beginning of DOX administration significantly reduced the percentage of mortality in DOX-treated animals on day 30 (27%v 64%, P < .05). In fact, the MST increased from around 18 days for DOX-treated animals to more than 42 days for the AcSDKP-DOX–treated group. In contrast, when the AcSDKP infusion started only 24 hours before DOX administration, mouse survival did not significantly differ from that observed in the control DOX-treated group. The optimal survival benefit was observed using the protocol starting with an AcSDKP infusion 48 hours before DOX.
Improvement of survival with AcSDKP in DOX-treated mice. Representative experiments showing the survival rates of mice (n = 30) treated with DOX either alone (closed squares) or in conjunction with AcSDKP given either 24 (open circles) or 48 hours (open squares) before the first DOX injection. (A) Continuous administration of AcSDKP (7.2 μg) by minipumps at a rate of 100 ng/h for 72 hours. (B) Discontinuous administration of AcSDKP in six injections (1.2 μg/injection) given twice daily beginning at the time indicated. The asterisk denotes a significant difference in day-30 survival compared with the control group treated with DOX alone (P < .05; Fisher's exact test).
Improvement of survival with AcSDKP in DOX-treated mice. Representative experiments showing the survival rates of mice (n = 30) treated with DOX either alone (closed squares) or in conjunction with AcSDKP given either 24 (open circles) or 48 hours (open squares) before the first DOX injection. (A) Continuous administration of AcSDKP (7.2 μg) by minipumps at a rate of 100 ng/h for 72 hours. (B) Discontinuous administration of AcSDKP in six injections (1.2 μg/injection) given twice daily beginning at the time indicated. The asterisk denotes a significant difference in day-30 survival compared with the control group treated with DOX alone (P < .05; Fisher's exact test).
A protocol using SC injections of AcSDKP was developed to investigate whether a more simple treatment protocol would also result in protection. AcSDKP was given 48 hours before DOX. When AcSDKP was administered by SC injection, the results were similar to those obtained with AcSDKP given by minipump (Fig 2B).
With the aim of optimizing the protocol of AcSDKP administration, comparative studies were performed using the same total dose of AcSDKP given in one or in multiple injections starting 48 hours before the beginning of DOX treatment. Results (not shown) indicated that 7.2 μg/mouse of AcSDKP given in six or nine injections significantly increased the survival of the mice both on day 30 (4% to 6% v28% deaths; P < .05) and on day 45 (22% to 25%v 67%; P < .05). The six- and nine-injection AcSDKP treatment almost doubled the MST from 35 days to up to 60 days for the AcSDKP-DOX–treated group. Survival improvement was not observed on day 30 when the same dose of AcSDKP was administered in only 1 or 3 injections.
The effect of AcSDKP on the recovery of CFU-GM, HPP-CFC, and CFU-S of mice given lethal doses of DOX: Importance of mode and dose of AcSDKP administration.
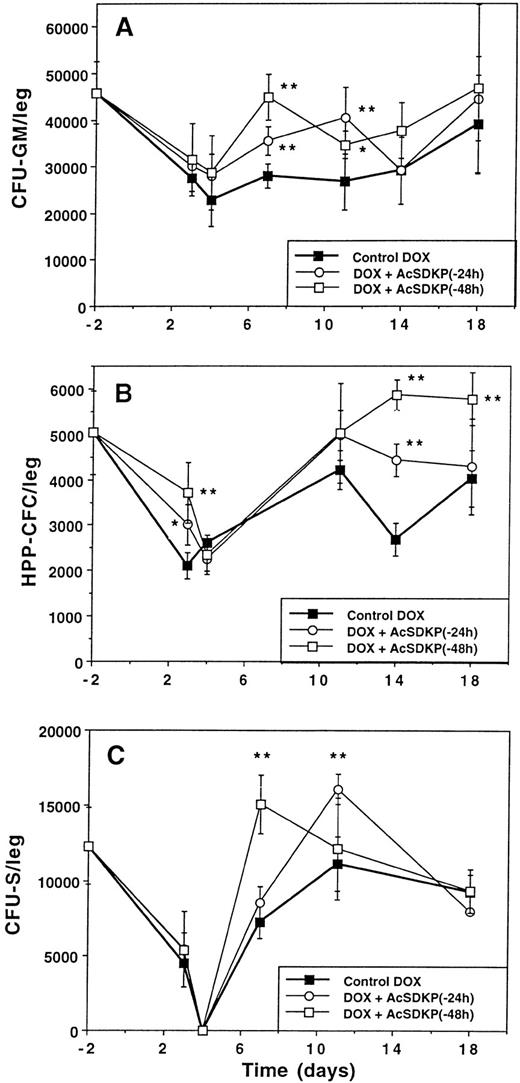
The kinetics of recovery were studied in parallel for three different cell populations (CFU-GM, HPP-CFC, and CFU-S) in mice given lethal doses of DOX alone or preceded by AcSDKP. As shown in Fig 3, DOX-alone treatment led to a nadir in all the cellular systems studied, which occurred around days 3 to 4, depending on the cell type. The number of cells returned progressively to normal values by day 18.
Improvement of the recovery of hematopoietic cells with AcSDKP in DOX-treated mice. AcSDKP was administered continuously by minipumps at a rate of 100 ng/h for 72 hours beginning 48 hours before the first DOX injection. In each experiment, mice (n = 5) were killed at different times, bone marrow was collected and pooled, and colony assays were performed: (A) CFU-GM, (B) HPP-CFC, (C) CFU-S. Results are expressed as the mean ± standard deviation of three (B and C) or four (A) experiments. Asteriks indicate significant differences between experimental and control groups: *P < .05; **P < .01 (Student's t-test).
Improvement of the recovery of hematopoietic cells with AcSDKP in DOX-treated mice. AcSDKP was administered continuously by minipumps at a rate of 100 ng/h for 72 hours beginning 48 hours before the first DOX injection. In each experiment, mice (n = 5) were killed at different times, bone marrow was collected and pooled, and colony assays were performed: (A) CFU-GM, (B) HPP-CFC, (C) CFU-S. Results are expressed as the mean ± standard deviation of three (B and C) or four (A) experiments. Asteriks indicate significant differences between experimental and control groups: *P < .05; **P < .01 (Student's t-test).
In mice given DOX plus AcSDKP, the recovery of CFU-GM (Fig 3A) was improved on day 7 and on day 11 (P < .01) as compared with that obtained with DOX alone. Recovery was more pronounced and faster when the tetrapeptide was given 48 hours rather than 24 hours before DOX. The same improvement in CFU-GM number was observed on day 7 when AcSDKP was given in six SC injections (results not shown). The highest doses of AcSDKP (7.2, 72, and 720 μg/mouse) were shown to significantly enhance CFU-GM recovery at day 7, whereas 0.072 and 0.72 μg/mouse were ineffective (results not shown).
A significant improvement of HPP-CFC recovery (Fig 3B) was observed on both days 14 and 18 or only on day 14 when AcSDKP was given respectively 48 or 24 hours before DOX. It should be pointed out that the number of HPP-CFCs was significantly increased on day 3 (day of the nadir) when AcSDKP was given 48 or 24 hours before DOX. Such a trend could also be observed for CFU-GM. Overall, the rate of recovery was faster when AcSDKP was given 48 hours before DOX.
The continuous 3-day perfusion of AcSDKP led to a faster recovery of the CFU-S compared with that observed with DOX alone (Fig 3C). A significant protective effect of AcSDKP against DOX toxicity on CFU-S was evidenced on day 7 and on day 11 (P < .01) when AcSDKP administration was started respectively 48 and 24 hours before DOX.
Protection of the primitive stem cells LTRC by AcSDKP.
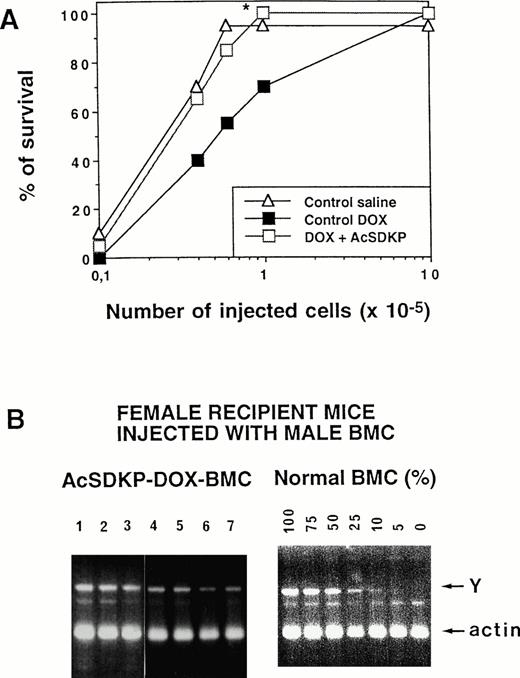
The in vivo ability of AcSDKP to protect LTRC in DOX-treated mice was evaluated when the tetrapeptide was given in a continuous regimen starting 48 hours before DOX. As shown in Fig 4A, the survival of lethally irradiated mice (5 months postgrafting), injected with DOX-AcSDKP-BMCs was higher than that of mice injected with similar numbers of DOX-BMCs. In fact, 6 × 104 control BMCs must be grafted to achieve a 100% survival of recipient mice; a 16-fold number of DOX-BMCs (106 cells) was necessary to induce a similar survival of recipients, whereas only 105 AcSDKP-DOX-BMCs were required. To check whether the hematopoietic reconstitution of mice grafted with DOX-AcSDKP-BMCs was of donor origin, the Y-chromosome fragment was amplified on DNA extracted from PBCs of surviving recipient mice, because sex-mismatched grafting (male donor/female recipient) was performed. As shown in Fig 4B, the presence of a 400-bp Y-chromosome–specific amplicon in seven of seven recipients grafted with DOX-AcSDKP-BMCs was observed. Comparison of the intensity of these bands to that of controls consisting of DNA extracted from mixtures of male and female BMCs (from 0% to 100% male cells) established that, on average, more than 75% of the PBCs were of donor origin.
LTRC protection with AcSDKP in DOX-treated mice. (A) Male donor mice given saline, DOX alone, or in association with AcSDKP administred as a continuous infusion beginning 48 hours before the first DOX injection were killed on day 7. Various numbers of their BMCs were then injected into lethally irradiated female recipients. (A) Recipient mice survival at 5 months postgrafting, as a function of the dose of cells injected, *P < .02 (Student'st-test) between AcSDKP-DOX– and DOX-alone–treated mice. (B) Left panel: Y chromosome PCR analysis of peripheral leukocyte DNA from 7 female mice grafted with bone marrow from male donors given both AcSDKP and DOX. Right panel: Controls consisted of Y chromosome PCR analysis of DNA from a mixture of male and female BMCs (from 0% to 100% male cells) and were used for quantification of the data. Mixed chimerism (an average of 75% donor cells) was seen in all long-term survivors tested.
LTRC protection with AcSDKP in DOX-treated mice. (A) Male donor mice given saline, DOX alone, or in association with AcSDKP administred as a continuous infusion beginning 48 hours before the first DOX injection were killed on day 7. Various numbers of their BMCs were then injected into lethally irradiated female recipients. (A) Recipient mice survival at 5 months postgrafting, as a function of the dose of cells injected, *P < .02 (Student'st-test) between AcSDKP-DOX– and DOX-alone–treated mice. (B) Left panel: Y chromosome PCR analysis of peripheral leukocyte DNA from 7 female mice grafted with bone marrow from male donors given both AcSDKP and DOX. Right panel: Controls consisted of Y chromosome PCR analysis of DNA from a mixture of male and female BMCs (from 0% to 100% male cells) and were used for quantification of the data. Mixed chimerism (an average of 75% donor cells) was seen in all long-term survivors tested.
Enhancement of the AcSDKP response on CFU-GM recovery by G-CSF.
The impact of a combined administration of G-CSF after DOX in the sequence of the AcSDKP-DOX protocol was next investigated. The recovery of CFU-GM in mice given AcSDKP in six SC injections initiated 48 hours before DOX administration was compared with that evaluated in mice receiving the combined AcSDKP-DOX administration and the subsequent four IP injections of G-CSF on days 3, 4, 5, and 6 after DOX treatment. The results presented in Table 1 show that AcSDKP or G-CSF given independently, in association with DOX, allowed the recovery of a higher number of CFU-GM. When compared with AcSDKP-DOX or G-CSF-DOX, the combination of AcSDKP with G-CSF resulted in an enhanced recovery of CFU-GM. This significant effect was observed at all G-CSF doses studied.
Mutual Enhancement of AcSDKP and G-CSF Activity on the Recovery of CFU-GM in DOX-Treated Mice
| . | Control . | DOX . | DOX AcSDKP . | DOX G-SCF 100 . | DOX AcSDKP G-CSF 100 . | DOX G-CSF 300 . | DOX AcSDKP G-CSF 300 . | DOX G-CSF 500 . | DOX AcSDKP G-CSF 500 . |
|---|---|---|---|---|---|---|---|---|---|
| Mean no. of CFU-GM/leg | 21,614 | 17,140 | 23,951 | 22,966 | 27,250 | 22,934 | 34,400 | 23,267 | 33,363 |
| Standard deviation | 3,823 | 2,140 | 4,751 | 6,287 | 7,349 | 5,972 | 10,744 | 4,627 | 6,970 |
| DOX toxicity | .0001 | ||||||||
| Effect of G-CSF alone | .01 | .01 | .01 | ||||||
| Effect of AcSDKP alone | .0001 | ||||||||
| Potentialization of AcSDKP by G-CSF | NS | .0001 | .0001 | ||||||
| Potentialization of G-CSF by AcSDKP | NS | .002 | .002 | ||||||
| Effect of increasing doses of G-CSF | NS |
| . | Control . | DOX . | DOX AcSDKP . | DOX G-SCF 100 . | DOX AcSDKP G-CSF 100 . | DOX G-CSF 300 . | DOX AcSDKP G-CSF 300 . | DOX G-CSF 500 . | DOX AcSDKP G-CSF 500 . |
|---|---|---|---|---|---|---|---|---|---|
| Mean no. of CFU-GM/leg | 21,614 | 17,140 | 23,951 | 22,966 | 27,250 | 22,934 | 34,400 | 23,267 | 33,363 |
| Standard deviation | 3,823 | 2,140 | 4,751 | 6,287 | 7,349 | 5,972 | 10,744 | 4,627 | 6,970 |
| DOX toxicity | .0001 | ||||||||
| Effect of G-CSF alone | .01 | .01 | .01 | ||||||
| Effect of AcSDKP alone | .0001 | ||||||||
| Potentialization of AcSDKP by G-CSF | NS | .0001 | .0001 | ||||||
| Potentialization of G-CSF by AcSDKP | NS | .002 | .002 | ||||||
| Effect of increasing doses of G-CSF | NS |
AcSDKP (SC injections, 7.2 μg/mouse) was administered 48 hours before DOX. G-CSF was injected IP (100, 300, and 500 ng/injection/mouse, respectively, 4, 5, and 5 experiments, altogether 6 experiments) once a day from day 3 to day 6 after DOX. CFU-GM were monitored at day 7. Student's t-test was performed for statistical analysis.
Abbreviation: NS, not significant.
DISCUSSION
Previous studies have shown the myeloprotective effect of the tetrapeptide AcSDKP against two cytotoxic drugs, cytosine arabinoside and cyclophosphamide.25 Moreover, clinical trials with this peptide (Goralatide) in patients undergoing monochemotherapy with similar drugs led to an improvement of the neutrophil recovery.34 These results prompted the initiation of the present studies to assess the potential of AcSDKP as a protector against marrow damage induced by doxorubicin, a widely used anticancer agent. In addition, the effect of the combined administration of AcSDKP and G-CSF on the CFU-GM recovery of DOX-treated mice was evaluated.
Preliminary experiments with DOX (results not shown) showed a dose-response curve for mouse survival. Based on these results, the total DOX dose of 2.65 mg/kg has been chosen to provide a mortality greater than 60% on day 30. AcSDKP administration before DOX appeared to protect mice from lethal doses of DOX and improved significantly their survival. Such an improvement in mice survival, because of the coadministration of AcSDKP and high doses of cytosine arabinoside or cyclophosphamide has already been observed.25 TGF-β, another negative regulator of hematopoiesis was also shown to be effective in vivo in protecting mice from acutely toxic doses of DOX.35 Interestingly, the mortality was not reversed by a subsequent bone marrow graft, suggesting that TGF-β protection was at least partially mediated by nonhematopoietic mechanisms.
The significant mortality induced by DOX administration in our experiments was shown to be associated with a marked hematological toxicity. The myelosuppressive effect of DOX was evaluated by ex vivo measurement of the level and duration of the nadir of primitive bone marrow cells. The recovery of pluripotent stem cells as well as of progenitors was faster and improved in AcSDKP-pretreated mice. The variable effects on the different progenitors and stem cells may be related to their specific cell cycle length. Because of the burden of our experiments, no attempt has been made to assess the megakaryocytic lineage, which has recently been reported to also be protected.27,28 Our present observations are in agreement with the protection of neutrophil, lymphocytes, CFU-GM, and colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) induced by AcSDKP in DOX-treated primates.36
The remarkable positive effect of AcSDKP observed on the protection of LTRC is worth emphasizing because, in the long- term, effects on these cells are of greater significance than protection of any other stem cells and progenitors. In fact, a bone marrow graft containing only 105 cells obtained from AcSDKP-DOX–treated donors allowed the survival of 100% of lethally irradiated recipients whereas 106 BMCs from DOX-alone–treated mice were required to achieve the same effect. At 5 months posttransplantation, the recipients had a hematopoietic reconstitution with cells from donor origin. Therefore, the increased survival of mice grafted with BMCs from AcSDKP-DOX–treated animals is probably because of the protection of the LTRC after administration of AcSDKP. These findings confirm the fact that primitive stem cell exhaustion, which often prevents the continuation of chemotherapy, can be circumvented both in terms of number and of function. Such a prevention has previously been shown for pre–CFU-S, an undefined cell population consisting of a mixture of CFU-S and long-term repopulating cells and for LTC-ICs with another negative marrow regulator, the pentapeptide pEEDCK in AraC-treated mice.37 Conversely, the chemokine MIP-1α, a potent inhibitor of hematopoietic stem cell proliferation, which does not protect stem cells more primitive than CFU-S and MIP-1α, given twice daily for 7 days, did not prevent the 10-fold LTRC depletion induced by 5FU in mice.38 Thus, whereas the protection reported for MIP-1α,38,39 TGF-β,35TNF-α,40 and pEEDCK37 41 appears to be restricted to certain hematopoietic compartments, such a preservation of various hematopoietic cell compartments, including LTRCs, in correlation with a survival improvement, as we describe with AcSDKP, has never been reported with any other negative regulator. AcSDKP appears to be quite unique in its ability to protect bone marrow.
To examine the protection achieved by AcSDKP several experiments varying the dose, timing, and route of administration of AcSDKP were undertaken. The best results were obtained with 7.2 μg of AcSDKP given by a continuous 3-day infusion initiated 48 hours before the first DOX injection. These conditions provided the best recovery of CFU-GM, CFU-S, and HPP-CFC as well as a significant improvement of mouse survival. Conversely, adequate protection was achieved against some other toxic agents with different modalities of AcSDKP administration. In vivo protective effect of AcSDKP against the hematological toxicity of sublethal irradiation was reported only when the tetrapeptide was given 24 hours before the irradiation.28 In monkeys, a 16-hour interval between AcSDKP and drug administration appeared to be sufficient to obtain protection against DOX-induced toxicity.36 The variable effects of AcSDKP associated with the use of different toxic agents and modalities of administration may be linked with the specificity of the mechanism of action of AcSDKP. In fact, the better mouse survival and progenitor recovery observed when AcSDKP was started 48 rather than 24 hours before DOX could be related with the longer interval allowed between the end of AcSDKP administration and the moment when stem cells return into cycle. A prolonged presence of AcSDKP may interfere negatively with the initiation of marrow recovery. The delay of the onset and duration of AcSDKP activity and, consequently, the duration of the quiescence of the hematopoietic target cells depend in part on the catabolism of the tetrapeptide in defined experimental conditions. Indeed, AcSDKP, a circulating natural peptide, is continuously degraded in vivo by ACE.12,13 It has been shown that both administration of cytotoxic drugs (unpublished results) and irradiation15 are followed by an accelerated catabolism of AcSDKP and a consequent transient decrease of its plasma level. Moreover, significant changes of plasma AcSDKP levels have been reported in patients with acute myeloid leukemia undergoing chemotherapy.42 In such a context, repeated administration of AcSDKP might lead to a brief but important increase of its concentration in vivo and be more effective than the continuous administration of low doses. To verify this hypothesis and to provide the simplest protocol for AcSDKP administration, the efficacy of the same total dose was evaluated when given in one, three, six, or nine injections. AcSDKP given through six or nine SC injections was as effective on survival as the continuous infusion, whereas the same dose given in one or three injections provided less or no protective effect. CFU-GM recovery was also improved no matter what mode of AcSDKP administration was used. This observation suggests that CFU-GM protection alone is unable to account for the survival improvement of DOX-treated mice.
As the concentration of AcSDKP was shown to be critical in vitro in obtaining an effect on hematopoietic progenitor cells, a broad range of doses was investigated. Only the highest doses of AcSDKP, varying from 7.2 μg to 720 μg, were able to improve significantly the recovery of CFU-GM. A bell-shaped response has been previously observed in several in vitro models, indicating an activity for AcSDKP at doses greater than 10−6 mol/L and less than 10−16 mol/L.16,18 Such a dose-dependent effect was also suggested by the results of a phase II clinical study in which AcSDKP appeared to better protect hematopoiesis at intermediate doses (results not published). This response profile suggests that accessory cells and/or release of other mediators may be involved. Cashman et al43 reported that adherent cells were required for the expression of the inhibitory effect of AcSDKP and that the activity of AcSDKP could be blocked by the addition of MIP-1β, an antagonist of MIP-1α and of other hematopoietic negative-regulating chemokines. However, the mechanism of AcSDKP activity cannot be entirely explained through the action of MIP-1α, because AcSDKP possesses a broader range of activities as shown by its protective effect on LTRCs. In vivo investigations on the effect of AcSDKP on hematopoiesis in a canine model indicate that AcSDKP exerts its inhibitory effect not only on hematopoietic precursors but also on the function of marrow-derived stromal cells.44 This finding is consistent with the earlier reports on the modulation of the adherence of hematopoietic stem cells to a stromal cell line in the presence of AcSDKP.8,45 Moreover, it has been shown in an in vitro purging model that human stromal cells could be selectively protected by AcSDKP from the toxicity of mafosfamide.46These observations concur to suggest that AcSDKP can protect very primitive hematopoietic stem cells in their natural environment and may decrease the long-term sequelae caused by chemotherapy.
Finally, we have been interested in determining whether the biological effect achieved by AcSDKP was additive to other types of myeloprotection, namely, to the use of G-CSF as suggested using GM-CSF (Bogden et al, unpublished data). The use of positive growth factors postchemotherapy may lead to stem cell depletion either by inducing the regenerating marrow to differentiate to such an extent that it would exhaust the pool of the primitive stem cells or by driving the primitive cells into cell cycle, making them more vulnerable to the subsequent courses of chemotherapy.4 Therefore, in a set of experiments AcSDKP preceded DOX administration where G-CSF was given after DOX. In these experiments, the administration of G-CSF resulted in a significant improvement of the CFU-GM recovery compared with that obtained with AcSDKP alone. In fact, the benefit from the combined sequence AcSDKP–G-CSF may be interpreted, first, as if AcSDKP ensured protection of the stem cell and progenitor compartments from the toxicity of chemotherapy and, second, as if G-CSF would induce more efficient recovery after progenitor depletion.
In summary, our preclinical studies using an inhibitor of hematopoietic stem cell proliferation, the tetrapeptide AcSDKP, have clearly shown that in vivo pretreatment with AcSDKP protects a broad range of stem cells and progenitor cells from the toxicity of DOX lethal doses, improves the survival of treated mice, and reduces the long-term toxic effects of chemotherapy as evidenced by LTRC studies. Ultimately, the combined administration of AcSDKP and G-CSF enhances the myeloprotection achieved by the application of either AcSDKP or G-CSF alone, suggesting that the use of a negative and positive regulator may be helpful for the prevention of the acute chemotherapy toxicity on hematopoiesis. All these results show the ability of AcSDKP alone or in combination with stimulating factors to reduce both short-term and long-term myelosupression. The results of phase I to II clinical trials have already showed a reduced period of neutropenia in cancer patients receiving Goralatide and either cytosine arabinoside or ifosfamide.34 Therefore, if applied to the clinics, the use of Goralatide should improve in the short term the therapeutic index of anticancer chemotherapy, and, in the long term, it should decrease the marrow sequelae.
ACKNOWLEDGMENT
The authors thank Drs E. Frindel, A. Bogden, F. Hérodin, L.L. Pritchard, and A. Riches for their helpful suggestions. We also thank P. Ardouin and A. Rouches for their excellent technical expertise. A special acknowledgment is directed to Prof M. Tubiana and Dr M. Guigon for the stimulating discussion and continuous support in the conduct of these studies.
Supported by Ipsen-Biotech, CNRS, INSERM, Jacques and Monique Roboh, Henry and Louise Marchal, Asclepios and Suzanne Axel funds, grants 4/95 from the Association pour la Recherche sur le Cancer (ARC), and Contrat de Recherche Clinique No. 95-6 from the Institut Gustave Roussy.
Address reprint requests to P. Carde, MD, Institut Gustave Roussy, 39, rue Camille Desmoulins, 94800 Villejuif, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal