To the Editor:
Retinoic acid (RA) is involved in hematopoiesis inducing in vitro myeloid differentiation in normal and leukemic progenitor cells.1 In vivo, the complete response to RA is typical of promyelocytic leukemia with PML-RARα rearrangement.2 An erratic and incomplete response to RA as been reported in myelodysplastic syndromes3 and in secondary leukemias combining ATRA with low doses of Ara-C.4 We report here 2 cases of secondary nonpromyelocytic leukemia that are completely responsive in vitro and in vivo to RA treatment and showing abnormal subcellular RARα protein localization. The first patient, a 55-year-old woman, was seen 23 years after treatment for advanced breast cancer with cyclophosphamide and radiotherapy. She developed an undifferentiated leukemia in 1992.
The patient was treated with conventional chemotherapy and achieved complete remission (CR) after 2 courses. At relapse, 13 months later, a new attempt of reinduction with chemotherapy was made without success. Because of poor clinical status, to attempt myeloid differentiation of the patient's leukemia cells, she was started on ATRA at 45 mg/m2/d, achieving CR after 2 months of treatment. ATRA was stopped for hepatotoxicity after 12 months; however, a further relapse occurred 1 month later. The patient was restarted on ATRA plus low-dose Ara-C, obtaining a partial remission lasting several months. The patient died July 1997 unresponsive to any chemotherapy.
The second patient, a 34-year-old woman who had been treated in 1989 for Hodgkin's disease with multiple courses of chemotherapy, including MOPP/ABVD/CAD, came to our observation in January 1996 with a diagnosis of acute leukemia after a myelodysplastic syndrome. The patient was started on low-dose Ara-C. After 2 courses, no hematological response was evident and the clinical status was worsening because of fungal infection; ATRA at 45 mg/m2/d was then added. After 1 month of therapy, the clinical condition improved and a CR was obtained. The patient remained in CR for 13 months, when she progressed to an unresponsive relapse. The cytogenetics of both patients were normal at the onset of the disease. In the first patient, the cytogenetics at relapse showed the presence of a jumping translocation involving, alternatively, bands p13 and q22 of chromosome 17 and band q31 of chromosome 2.
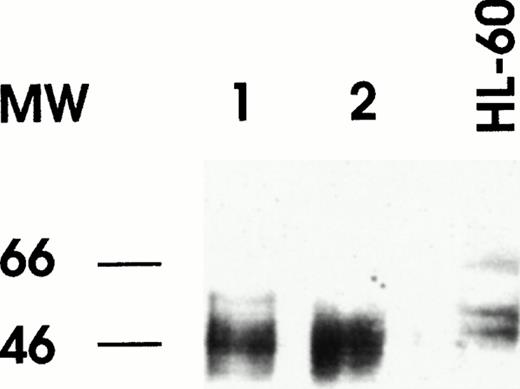
Leukemic blasts of the patients were labeled with fluorescent antibodies (Abs) specific for RARα, RXRα, and PML.5Leukemic cells of both cases displayed cytoplasmatic perinuclear reactivity with anti-RARα (Fig 1A); this reactivity was apparent as small granulations forming a ring around the nucleus of the cells. In contrast, labeling of the cells with RXRα and PML Abs displayed the typical nuclear reactivity, the former under form of diffuse nuclear staining and the latter under form of the nuclear bodies staining (Fig 1B). Several controls were performed in normal hematopoietic cells or in leukemic non-APL cell lines to verify the specificity of the staining obtained with anti-RARα antibody. Specifically, in HL-60 cells and in normal myeloid and in several (30) cases of primary AML (excluding M3), the antibody gives rise to the expected nuclear reactivity. Western blotting analysis of RARα performed on cellular lysates derived from the leukemic blasts of the 2 patients displayed a major band with an apparent molecular weight slighly lower than the 2 RARα bands observed in cell lysates derived from controls (Fig 2). This observation was confirmed on several occasions Southern blotting analysis with different restriction enzymes performed under conditions appropriate either for detection of RARα gene rearrangement observed in PML-RARα+ APL or of PML-RARα mRNA failed to detect a gross deletion of rearrangement of the RARα gene (data not shown). Furthermore, several different regions of the RAR-α mRNA have been amplified, reverse transcribed, and sequenced and no abnormalities were observed (data not shown).
RARα (A) and PML (B) immunofluorescence labeling of bone marrow cells derived from one of the two patients.
RARα (A) and PML (B) immunofluorescence labeling of bone marrow cells derived from one of the two patients.
Western blot analysis of RARα in nuclear extracts of bone marrow cells from patient no. 1 (lane 1) and patient no. 2 (lane 2). Nuclear extract was prepared from HL60 cell line as a control of normal RARα expression.
Western blot analysis of RARα in nuclear extracts of bone marrow cells from patient no. 1 (lane 1) and patient no. 2 (lane 2). Nuclear extract was prepared from HL60 cell line as a control of normal RARα expression.
The impressive therapeutic results obtained in promyelocytic leukemia encouraged the exploration of the RA therapy in patients with different forms of leukemia. In this context, some studies have been performed on relapsed and refractory or poor prognosis AMLs, in which RA was used along with either standard or low-dose Ara-C; in these studies, a variable, but significant proportion of the patients exhibited a therapeutic response to treatment.3,4 However, in these studies, the pattern of RARα expression as well as the integrity of RARα alleles were not investigated. Neither of our patients exhibited morphological, cytogenetic, or molecular abnormality, suggesting an APL or APL variant. However, investigation of the cellular localization of RARα by immunofluorescence showed an aberrant protein distribution, altering expression from the nucleus to the cytoplasm. Of interest, both APL and these 2 cases of secondary AML exhibit an abnormal pattern of nuclear protein localization (PML and RARα in APL and only RARα in secondary leukemias) that may be used for rapid diagnosis. However, an interesting difference between APL and the 2 secondary AMLs of the present study consists in the relocalization of both PML and RARα after RA treatment observed in APL,6 as compared with the permanently delocalized cytoplasmatic pattern in secondary AML after RA treatment. It remains to be determined whether the delocalization of RARα protein from the nucleus to the cytoplasm observed in these 2 patients is dependent on an alteration of 1 of the 2 RARα alleles. In this context, Western blot analysis of cell lysates showed, with a specific anti-RARα antiserum, the presence in both patients of an RARα protein exhibiting a slightly reduced apparent molecular weight. This alteration could be dependent either on a mutation in the coding sequence of 1 of the 2 RARα alleles or on a posttraslational modification. In conclusion, our study indicates that secondary AML can be responsive to differentiating therapy with ATRA; these leukemias are characterized by an anomalous RARα subcellular localization.
ACKNOWLEDGMENT
E.O.L.B. was supported by a fellowship from FIRC (Fondazione Italiana per la Ricerca sul Cancro).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal