Abstract
Plasmodium falciparum-infected erythrocytes (IRBC) roll on the adhesion molecule P-selectin in vitro under flow conditions that approximate the shear stress in capillary and postcapillary venules in which cytoadherence occurs in vivo. The pathological significance of this adhesive interaction is currently unknown. In this study, we further investigated the molecular interactions between IRBC and P-selectin by using a laminar flow system that allowed for the direct visualization of IRBC-substratum interactions. The results showed that the IRBC–P-selectin interaction was Ca2+-dependent and involved the lectin domain of P-selectin and a sialic acid residue on IRBC. The sialylated P-selectin ligand was trypsin-sensitive, which suggests that it could be part of the parasite antigen PfEMP1 that interacts with CD36 and intercellular adhesion molecule-1 (ICAM-1), but different from a trypsin-resistant IRBC ligand that adheres selectively to chondroitin sulfate A. Studies on the rolling and adhesion of IRBC on activated platelets that express both CD36 and P-selectin showed that inhibition of rolling on P-selectin reduced the adhesion of some clinical parasite isolates to CD36, whereas other parasite isolates appeared to interact directly with CD36. Thus, cytoadherence under physiological flow conditions may be mediated by multiple IRBC ligands that interact with different adhesion molecules in a cooperative fashion. These findings underscore the complexity of the interactions betweeen IRBC and vascular endothelium.
PLASMODIUM FALCIPARUM malaria is a major cause of death in the tropics. The pathology is due mainly to the sequestration of parasitized erythrocytes (IRBC) in the microcirculation leading to alterations in blood flow, metabolic dysfunction, and local toxicity.1 In the effort to develop therapeutic strategies that would inhibit or reverse sequestration, considerable interest has been focused on the mechanisms of the underlying cytoadherent process whereby IRBC adhere to capillary and postcapillary venular endothelium. In studies using a parallel plate flow chamber in which IRBC were infused under shear conditions over mouse L-cells transfected with individual adhesion molecules or immobilized receptor proteins, we and others demonstrated previously that IRBC rolled and adhered to CD36.2 3 The interaction with intercellular adhesion molecule-1 (ICAM-1) was mainly one of rolling. In addition, we showed that IRBC rolled on P-selectin and vascular adhesion molecule-1 (VCAM-1). Furthermore, although adhesion of IRBC to CD36 could occur without prior rolling on another adhesion molecule, rolling on ICAM-1 enhanced subsequent adhesion of some parasite isolates to CD36 on C32 melanoma cells that constitutively express both molecules. Clearly, IRBC can use different adhesion molecules in a cooperative fashion to make contact with and adhere to vascular endotheium.
The initial capture and rolling of leukocytes on endothelium, a prerequisite for adhesion, are mediated by the selectin family of adhesion molecules.4 Although IRBC also roll on P-selectin, whether this adhesion molecule serves a similar role in the adhesive interactions between IRBC and endothelium remains unknown. Therefore, the first objective of the present study was to characterize the molecular interactions of IRBC and P-selectin using clinical parasite isolates and immobilized recombinant soluble P-selectin. Second, we examined if IRBC rolling on P-selectin enhanced adhesion to CD36 expressed on platelet monolayers. We showed that IRBC–P-selectin interaction involved the lectin domain of P-selectin and a trypsin-sensitive sialylated ligand on IRBC. Furthermore, IRBC rolling on P-selectin of some clinical parasite isolates enhanced their subsequent adhesion to CD36, whereas other parasite isolates interacted with CD36 independently of P-selectin. These results underscore the complexity of the interaction between IRBC and vascular endothelium under physiological shear stress.
MATERIALS AND METHODS
Parasites.
Clinical isolates of P falciparum obtained from acutely infected patients admitted to the Hospital for Tropical Diseases (Bangkok, Thailand) were studied. The study protocol was approved by the ethics committee of the Faculty of Tropical Medicine, Mahidol University (Bangkok, Thailand). P falciparum malaria was diagnosed by the demonstration of asexual parasites in peripheral blood smears. Fifteen milliliters of blood was taken on admission before antimalarial therapy was initiated. Washed erythrocytes were cryopreserved in glycerolyte5 and stored in liquid nitrogen after the removal of the buffy coat and platelets. They were transported to Calgary on dry ice.
A fresh aliquot of cryopreserved IRBC was used for each of the following experiments. Thawed parasites were grown for 24 to 36 hours in RPMI 1640 medium (GIBCO BRL Life Technologies, Gaithersburg, MD) supplemented with 25 mmol/L HEPES (Sigma Co, St Louis, MO), 100 U/mL of penicillin and 100 g/mL streptomycin (Sigma), 2 mmol/L glutamine (Sigma), and 10% normal AB serum from a normal Thai donor. IRBC were harvested for laminar flow studies when the parasites had reached the late trophozoite/early schizont stage as judged by light microscopy. IRBC suspensions of 1% hematocrit in RPMI and 1% AB serum were prepared. To validate the use of cryopreserved parasites, experiments performed using fresh and frozen IRBC from a parasite isolate obtained in Calgary showed that they interacted equally with immobilized P-selectin protein and platelet monolayers.
Soluble P-selectin.
Recombinant soluble P-selectin was kindly provided by Dr R.P. McEver (University of Oklahoma, Oklahoma City, OK).6 One hundred microliters of a solution at 5 μg/mL Hanks' balanced salt solution (HBSS; GIBCO BRL) was immobilized on 25 × 50 mm glass coverslips (Fisher Scientific Co, Nepean, Ontario, Canada) for 2 hours at 37°C and then overnight at 4°C. The coverslips were blocked with 1% bovine serum albumin (BSA) in HBSS for 2 hours at 37°C before use. Coverslips coated with BSA alone were prepared as controls.
Platelet monolayers.
Platelets from normal donors were harvested from citrated whole blood by centrifugation. They were resuspended in HBSS at 2 × 108/mL. Platelet monolayers were prepared on 25 × 50 mm glass coverslips that had been precoated with type 1 rat tail collagen (Collaborative Biomedical Products, Bedford, MA) at 37°C for 1 hour. One milliliter of platelet suspension was added to each coverslip after the collagen had been gently pipetted off and was incubated at 37°C for 1 hour. Unattached platelets were removed by gently rinsing the monolayer three times in HBSS. Platelet monolayers were fixed in 1% buffered formalin (Sigma) at room temperature for 1 hour and stored at 4°C for a maximum of 2 weeks. IRBC interacted equally with freshly immobilized platelets and fixed platelet monolayers.
CD36 transfectant.
A stable transfectant of mouse LA9 cells (CCL 1.4, HPRT−APRT−; ATCC, Rockville, MD) permanently expressing CD36 was constructed as described.2Transfected cells were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO BRL) supplemented with 4 mmol/L glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% fetal calf serum (FCS; GIBCO BRL). Monolayers of CD36 transfectants were prepared by plating 1 mL of cells at 5 × 105/mL on 25 × 50 mm glass coverslips in 60 × 100 mm Petri dishes. The cells were allowed to adhere for 2 hours at 37°C, 5% CO2, after which 6 mL of supplemented medium was added to each dish. After 40 to 48 hours of culture, confluent monolayers were rinsed in HBSS and used immediately in laminar flow studies.
Antibodies.
The following specific monoclonal antibodies (MoAbs) were used in the studies: OKM5, an anti-CD36 MoAb known to inhibit both rolling and adhesion of IRBC on CD362 (kind gift of Ortho Diagnostics System, Raritan, NJ); G1, an anti–P-selectin MoAb that inhibits neutrophil rolling; and S12, an anti–P-selectin MoAb that binds but does not inhibit neutrophil–P-selectin interaction.7 Both anti–P-selectin MoAb were kind gifts of Dr R.P. McEver. A polyclonal anti–P-selectin antibody was raised in a NZW female rabbit by immunizing with an intramuscular injection of 50 μg/mL of purified human P-selectin in Freund's complete adjuvant, followed in 4 weeks by a second subcutaneous injection in incomplete Freund's adjuvant. The rabbit was terminally bled two weeks after the second dose. The titer of the antiserum against P-selectin was found to be greater than 1:20,000 by enzyme-linked immunosorbent assay (ELISA), but it did not react with purified CD36 at 1:100 dilution. A mouse IgG1 (Becton Dickinson Immunocytometry Systems, San Jose, CA) and normal rabbit serum (NRS) were used as controls.
In experiments in which the inhibitory effect of antibodies was studied, the P-selectin spots and platelet monolayers were preincubated with antibody at 37°C for 30 minutes. The same concentration of antibody was maintained in the perfusate throughout the experiment.
Parallel plate flow chamber system.
A laminar flow system to study the dynamic interaction between IRBC and various cellular substrata was established as previously described.8 Briefly, cells or recombinant protein on glass coverslips were assembled in a parallel plate flow chamber of 220-μm gap thickness in which a uniform wall shear stress was generated. The flow chamber was mounted on the stage of an inverted phase contrast microscope that is kept at 37°C by a temperature regulator. A suspension of IRBC in RPMI 1640, pH 7.2, and 1% AB serum was drawn through the flow chamber at varying rates with a Harvard infusion pump attached to the outlet. At this concentration of IRBC, attached IRBC and their motion were clearly observed with phase contrast objectives (200×) and quantitated by analysis of videotaped images. Experiments were conducted at 1 dyne/cm2, which we have found previously to be optimal for IRBC interactions with both P-selectin and CD36.2
Effect of Ca2+ on IRBC rolling.
Adhesive interactions of leukocytes and all selectins are Ca2+-dependent.9 To study the effect of Ca2+ on IRBC rolling on P-selectin, soluble P-selectin was immobilized in Ca2+ and Mg2+ free HBSS to which 1.8 mmol/L MgCl2 and 1 mmol/L EGTA were added (Ca2+-free HBSS). The spots were blocked with 1% BSA in the same buffer. Before IRBC infusion into the parallel plate flow chamber, the flow system was flushed with Ca2+-free HBSS for 5 minutes. IRBC were washed three times in Ca2+-free HBSS and resuspended at 1% hematocrit in the same buffer.
Effect of fucoidin.
Sulfated polysaccharides interfere with leukocyte rolling by binding to the lectin domain of selectins.10 To determine if IRBC interacts with the lectin domain of P-selectin, we examined the effect of fucoidin, a homopolymer of fucose, on IRBC rolling. Fucoidin (Sigma) at 4 to 100 μg/mL HBSS was prepared just before use. For each concentration tested, fucoidin was infused for 5 minutes over the immobilized P-selectin before an IRBC suspension containing the same concentration of fucoidin. The number of rolling IRBC in the presence or absence of fucoidin were compared. Dextran (Sigma) and chondroitin sulfate A (Sigma), two other carbohydrates with no known interactions with P-selectin, were used as controls.
Role of sialic acid residue in IRBC rolling.
To determine if IRBC rolling on P-selectin is mediated by a sialic acid residue, a suspension of IRBC at 5% hematocrit was incubated with neuraminidase from Arthrobacter ureafaciens(Calbiochem-Nevabiochem Corp, La Jolla, CA) at 1 U/mL of 0.15 mol/L NaCl, 10 mmol/L HEPES, 5 mmol/L CaCl2, pH 6.5, for 1 hour at 37°C. A similar IRBC suspension was incubated in the same volume of buffer alone as control. At the end of the incubation, IRBC were washed three times in 10 mL of RPMI and resuspended at 1% hematocrit in RPMI + 1% AB serum.
In other experiments, 2.5 mmol/L of the tetrasaccharide sialyl Lewisx was added to the IRBC suspension after baseline rolling was established. The sugar was a kind gift of Dr Robert D. Larsen (Glycomed Inc, Alameda, CA). The number of rolling cells before and after the addition of the sugar were compared.
Effect of proteases on IRBC rolling.
To determine whether the P-selectin ligand on IRBC is associated with a protein, IRBC at 5% hematocrit in RPMI were incubated with 100 μg/mL of TPCK-treated trypsin (Sigma) or TLCK-treated chymotrypsin (Sigma) at 37°C for 30 minutes. The trypsin reaction was terminated by the addition of 1 mg/mL of soybean trypsin inhibitor (Sigma). Trypsin inhibitor alone was added to control IRBC. The chymotrypsin reaction was stopped by the addition of 10% human serum. The enzyme-treated cells were washed twice with RPMI before use in flow experiments.
Rolling and adhesion of IRBC on activated platelets.
Immobilized activated platelets express both CD363 and P-selectin,11 allowing us to use this substratum as a dual adhesion molecule expression system. To confirm that the platelet monolayers used in these studies expressed functional P-selectin, neutrophils were perfused over the platelets. Neutrophil rolling was observed and was completely inhibited by the MoAb G1 (10 μg/mL) and the polyclonal anti–P-selectin antibody (1:100).
To determine the respective roles of CD36 and P-selectin in IRBC rolling and adhesion when both molecules are present on the same cell surface, IRBC were infused over platelet monolayers in the presence of the CD36-specific MoAb OKM5 at 10 μg/mL or the polyclonal anti–P-selectin at 1:100 dilution. Mouse IgG1 and NRS at the same concentrations were used as controls.
Experimental analysis and statistics.
Rolling and adhesion of IRBC on immobilized receptor protein and platelet monolayers were determined for each minute of perfusion. A rolling cell was defined as one that was in contact with the substratum but was not stationary. These cells could be seen to roll end over end across the substratum surface. The flux of rolling IRBC on soluble P-selectin was expressed as the number of cells that rolled past a fixed line on the monitor screen during a 1-minute interval. IRBC rolling velocity was calculated from the time required for a given IRBC to travel 100 μm along the chamber, expressed as the mean velocity ± SD for 50 cells counted in consecutive frames. On platelet monolayers, an IRBC was considered adherent if it remained stationary for at least 10 seconds. The total number of rolling and adherent cells were counted for a 10-minute period and the results were expressed as the number of rolling or adherent IRBC per minute per square millimeter of surface area. All values were expressed as the mean ± SEM or mean ± SD, as indicated. The significance of correlation was determined by Spearman's rank sum test. A P value of less than .05 was considered statistically significant.
RESULTS
IRBC rolling on P-selectin.
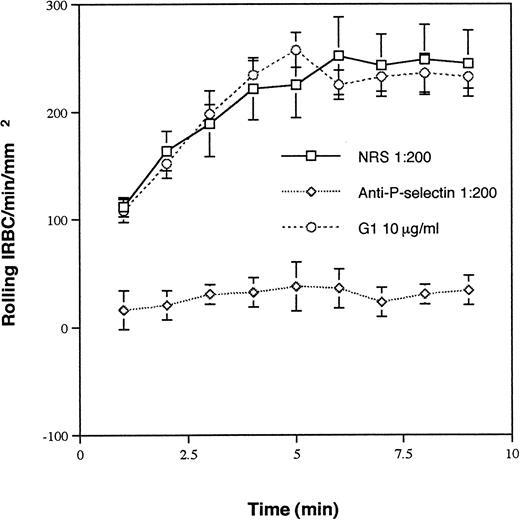
Ten randomly selected clinical P falciparum isolates were studied. The parasitemia ranged from 2.1% to 9.5%. All of the isolates tethered and rolled on immobilized soluble P-selectin. The rolling interaction was specific, because it was completely inhibited by a polyclonal antibody raised against soluble human P-selectin at 1:200 dilution, but not by the same concentration of NRS (Fig 1). However, the anti–P-selectin MoAb G1, which completely inhibits neutrophil rolling at 10 μg/mL, had no effect on IRBC rolling. A nonblocking MoAb S12 at the same concentration also had no effect (data not shown).
Rolling of IRBC on immobilized soluble P-selectin in the presence of NRS, G1, a MoAb that inhibits neutrophil rolling on P-selectin, and an anti–P-selectin polyclonal Ab. Immobilized P-selectin was preincubated with each of the Ab at the concentrations indicated at 37°C for 30 minutes. The same concentration of Ab was maintained in the perfusate. Values represent the mean number of rolling cells ± SEM of 10 clinical P falciparum isolates studied.
Rolling of IRBC on immobilized soluble P-selectin in the presence of NRS, G1, a MoAb that inhibits neutrophil rolling on P-selectin, and an anti–P-selectin polyclonal Ab. Immobilized P-selectin was preincubated with each of the Ab at the concentrations indicated at 37°C for 30 minutes. The same concentration of Ab was maintained in the perfusate. Values represent the mean number of rolling cells ± SEM of 10 clinical P falciparum isolates studied.
The mean rolling velocities of the IRBC from the 10 parasite isolates showed considerable variability (Table 1). For a given isolate, the degree of rolling, as indicated by the number of rolling IRBC after equilibrium was reached, correlated positively with parasitemia (r = .621, P < .05) and inversely with rolling velocity (r = −.830, P < .01).
Rolling Velocity of IRBC From 10 Clinical P falciparum Isolates on Immobilized Soluble P-Selectin (5 μg/mL) at 1 dyne/cm2
| Isolate . | Parasitemia . | Rolling IRBC/min/mm2 * . | Velocity (μm/sec)-151 . |
|---|---|---|---|
| 96-11 | 2.1% | 54 ± 11 | 144 ± 51 |
| 96-14 | 3.2% | 126 ± 34 | 96 ± 35 |
| 96-8 | 4.5% | 144 ± 24 | 82 ± 40 |
| 96-26 | 4.8% | 54 ± 14 | 116 ± 54 |
| 96-12 | 5.0% | 180 ± 17 | 62 ± 39 |
| 97-19 | 5.9% | 252 ± 68 | 83 ± 52 |
| 97-15 | 6.5% | 342 ± 70 | 90 ± 54 |
| 96-39 | 6.9% | 540 ± 39 | 42 ± 27 |
| 96-32 | 9.2% | 90 ± 9 | 92 ± 54 |
| 96-34 | 9.5% | 468 ± 87 | 62 ± 42 |
| Isolate . | Parasitemia . | Rolling IRBC/min/mm2 * . | Velocity (μm/sec)-151 . |
|---|---|---|---|
| 96-11 | 2.1% | 54 ± 11 | 144 ± 51 |
| 96-14 | 3.2% | 126 ± 34 | 96 ± 35 |
| 96-8 | 4.5% | 144 ± 24 | 82 ± 40 |
| 96-26 | 4.8% | 54 ± 14 | 116 ± 54 |
| 96-12 | 5.0% | 180 ± 17 | 62 ± 39 |
| 97-19 | 5.9% | 252 ± 68 | 83 ± 52 |
| 97-15 | 6.5% | 342 ± 70 | 90 ± 54 |
| 96-39 | 6.9% | 540 ± 39 | 42 ± 27 |
| 96-32 | 9.2% | 90 ± 9 | 92 ± 54 |
| 96-34 | 9.5% | 468 ± 87 | 62 ± 42 |
*Results represent the mean ± SD of two to four experiments with each isolate.
Results represent the mean ± SD for 50 cells from each of duplicate experiments for each parasite isolate.
Ca2+ dependency.
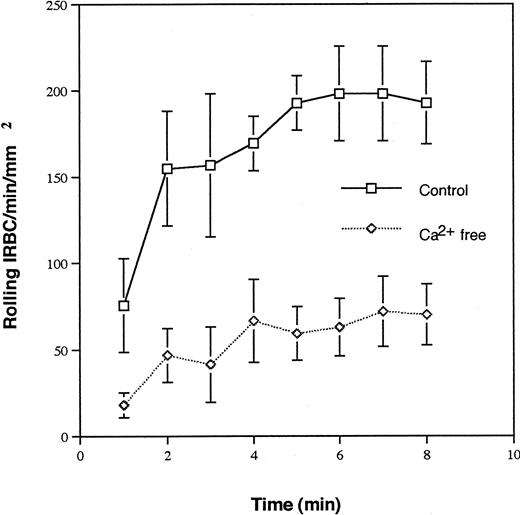
The lectin domain of selectins binds Ca2+ and undergoes conformational changes before interacting with its ligands.12 The role of Ca2+ in IRBC rolling on P-selectin was studied with 5 parasite isolates. IRBC rolling was inhibited by 70% in the absence of Ca2+ in all cases (Fig 2), consistent with the requirement for Ca2+ in the interaction of P-selectin with other ligands.
Requirement of Ca2+ for IRBC rolling on immobilized soluble P-selectin. IRBC suspended in Ca2+free HBSS were infused over soluble P-selectin immobilized in the same buffer. Results represent the mean number of rolling cells ± SEM for 5 clinical Pfalciparum isolates studied.
Requirement of Ca2+ for IRBC rolling on immobilized soluble P-selectin. IRBC suspended in Ca2+free HBSS were infused over soluble P-selectin immobilized in the same buffer. Results represent the mean number of rolling cells ± SEM for 5 clinical Pfalciparum isolates studied.
Effect of fucoidin on IRBC rolling on P-selectin.
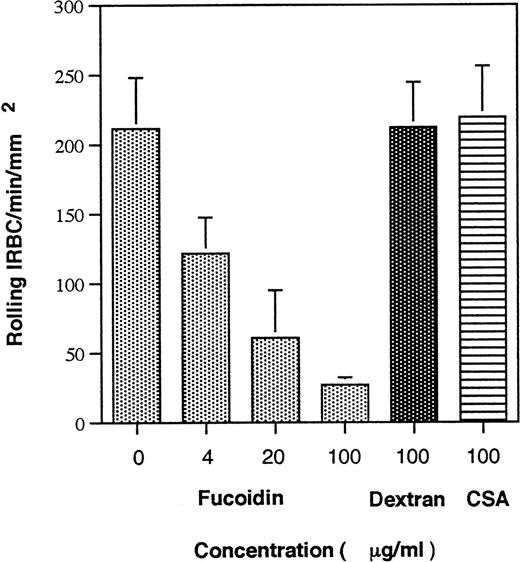
Fucoidin is a fucosylated sugar that binds to the lectin domain of P-selectin and is known to interfere with the interaction between P-selectin and its natural ligand PSGL-1 on neutrophils. We observed that fucoidin inhibited the interaction between IRBC from all 5 parasite isolates tested and immobilized soluble P-selectin in a dose-dependent fashion (Fig 3). Two other carbohydrates, namely dextran and chondroitin sulfate A, at 100 μg/mL had no effect on IRBC rolling.
Effect of fucoidin on IRBC rolling on immobilized soluble P-selectin. IRBC suspended in increasing concentrations of fucoidin, a sulfated homopolymer of fucose, in HBSS were infused over immobilized P-selectin. Dextran and chondroitin sulfate A, two other carbohydrates with no known interaction with P-selectin, were used as controls. Values represent the mean number of rolling cells ± SEM for 5 clinical P falciparum isolates studied.
Effect of fucoidin on IRBC rolling on immobilized soluble P-selectin. IRBC suspended in increasing concentrations of fucoidin, a sulfated homopolymer of fucose, in HBSS were infused over immobilized P-selectin. Dextran and chondroitin sulfate A, two other carbohydrates with no known interaction with P-selectin, were used as controls. Values represent the mean number of rolling cells ± SEM for 5 clinical P falciparum isolates studied.
Effect of proteases on IRBC rolling on P-selectin.
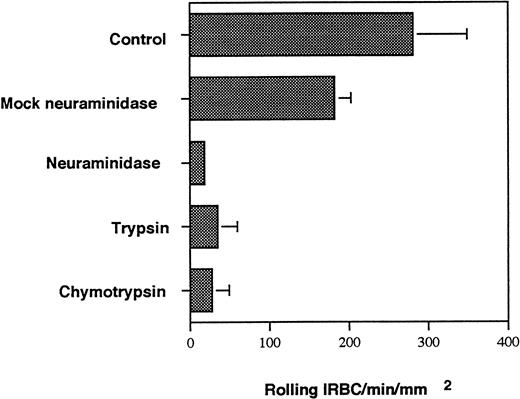
To further delineate the biochemical nature of the P-selectin ligand on IRBC, IRBC from 5 parasite isolates were treated with trypsin and chymotrypsin. Enzyme treatment for 30 minutes at 37°C markedly inhibited the interaction of all 5 parasite isolates with P-selectin (Fig 4). The enzyme treatment also inhibited both rolling and adhesion of IRBC on CD36 transfectants (data not shown).
Effect of neuraminidase and proteases on IRBC rolling on immobilized soluble P-selectin. A 5% suspension of IRBC was treated with 1 U/mL of neuraminidase from Arthrobacter ureafaciens for 1 hour at 37°C. Control IRBC were incubated in buffer alone (mock neuraminidase). IRBC were also treated with 100 μg/mL of TPCK-treated trypsin and TLCK-treated chymotrypsin at 37°C for 30 minutes. Values represent the mean number of rolling cells ± SEM for 5 clinical P falciparum isolates studied.
Effect of neuraminidase and proteases on IRBC rolling on immobilized soluble P-selectin. A 5% suspension of IRBC was treated with 1 U/mL of neuraminidase from Arthrobacter ureafaciens for 1 hour at 37°C. Control IRBC were incubated in buffer alone (mock neuraminidase). IRBC were also treated with 100 μg/mL of TPCK-treated trypsin and TLCK-treated chymotrypsin at 37°C for 30 minutes. Values represent the mean number of rolling cells ± SEM for 5 clinical P falciparum isolates studied.
Role of sialic acid residue in IRBC rolling on P-selectin.
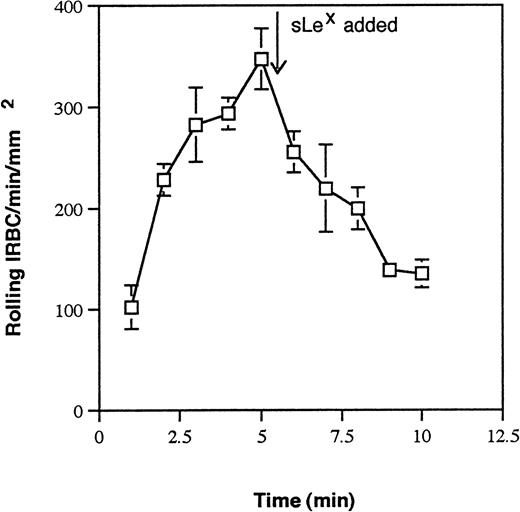
P-selectin is known to bind to carbohydrates or sialoglycoproteins. To determine if a sialylated residue on IRBC is involved in the interaction with P-selectin, IRBC were treated with neuraminidase and then infused over immobilized P-selectin. The results are shown in Fig4. There was complete inhibition of rolling on immobilized P-selectin for neuraminidase-treated IRBC from all isolates tested, suggesting the expression of a sialic acid moiety on IRBC. The involvement of a sialic acid residue was confirmed by the inhibition of IRBC rolling on P-selectin by sialyl Lewisx(Fig 5).
Effect of the tetrasaccharide sialyl Lewisx(sLex) on IRBC rolling on immobilized soluble P-selectin. IRBC were infused over immobilized P-selectin until equilibrium was reached. sLex (2.5 mmol/L) was then added to the IRBC suspension. Values represent the mean number of rolling cells ± SEM for 3 parasite isolates studied.
Effect of the tetrasaccharide sialyl Lewisx(sLex) on IRBC rolling on immobilized soluble P-selectin. IRBC were infused over immobilized P-selectin until equilibrium was reached. sLex (2.5 mmol/L) was then added to the IRBC suspension. Values represent the mean number of rolling cells ± SEM for 3 parasite isolates studied.
Rolling and adhesion of IRBC on platelets.
IRBC from all six clinical isolates tested rolled and adhered to activated platelets that expressed both CD36 and P-selectin. The two types of adhesive interactions were sequential, in that greater than 90% of adherent cells rolled for varying distances before arresting on the cell surface. Only an occasional IRBC tethered and adhered immediately at that position.
Receptor-specific antibodies were used to determine the respective roles of CD36 and P-selectin on IRBC rolling and adhesion on activated platelets. The inhibition of IRBC rolling on platelets by OKM5 was variable (57% to 77%; mean ± SD, 69% ± 7.3%). In contrast, adhesion to platelet monolayers was inhibited by greater than 95% in all cases. The same concentration of OKM5 completely inhibited all rolling and adhesive interactions between IRBC and CD36 transfectants.2
IRBC from three of six parasite isolates studied showed reduced rolling and subsequent adhesion on immobilized platelets in the presence of an anti–P-selectin antibody. The antibody had no effect on the adhesive interactions between platelets and IRBC from three other isolates. The results of duplicate experiments with each of the isolates are summarized in Table 2. The inhibitory effect of the anti–P-selectin antibody was receptor specific, because it had no effect on the rolling and adhesion of the same isolates to CD36 transfectants at 1:100 dilution (data not shown).
Effect of Anti–P-Selectin Antibody on IRBC Rolling and Adhesion to Activated Platelets
| Isolate . | Rolling IRBC/min/mm2 . | Adherent IRBC/min/mm2 . | ||||
|---|---|---|---|---|---|---|
| NRS . | Anti–P-Selectin . | % Inhibition (SD) . | NRS . | Anti–P-Selectin . | % Inhibition (SD) . | |
| Responders | ||||||
| 96-12 | 94, 128 | 75, 89 | 25 (7) | 99, 85 | 57, 59 | 40 (12) |
| 96-26 | 118, 97 | 93, 76 | 21 (1) | 81, 93 | 66, 77 | 18 (1) |
| 96-32 | 123, 91 | 90, 69 | 26 (2) | 81, 102 | 42, 72 | 39 (13) |
| Nonresponders | ||||||
| 96-14 | 73, 95 | 80, 92 | 69, 52 | 71, 58 | ||
| 96-34 | 309, 291 | 298, 295 | 239, 260 | 287, 271 | ||
| 96-39 | 264, 231 | 264, 259 | 125, 103 | 154, 137 | ||
| Isolate . | Rolling IRBC/min/mm2 . | Adherent IRBC/min/mm2 . | ||||
|---|---|---|---|---|---|---|
| NRS . | Anti–P-Selectin . | % Inhibition (SD) . | NRS . | Anti–P-Selectin . | % Inhibition (SD) . | |
| Responders | ||||||
| 96-12 | 94, 128 | 75, 89 | 25 (7) | 99, 85 | 57, 59 | 40 (12) |
| 96-26 | 118, 97 | 93, 76 | 21 (1) | 81, 93 | 66, 77 | 18 (1) |
| 96-32 | 123, 91 | 90, 69 | 26 (2) | 81, 102 | 42, 72 | 39 (13) |
| Nonresponders | ||||||
| 96-14 | 73, 95 | 80, 92 | 69, 52 | 71, 58 | ||
| 96-34 | 309, 291 | 298, 295 | 239, 260 | 287, 271 | ||
| 96-39 | 264, 231 | 264, 259 | 125, 103 | 154, 137 | ||
DISCUSSION
We have shown previously that P falciparum-infected erythrocytes roll on P-selectin under flow conditions at shear rates that approximate those encountered by IRBC in capillaries and post-capillary venules in vivo.2 In this study, we extended the observation in demonstrating that IRBC interact with the lectin domain of P-selectin, because rolling is Ca2+-dependent and inhibitable by fucoidin. Fucoidin and other sulfated glycoconjugates are known to interfere with sporozoite invasion of hepatocytes, merozoite reinvasion of erythrocytes, and rosetting of uninfected erythrocytes around IRBC.13 Our finding of IRBC rolling on P-selectin adds to the growing list of glycoconjugate-lectin interactions that are important in cell adhesion at different stages of parasite development.
The exact site of IRBC interaction with P-selectin has not been determined. It does not appear to involve the same epitope as for PSGL-1, the natural ligand for P-selectin. A similar situation has been shown for the interaction between IRBC and ICAM-1 in which the epitope recognized by IRBC in the first Ig-domain of the molecule is distinct from the binding sites of either LFA-1 or rhinovirus.14 The lack of inhibition by the MoAb S12 shows that IRBC are not interacting directly with the fifth complement regulatory protein-like short consensus repeat unit of P-selectin.15
On the surface of the infected erythrocyte, we showed that IRBC rolling on P-selectin requires a sialic acid residue by demonstrating that the process is neuraminidase sensitive and inhibitable by sialyl Lewisx to the same extent as it did neutrophil rolling on immobilized P-selectin (Kubes et al, unpublished data). Neuraminidase treatment of IRBC actually increases their adhesion to CD36 in static adhesion assays,16,17 confirming that the enzyme treatment per se does not adversely affect ligand-receptor interaction. The sensitivity of the interaction to proteases indicate that the P-selectin ligand is likely to be located on a glycoprotein and not a glycolipid associated with the cell surface. Whether P falciparum erythrocyte membrane protein 1 (PfEMP1), the parasite-derived variant cytoadherent ligand that has already been shown to interact with CD36,18 ICAM-1,18thrombospondin,18 and complement-receptor 1 (CR1),19 is involved in the IRBC–P-selectin interaction remains to be determined. Clinical parasite isolates may be made up of parasite subpopulations each expressing a different cytoadherent ligand, or individual IRBC can express multiple ligands that interact with different adhesion molecules on activated endothelium. IRBC expressing at least two ligands with different sensitivity to trypsin have been shown previously for laboratory-adapted parasite lines selected on CHO cells, one of which is specific for chondroitin sulfate A.20 Further work is necessary to establish whether the IRBC ligand for P-selectin is of parasite origin or a red blood cell antigen that has been exposed by conformational changes induced by the presence of an intracellular parasite.
In this study, activated platelets were used as a model for CD36 and P-selectin expression on a single cell. Other adhesion molecules or adhesive proteins expressed on activated platelets might have contributed to the observed IRBC-platelet interaction. In particular, the adhesive glycoprotein thrombospondin (TSP) has been shown to mediate cytoadherence of IRBC to rat endothelium under flow conditions.21 However, because ligand-receptor interactions are likely to be species-specific, it is difficult to assess if the findings in an ex vivo animal model are directly relevant to the human infection. Cooke et al3 have shown that both IRBC rolling and adhesion on purified TSP is essentially absent under flow conditions in vitro. The data in this report confirmed that IRBC adhesion to activated platelets is almost exclusively via CD36, whereas approximately 30% of rolling IRBC may be interacting with other molecules.
IRBC rolled and then adhered on activated platelets. Inasmuch as these adhesive interactions appeared to be sequential steps in IRBC cytoadherence under flow conditions, any reduction in rolling would likely adversely affect adhesion. We showed that an antibody that inhibited IRBC rolling on P-selectin partially inhibited the rolling and, as a result, adhesion of IRBC from some clinical parasite isolates on platelet monolayers. The same antibody had no effect on the rolling and adhesion of the same isolates on CD36 transfectants. This observation suggests that rolling on P-selectin may enhance subsequent adhesion to CD36 and is in keeping with our hypothesis that cytoadherence of IRBC on vascular endothelium under physiological shear stress is a cooperative process involving different parasite ligands and endothelial receptors.2 The cooperation or synergy between selectins in the recruitment of leukocytes is well documented.22 The rolling interactions with molecules such as P-selectin in this study and ICAM-12 appear to facilitate adhesion to CD36, even if they individually are of much lower avidity than that required to allow attachment. On the other hand, some parasite isolates could interact with CD36 exclusively on platelet monolayers. These findings highlight the diversity of the adhesive properties of clinical P falciparum isolates relative to the much more homogeneous adhesive mechanisms used by leukocytes. The observation is not entirely unexpected, because the expression of the various domains of PfEMP1 that interact with different adhesive molecules is highly variable among parasite isolates.17
The clinical significance of IRBC rolling on P-selectin remains to be determined. P-selectin may be important in mediating capture and fast rolling of IRBC followed by slower rolling and attachment on CD36 early in the infective process as P-selectin is readily mobilized from intracellular stores in postcapillary venular endothelium.23 As the infection progresses, additional adhesion molecules are likely to become involved as they are upregulated both directly by parasite products24 and through the proinflammatory cytokines that they induce.25The cooperative interaction of IRBC with a number of different adhesion molecules is particularly important in view of the fact that, whereas the expression of selectins and integrins may be readily modulated by cytokines and pharmacological agents,26 CD36 expression on microvascular endothelium is not affected by these mediators.27 In other words, the degree of cytoadherence ofP falciparum on vascular endothelium may be regulated at the level of the expression of adhesion molecules such as P-selectin and ICAM-1 rather than that of CD36. Therapeutic intervention with receptor-specific peptides or soluble ligands may inhibit or reverse cytoadherence in the same manner that they reduce leukocyte recruitment in experimental models of acute inflammation.28 29
ACKNOWLEDGMENT
The authors are grateful to the nurses and patients of the Hospital for Tropical Diseases (Bangkok, Thailand) for their cooperation and Dr J.G. Geng (Upjohn Laboratories, Kalamazoo, MI) for technical advice.
Supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, the Medical Research Council of Canada, the Alberta Heritage Foundation for Medical Research, Alberta, Canada, and the Wellcome-Mahidol University, Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
Address reprint requests to May Ho, MD, Department of Microbiology & Infectious Diseases, Health Sciences Centre, 3330 Hospital Dr NW, Calgary, Alberta, Canada T2N 4N1.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal