Abstract
Eosinophils participate in the inflammatory response seen in allergy and parasitic infestation, but a role in host defense against bacterial infection is not settled. The bactericidal/permeability-increasing protein (BPI) has been demonstrated in neutrophils and it exerts bacteriostatic and bactericidal effects against a wide variety of Gram-negative bacterial species. Using the Western blot technique, a 55-kD band, corresponding to BPI, was detected in lysates from both neutrophils and eosinophils. The localization of BPI in immature and mature eosinophils was investigated using immunoelectron microscopy. BPI was found in immature and mature specific granules of eosinophils and was detected in phagosomes as well, indicating release of the protein from the granules into the phagosomes. Using a specific enzyme-linked immunosorbent assay, eosinophils were shown to contain 179 ng of BPI/5 × 106 eosinophils compared with 710 ng BPI/5 × 106 neutrophils. The presence of BPI in eosinophils suggests a role for these cells in host defense against Gram-negative bacterial invasion or may suggest a role for BPI against parasitic infestation.
EOSINOPHILS ARE believed to participate in host defense against helminthic infections and play a pathophysiological role in asthma and allergy.1 They have a characteristic content of highly cationic proteins in their cytoplasmic granules1 and bactericidal effects have been shown for three of these: eosinophil cationic protein, major basic protein, and eosinophil peroxidase.2 However, the eosinophil count of peripheral blood is not typically raised in bacterial infection, but eosinophils may be recruited to sites of bacterial invasion, whereas serum concentration of characteristic eosinophil proteins are raised in patients with bacterial infection.3
The bactericidal/permeability-increasing protein (BPI) is a 55-kD highly cationic protein4 that is stored in the population of azurophil granules of neutrophils.5 BPI has a high target cell specificity for Gram-negative bacteria and binds to the lipopolysaccharides (LPS)6 present in the outer envelope of these bacteria. Through this binding, it exerts immediate bacteriostatic effects and, later, bactericidal effects.7Both isolated BPI and intact neutrophils have similar actions on target bacteria, suggesting that BPI is of major importance in the host defense functions of neutrophils against BPI-sensitive Gram-negative bacteria.8 BPI is thought to be stored in association with the granule membrane of azurophil granules, and transmembranous anchoring by the hydrophobic carboxy-terminal portion has been suggested.5 9
In the present study, we show that BPI is present in both neutrophils and eosinophils. We also show the subcellular distribution of BPI in eosinophils. Our finding suggests a role for eosinophils in host defense against Gram-negative infection.
MATERIALS AND METHODS
Serum-treated zymosan (STZ) was prepared as described before.10 Interleukin-5 (IL-5) was from Amersham (Buckinghamshire, UK). Incubation medium for the cell incubations contained 132 mmol/L NaCl, 6.0 mmol/L KCl, 1.0 mmol/L CaCl2, 1.0 mmol/L MgSO4, 1.2 mmol/L potassium phosphate, 20 mmol/L HEPES, 5.5 mmol/L glucose, and 0.5% (wt/vol) human albumin, pH 7.4.
Cell isolation.
Blood was obtained from healthy volunteers and was collected in 13 mmol/L trisodium citrate (pH 7.4) for immediate anticoagulation.
Granulocytes were obtained as described11 and then incubated with isotonic NH4Cl-solution to lyse the erythrocytes. The remaining leukocytes were washed twice in cold phosphate-buffered saline (PBS) containing 0.5% human serum albumin. The polymorphonuclear cells (PMN) had a purity of greater than 95%, consisting mainly of neutrophils, and a viability of greater than 98%. Cell viability was tested by lactate dehydrogenase release or Trypan blue exclusion.
Eosinophils were isolated as previously described,12 with slight modifications. In short, the mononuclear cells were separated from the granulocytes by centrifugation of the blood over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). The granulocytes were washed twice in incubation medium and resuspended. For the immunomagnetic isolation,12 the cells were incubated with MACS microbeads conjugated to a monoclonal antibody to CD16 (Miltenyi Biotec GmbH, Bergish Gladbach, Germany) at 4°C for 30 minutes. Thereafter, the cell-suspension was run through a magnetic column (VarioMACS; Miltenyi Biotec GmbH) to remove neutrophils from the cell suspension. The eosinophils (purity, > 98%; contaminating cells were mainly lymphocytes) were washed and suspended in incubation medium.
To purify lymphocytes, the mononuclear cells obtained after Ficoll-Paque separation were washed and incubated in RPMI 1640 supplemented with 10% fetal bovine serum (Biofluids, Rockville, MD) on plastic Petri dishes to let the monocytes adhere. After 1 hour of incubation at 37°C, the nonadherent lymphocytes were poured off and collected for lysis as described below. The lymphocytes had a purity of greater than 99%.
In initial experiments, lysis of neutrophils by Triton X-100 was compared with lysis by sonication and repeated freeze/thaw cycles. Lysis using Triton X-100 gave the highest yield of BPI and results similar to the detergent Igepal (Sigma, St Louis, MO) used at the same concentration. Therefore, in subsequent experiments, 1% Triton X-100 in PBS was used. To inhibit proteases and possible degradation of BPI, benzamidine was added at 10 mmol/L. Lysis of cells was performed for 20 minutes on ice. The lysates were stored at −80°C until analyzed by enzyme-linked immunosorbent assay (ELISA).
Some cells were collected for electron microscopy, and a part of these cells were pretreated with IL-5 at 10−10 mol/L and subsequent addition of STZ. All the cells were fixed in a mixture of 0.5% glutaraldehyde and 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer (pH 7.2) for 2 hours.
Immature myeloid cells were obtained from bone marrow aspirate after enrichment by density gradient centrifugation over Percoll (density of 1.074 g/mL) as described.13 The cells at the interphase were collected and, after washing, fixed as described above.
Immunoelectron microscopy.
Fixed cells were pelleted in 10% gelatin in PBS. Approximately 80-nm cryosections were made on an MT-7 ultracryomicrotome (Research and Manufacturing Co, Tucson, AZ) and incubated at room temperature with rabbit anti-BPI (dilution 1/600), followed by incubation with 10-nm gold-conjugated goat antirabbit IgG (dilution 1/40). Both incubations were for 1 hour. As control, the primary antiserum was replaced by a nonrelevant rabbit antiserum. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate. All sections were examined with a Philips CM 10 electron microscope (Eindhoven, The Netherlands).
Antibodies.
A polyclonal rabbit antibody to BPI was supplied by Dr Inge Olsson (Lund, Sweden). The production and characterization of this antibody has been described.5 Goat antirabbit IgG linked to 10-nm gold was from Amersham Nederland ('s-Hertogenbosch, The Netherlands).
BPI ELISA.
A previously described sandwich ELISA, specific for BPI,14was used to determine BPI in cell lysates.
Western blot.
Eosinophils obtained by immunomagnetic isolation (purity, >98%; contaminating cells were mainly lymphocytes), neutrophils (purity, >98%), and lymphocytes (purity, >99%) were lysed with Triton X-100 at a final concentration of 1 × 107 cells/mL and subsequently subject to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 14% tris-glycine gels (Novel Experimental Technologies, San Diego, CA) followed by Western blotting using standard procedures15; the primary antibody was used at a 1:1,000 dilution followed by a 1:1,000 dilution of goat antirabbit IgG (Biorad, Richmond, CA).
In some experiments, eosinophils, at a final concentration of 106 eosinophils/mL, were prewarmed for 10 minutes at 37°C before incubation with IL-5 at 10−10 mol/L for 20 minutes and subsequent incubation with STZ at a final concentration of 1 mg/mL. Cells were collected at different time points and treated as described above.
RESULTS
Detection of BPI in eosinophil lysates by Western blotting.
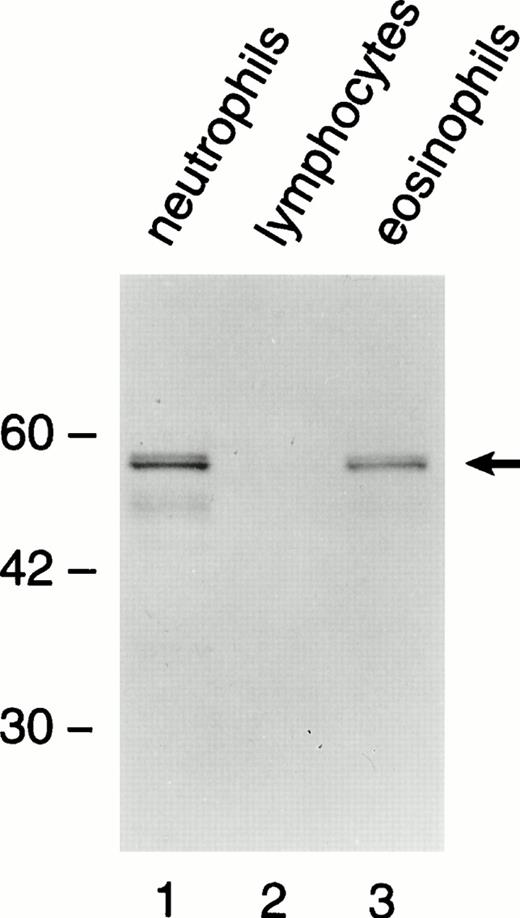
Western blot technique was used to detect BPI in lysates from highly purified neutrophils, eosinophils, and lymphocytes (Fig 1). A doublet 55-kD band, corresponding to BPI, was detected in the lanes of neutrophils and eosinophils, but not in lymphocytes. The immunoreactive band in the lane of eosinophils was less intense than that of neutrophils. This could correspond to a lower content of the protein in eosinophils compared with neutrophils. The doublet pattern of the 55-kD band has been shown to be due to variable glycosylation of BPI in neutrophils.16
A doublet 55-kD immunoreactive band corresponding to BPI is present in neutrophils (lane 1) and eosinophils (lane 3), but not in lymphocytes (lane 2). Highly purified cells were lysed and subject to SDS-PAGE/Western blotting. The primary antibody was detected by a secondary goat antirabbit antibody conjugated with alkaline phosphatase.
A doublet 55-kD immunoreactive band corresponding to BPI is present in neutrophils (lane 1) and eosinophils (lane 3), but not in lymphocytes (lane 2). Highly purified cells were lysed and subject to SDS-PAGE/Western blotting. The primary antibody was detected by a secondary goat antirabbit antibody conjugated with alkaline phosphatase.
Localization of BPI in eosinophils from human peripheral blood and in eosinophil progenitors.
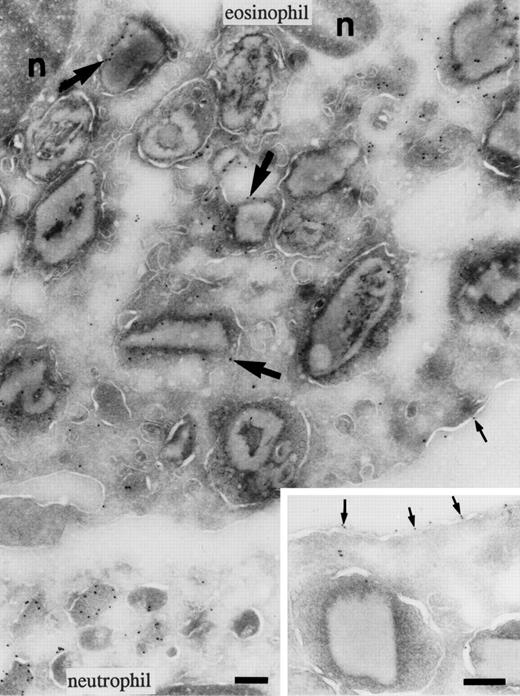
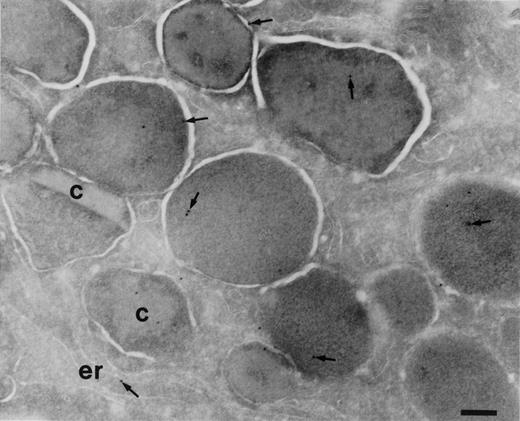
To determine the subcellular localization of BPI in eosinophils, cryosections of eosinophils isolated from peripheral blood and immature cells from bone marrow were incubated with anti-BPI serum. Compared with neutrophils, eosinophils showed less intense labeling for BPI. In mature eosinophils (Fig 2), BPI was present in the specific granules. The majority of these granules possess crystalloids. The cell surface was also weakly labeled, as has been demonstrated for neutrophils.16 In eosinophil promyelocytes and myelocytes of the bone marrow, the granules are round and do not yet possess crystalloids (promyelocytes) or have few (myelocytes) and the endoplasmic reticulum is abundant. The BPI in these cells was localized to some granules and in the endoplasmic reticulum (Fig 3). Lymphocytes, monocytes, and platelets present in the same sections were unlabeled. No labeling was found in the control of each experiment in which the primary antibody was replaced by a nonrelevant rabbit antiserum.
Localization of BPI in eosinophils. Cryosections of eosinophils labeled to detect BPI. Labeling of the core-containing specific granules of eosinophils was heterogeneous. The top of the micrograph shows an eosinophil with labeled granules (large arrows); however, the labeling was less intense than in the neutrophil at the bottom. Some labeling (small arrows) was also observed on the surface membrane of the eosinophil (insert). The nucleus (n) was unlabeled. Bars = 200 nm.
Localization of BPI in eosinophils. Cryosections of eosinophils labeled to detect BPI. Labeling of the core-containing specific granules of eosinophils was heterogeneous. The top of the micrograph shows an eosinophil with labeled granules (large arrows); however, the labeling was less intense than in the neutrophil at the bottom. Some labeling (small arrows) was also observed on the surface membrane of the eosinophil (insert). The nucleus (n) was unlabeled. Bars = 200 nm.
Localization of BPI in eosinophilic myelocytes. Cryosections of bone marrow cells incubated with anti-BPI and visualized by secondary gold-conjugated antibody. An area of an eosinophilic myelocyte is shown. Most of the granules do not possess a crystalloid, but two crystalloid-containing mature specific granules are present (c). BPI (arrows) is present in almost all granules and in the endoplasmic reticulum (er). Bars = 400 nm.
Localization of BPI in eosinophilic myelocytes. Cryosections of bone marrow cells incubated with anti-BPI and visualized by secondary gold-conjugated antibody. An area of an eosinophilic myelocyte is shown. Most of the granules do not possess a crystalloid, but two crystalloid-containing mature specific granules are present (c). BPI (arrows) is present in almost all granules and in the endoplasmic reticulum (er). Bars = 400 nm.
Measurement of the BPI content in neutrophils and eosinophils.
Using specific ELISA for measurement of BPI, the content in highly purified preparations of neutrophils and eosinophils was determined from whole cell extracts. Eosinophils were found to hold 179 ± 44 ng (mean ± SEM; range, 77 to 280 ng, n = 4)/5 × 106 eosinophils compared with 710 ± 83 ng (mean ± SEM; range, 510 to 901 ng, n = 4)/5 × 106neutrophils. In the literature, varying amounts of BPI in neutrophils have been reported: 1,650 ± 125 ng (mean ± SEM, range, 435 to 2,200 ng, n = 17)/5 × 106 neutrophils17; 910 ± 505 ng (mean ± SEM, n = 4)/5 × 106neutrophils18; and 3,250 ± 315 ng (mean ± SEM, range, 2,050 to 4,400 ng, n = 5)/5 × 106neutrophils.5 Although the extraction procedure is principally the same in these studies, the variation most likely is due to variations in the sensitivity and calibration of the methods of measurement used. There also seems to be a large donor variation in the BPI content of neutrophils. In any case, our data show that the BPI content of eosinophils is about one fourth of that found in neutrophils.
Redistribution of BPI in eosinophils stimulated with STZ.
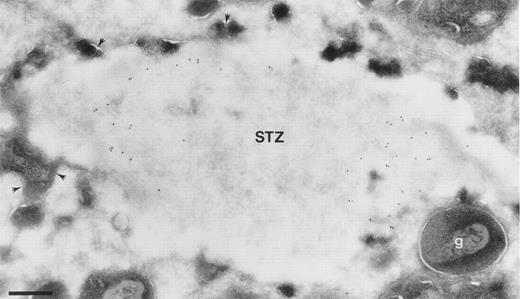
Highly purified eosinophils were prewarmed and preincubated with IL-5 at 10−10 mol/L for 20 minutes at 37°C before the addition of STZ for 10 minutes. The pretreatment with IL-5 activates eosinophils and increase their interaction with STZ.19After incubations, cells were fixed and processed for immunoelectron microscopy. Electron microscopic investigation show extensive phagocytosis of STZ (Fig 4). Detection of BPI by immunogold technique show BPI present within phagosomes (Fig 4), indicating release into the phagosome.
Localization of BPI in eosinophils after coincubation with STZ. Cells were prewarmed for 10 minutes at 37°C and activated with IL-5 at 0.1 nmol/l for 20 minutes before the addition of STZ and coincubation for 10 minutes. Ultrathin cryosections were incubated with anti-BPI and visualized by secondary gold-conjugated antibody. Area of an eosinophil showing a phagosome with a STZ containing BPI. Granules are seen fusing with the phagosome (arrowheads), whereas some intact granules (g) are seen close to the membrane of the phagosome. Bar = 400 nm.
Localization of BPI in eosinophils after coincubation with STZ. Cells were prewarmed for 10 minutes at 37°C and activated with IL-5 at 0.1 nmol/l for 20 minutes before the addition of STZ and coincubation for 10 minutes. Ultrathin cryosections were incubated with anti-BPI and visualized by secondary gold-conjugated antibody. Area of an eosinophil showing a phagosome with a STZ containing BPI. Granules are seen fusing with the phagosome (arrowheads), whereas some intact granules (g) are seen close to the membrane of the phagosome. Bar = 400 nm.
DISCUSSION
It is a novel finding that eosinophils possess BPI. About one fourth of the amount was found in eosinophils compared with neutrophils. BPI was detected in the specific crystalloid-containing granules of eosinophils.
In a previous study, using immunofluorescence for the detection of BPI, it was not possible to detect the presence of BPI in eosinophils due to their autofluorescent properties.5 In the present study, this problem was circumvented by the use of immunoelectron microscopy. The finding of BPI in both immature and mature eosinophils by immunoelectron microscopy suggests that the protein is synthesized at the promyelocytic stage of differentiation in the bone marrow, because we were able to detect BPI in both core-less and core-containing specific granules. The core-less granules of eosinophil promyelocytes have been suggested to mature into the core-containing specific granules of mature eosinophils.20
Eosinophils are generally considered to be effector cells in host defense against parasitic infection.1 However, the presence of BPI in eosinophils suggests an additional role for this cell in host defense to some bacterial infections. Beside the bactericidal activity from BPI, the characteristic cationic proteins of eosinophil granules, such as major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil peroxidase (EPO), do possess bactericidal activity beside their activity shown in vitro against several parasites and mammalian cells.2 Taken together, this further indicates that eosinophils may have an additional role in host defense to bacterial infection. On the other hand, the presence of BPI in eosinophils could suggest a function for BPI in the defense against parasitic infestation. To our knowledge, possible effects from BPI on parasites have not been investigated.
Eosinophils have been regarded as poor phagocytes, but this might be because, in previous studies, mainly resting populations of eosinophils have been investigated. In one study, normal human eosinophils were shown to ingest and kill Escherichia coli less efficiently than neutrophils.21 Recently, it was shown that, after incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, or IL-5, their phagocytic capacity increases dramatically.22 In fact, tissue-derived eosinophils, which presumably are activated, were more efficient in phagocytosing E coli than either neutrophils or macrophages.23 In mice,E coli attracts eosinophils to inflammatory sites,24 and in guinea pigs, LPS was shown to induce accumulation of eosinophils in vivo in guinea pig skin,25speaking for a role for eosinophils against Gram-negative infection in these animals.
The biological effects of BPI can be exerted by a 25-kD N-terminal fragment,26,27 and the C-terminal hydrophobic portion of BPI has been suggested to serve as an anchor into the granule membrane.9 Moreover, the middle of the BPI molecule contains a hydrophilic proline-rich region that is protease-sensitive and provides potential cleavage sites for elastase.9 We were not able to detect a smaller fragment of BPI in lysates from activated and phagocytosing eosinophils (data not shown). In line with this is that neutrophils upon stimulation release 55-kD BPI to the supernatant, suggesting that, at least partly, BPI is not transmembranously anchored and, furthermore, is not subject to proteolytic cleavage.17
In conclusion, eosinophils possess BPI, suggesting a role for eosinophils in protection against Gram-negative infection. Therefore, eosinophils may be specialized to participate in host defense against parasites, but could have an additional role in the protection against Gram-negative infection. Especially because eosinophils are numerous in colonic mucosa.23 Further studies are needed to elucidate how efficient activated eosinophils are in killing phagocytosed Gram-negative bacteria.
Supported by grants from the Th. C. Berg Foundation, the Greta & Johan Kock Foundations, the Ernhold Lundström Foundation, and the Alfred Österlund Foundation.
Submitted August 1, 1997; accepted February 10, 1998.
Address reprint requests to Arne Egesten, MD, Department of Medicine, University Hospital MAS, S-205 02 Malmö, Sweden; e-mail:Arne.Egesten@medforsk.mas.lu.se.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal