Abstract
Acute promyelocytic leukemia (APL) has a specific genetic rearrangement between the retinoic acid receptor (RAR)-α gene and the pml nuclear protein gene. All-trans retinoic acid (ATRA) induces granulocytic differentiation of APL-derived cells and is used to treat APL patients. However, ATRA interacts with normal cells with RAR throughout the entire body, and when used at high doses or over a long duration, it induces several adverse effects. The development of drugs that selectively act on APL cells may contribute to increasing the therapeutic efficacy of APL treatment as well as elucidating the mechanisms of response to ATRA. In this study, 9-cis retinoic acid α-tocopherol ester (9CTT) inhibited the proliferation of APL-derived NB4 and HT93 cells and induced differentiation markers, such as granulocytic maturation, nitroblue tetrazolium reduction, and CD11b expression, in these cells. The effects of 9CTT on non-APL cells, including HL-60 and U937 cells, were much weaker than those on APL cells, and tretinoin tocoferil (TT), which is an α-tocopherol ester of ATRA, did not induce the differentiation of APL cells as effectively as 9CTT. The differentiation-inducing effects of 9CTT were inhibited by RAR antagonists. 9CTT and TT similarly induced the transactivating activity of RARs, but were not effective on RXRs. 9CTT downregulated the expression of PML/RAR-α protein more effectively than TT, which suggests that it may be involved in the selectivity of 9CTT against APL cells. Interestingly, 9CTT enhanced the differentiation of APL cells induced by ATRA, 9-cis retinoic acid, and synthetic retinobenzoic acids. Combined with 1α,25-dihydroxyvitamin D3 (VD3), 9CTT also more than additively induced the differentiation of APL cells. Thus, 9CTT, alone or in combination with other retinoids or VD3, may be useful for the treatment of APL.
ACUTE PROMYELOCYTIC leukemia (APL; M3 in the French-American-British [FAB] classification) has a specific chromosomal abnormality t(15;17), which involves a genetic rearrangement between the retinoic acid receptor (RAR)-α gene and the pml gene coding a nuclear protein,1,2 and can be successfully treated with all-trans retinoic acid (ATRA).3 ATRA induces granulocytic differentiation of APL-derived cells.4,5 The biological actions of retinoic acid are mediated through specific nuclear receptors. ATRA binds to three types of receptors; RAR-α, RAR-β, and RAR-γ.6,7Another natural retinoid, 9-cis retinoic acid (9CRA), binds to RARs and other receptors: retinoid X receptor (RXR)-α, RXR-β, and RXR-γ.6,7 The PML/RAR-α fusion protein retains the ligand-binding and DNA-binding domains of RAR-α and can induce the expression of responsive genes in response to ATRA.1,2However, the PML/RAR-α protein exhibits a dominant negative action on RAR-α, and expression of the pml/RAR-α gene that has been introduced into human myeloid leukemia U937 and HL-60 cells interferes with the induction of their differentiation by ATRA.8,9 The PML protein is physiologically localized within nuclear organella called nuclear bodies10,11 and has been reported to suppress oncogenic transformation.12 In APL cells, PML/RAR-α protein delocalizes the wild-type PML protein and disrupts nuclear bodies; treatment with ATRA restores the structure of nuclear bodies.10 11 Thus, the PML/RAR-α fusion protein has been suggested to contribute to leukemogenesis and responsiveness to ATRA, although their precise mechanisms have not been elucidated.
Retinoids are involved in several biological phenomena, such as embryogenesis and the control of cell proliferation and differentiation. Although ATRA induces remission in APL patients without significant toxicities, the administration of retinoids at higher doses or over a long duration causes several adverse effects in other organs, including the skin, liver, and central nervous system.13-15 Because retinoids are teratogenic, their use in fertile women is limited.16,17 Several retinoid analogues have been synthesized, but their biologic activities are associated with toxicities.18 Because expression of the oncogenic PML/RAR-α protein is limited to APL cells, the fusion protein might be a good target for chemotherapeutic drugs in APL treatment. The development of retinoid derivatives that selectively act on APL cells may overcome retinoid-related drawbacks and contribute to improving the effects of therapy in APL as well as elucidating the mechanisms for response to retinoids.
MATERIALS AND METHODS
Materials.
Tretinoin tocoferil [TT; tocoretinate/(±)-3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-yl (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoate] and its Z-isomer, 9-cis retinoic acid α-tocopherol ester [9CTT; 9-cis tretinoin tocoferil/(±)-3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-yl (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoate], were synthesized and donated by Pharmaceutical Research Laboratories, Nisshin Flour Milling Co (Saitama, Japan). The chemical structures of TT and 9CTT are shown in Fig 1. The concentration of both stock solutions was 3.5 × 10−3 mol/L in ethanol. ATRA was purchased from Sigma (St Louis, MO), 9CRA was from Biomol Research Laboratories (Plymouth Meeting, PA), and 1α,25-dihydroxyvitamin D3(VD3) was from Wako Pure Chemical Industry (Osaka, Japan). The stock solutions for ATRA and 9CRA were 4 × 10−3 mol/L and that for VD3 was 1.2 × 10−3 mol/L in ethanol. Stock solutions for synthetic retinoids (Am80, Am580, Ch55, Re80, Am555s, and LE540) were 1 × 10−2 mol/L and that for LGD1069 was 3 × 10−3 mol/L in ethanol. The highest concentration of 9CTT in this study was 1.5 × 10−5 mol/L and the maximal final concentrations of ethanol was 0.5%, which did not affect cell proliferation or differentiation.
Chemical structures of tretinoin tocoferil (TT) and its Z-isomer (9-cis isomer), 9-cis retinoic acid α-tocopherol ester (9CTT). TT and 9CTT are α-tocopherol esters of ATRA and 9CRA, respectively.
Chemical structures of tretinoin tocoferil (TT) and its Z-isomer (9-cis isomer), 9-cis retinoic acid α-tocopherol ester (9CTT). TT and 9CTT are α-tocopherol esters of ATRA and 9CRA, respectively.
Cell lines and cell culture.
Human myeloid leukemia HL-60, U937, ML-1, THP-1, P39/TSU, P31/FUJ, NB4,5 and HT93 cells19 were cultured in suspension in RPMI 1640 medium containing 10% fetal bovine serum and 80 μg/mL gentamicin at 37°C in a humidified atmosphere of 5% CO2 in air.20 We confirmed the expression of the pml/RAR-α gene in NB4 and HT93 cells but not in HL-60, U937, ML-1, THP-1, P39/TSU, or P31/FUJ cells by a previously described reverse transcription-polymerase chain reaction (RT-PCR) technique1 (data not shown). Monkey kidney CV-1 and COS-7 cells were cultured in Dulbecco's modified Eagle medium or RPMI 1640 medium containing 10% fetal bovine serum and 80 μg/mL gentamicin.
Cell growth and differentiation.
Suspensions of cells were cultured with or without the test compounds in multidishes. The cells were counted in a Model ZM Coulter Counter (Coulter Electronics, Luton, UK). Nitroblue tetrazolium (NBT) reduction was assayed colorimetrically by a method reported by Takuma et al21 and modified by our laboratory.20 Briefly, cells were incubated with 1 mg/mL NBT (Sigma) and 100 ng/mL phorbol-12-myristate 13-acetate (Sigma) in RPMI1640 medium at 37°C for 30 minutes, and the reaction was stopped by adding HCl. Formazan deposits were solubilized in dimethyl sulfoxide (Wako), and absorption of the formazan solution at 560 nm per 107 cells was measured in a spectrophotometer (U-2000; Hitachi, Tokyo, Japan). Lysozyme activity in the conditioned medium was determined using a lysoplate containing 1% agar, 66.7 mmol/L sodium phosphate buffer (pH 6.6), 50 mmol/L NaCl, and 0.5 mg/mL heat-killed Micrococcus lysodeikticus (Sigma).20 One unit is equivalent to 1 μg/mL egg-white lysozyme. Cell morphology was examined in cell smears stained with May-Grünwald and Giemsa solutions (Merck, Darmstadt, Germany) by examination of more than 200 cells. α-Naphthyl acetate esterase was determined cytochemically with an esterase kit (Sigma).
Flow cytometry.
Expression of the granulocyte- and macrophage-specific antigens CD11b and CD14 on the cell surface was determined by indirect immunofluorescent staining and flow cytometry.22 Mouse monoclonal antibodies to CD11b (2LPM19c), CD14 (TÜK4), and control mouse IgG1 and IgG2a were obtained from Dako (Glostrup, Denmark). Cells were treated with the mouse monoclonal antibody in IFA buffer (10 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 4% fetal bovine serum, and 0.1% NaN3) plus 2% Block Ace (Snow Brand Milk Products, Sapporo, Japan) and stained with fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of goat antimouse IgG (Dako) in IFA buffer plus 2% Block Ace. The stained cells were assayed using a flow cytometer (Epics XL; Coulter Electronics). The mean fluorescence intensity was calculated using the Immuno-4 histogram analysis program (Coulter) with mouse Ig of the same isotype as a negative control. The Immuno-4 program subtracts a control histogram from a test histogram to calculate the percentage of positive cells and mean fluorescence intensity in the test histogram.23
Cell cycle analysis.
The cell cycle was analyzed using propidium iodide staining.24 Briefly, cells were fixed by the addition of cold ethanol, suspended with 250 μg/mL RNase A in 1.12% sodium citrate at 37°C for 30 minutes, and stained with 50 μg/mL propidium iodide (Sigma) on ice for more than 30 minutes. The stained nuclei were analyzed with a Epics XL flow cytometer (Coulter).
Transactivation assays for retinoid receptors.
To assay ligand-binding specificity, CV-1 cells were transfected with 100 ng of receptor plasmid (pCMX-hRARs, pCMX-hRXR-α, or pCMX-PML-RAR-α), 250 ng of reporter plasmid, 200 ng of pCMX-β-gal, and 450 ng of carrier pGEM using Lipofectin reagent (GIBCO BRL, Gaithersburg, MD).1,25 The reporters used were TK-TREpx2-LUC25 and mRARβx2-LUC, which contains the promoter region of the mouse RAR-β gene,2 for RARs and TK-CRBPII-LUC for RXR-α.26 The cells were transfected for 18 hours, and after removing the DNA-containing medium, they were incubated with a test compound in medium containing 10% resin-charcoal-stripped fetal bovine serum for 24 hours. Luciferase and β-galactosidase activities were analyzed using a luminescence reader (BLR-201; Aloka, Tokyo, Japan) and a spectrophotometer (Hitachi), respectively. All transfection data were normalized using an internal β-galactosidase marker. To investigate the effect of PML/RAR-α protein on transactivation by RAR, CV-1 cells were cotransfected with 100 ng of pCMX-PML-RAR-α, 250 ng of reporter plasmid (TK-TREpx2-LUC for exogenous receptor or TK-βREx3-LUC1,2 for endogenous receptor), and pGEM carrier to give 1 μg of DNA/well. A GAL4-receptor hybrid system was also used to assay ligand-binding specificity. Chimeric GAL4-receptor expression plasmids (CMX-GAL4-hRARs and CMX-GAL4-mRXRs) were constructed by fusion of the ligand-binding domains of retinoid receptor and the DNA-binding domain of the yeast GAL4 to pCMX vector.25,27 CV-1 cells were transfected with TK-GAL4-UASx4-LUC reporter, pCMX-β-gal, each receptor expression plasmid, and pGEM carrier in medium containing 10% resin-charcoal-stripped fetal bovine serum by the calcium phosphate coprecipitation technique.25 To assay the transactivating activity of endogenous retinoid receptor in NB4 and U937 cells, cells were transfected with 5 μg of TK-βREx3-LUC reporter, 5 μg of pCMX-β-gal, and 10 μg of pGEM carrier by electroporation using a Gene Pulser (Bio-Rad, Hercules, CA).1
Western blot for PML/RAR-α protein.
PML/RAR-α protein was expressed in COS-7 cells using Lipofectin (GIBCO BRL). After the cells were treated with a test compound for 2 days, total cellular protein was extracted28 and separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred from the gel onto a polyvinylidene difluoride microporous membrane (Immobilon; Millipore, Bedford, MA). After blocking with Block Ace (Snow Brand Milk Products), the membrane was immunoblotted with a rabbit polyclonal antibody to RAR-α (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized with a biotin-avidin-alkaline phosphatase system (Vectastain ABC system; Vector, Burlingame, CA). To examine the expression of PML/RAR-α protein in NB4 cells, crude nuclear extracts were prepared and a rabbit polyclonal antibody to PML was used.29
Statistical evaluation.
Statistical analyses were performed using an unpaired two-tailed Student's t-test.
RESULTS
Effects of 9CTT on the growth and differentiation of human leukemia cells.
TT, an α-tocopherol ester of ATRA, induces granulocytic differentiation in human promyelocytic leukemia HL-60 cells.20 Because ATRA is clinically used to treat APL, we studied the effects of TT on the differentiation of APL-derived NB4 and HT93 cells. TT at concentrations of up to 1.5 × 10−5mol/L only slightly affected proliferation and NBT reduction, which is a marker of myelomonocytic differentiation, in NB4 cells, but did not affect either proliferation or NBT reduction in HT93 cells (Table 1 and Fig 2A). Next, we examined the effect of 9CTT, which is an α-tocopherol ester of 9CRA and a Z-isomer of TT (Fig 1), on the proliferation and differentiation of APL cells. 9CTT at 1.5 × 10−5 mol/L inhibited the proliferation of NB4 and HT93 cells to 44% and 36% of that in the control, respectively, and increased the percentage of cells in G1 phase (Table1). In contrast to TT, 9CTT at up to 1.5 × 10−5mol/L effectively and concentration-dependently induced the NBT-reducing activity of NB4 and HT93 cells (Fig 2A). Morphologically, 9CTT induced the differentiation of these cells into myelocyte- and metamyelocyte-like cells, in that the cytoplasm became less basophilic, nucleoli disappeared, and nuclei were slightly lobulated, whereas TT did not affect the morphology (Table 1). The expression of CD11b antigen, another marker of myelomonocytic differentiation, was assayed by a flow cytometer with a very sensitive Immuno-4 histogram analysis program.23 TT and 9CTT increased CD11b expression by NB4 and HT93 cells, and 9CTT was more potent than TT in both cell lines (Table 1). Thus, 9CTT is more potent in inducing the differentiation of APL-derived NB4 and HT93 cells than TT.
Effects of TT and 9CTT on the Proliferation and Differentiation of Human Myeloid Leukemia Cells
| Cell . | Treatment . | Growth (% of control) . | Cell Cycle (%) . | Morphological Differentiation-150 (%) . | CD11b (mean intensity) . | ||
|---|---|---|---|---|---|---|---|
| G1 . | G2/M . | S . | |||||
| NB4 | None | 100 | 38 ± 2 | 13 ± 1 | 49 ± 1 | 1 ± 0 | 1.7 ± 0.0 |
| TT | 85 ± 8 | 36 ± 1 | 16 ± 1 | 48 ± 1 | 2 ± 1 | 3.8 ± 0.0 | |
| 9CTT | 44 ± 5 | 58 ± 2 | 11 ± 1 | 30 ± 1 | 49 ± 8 | 4.5 ± 0.2 | |
| HT93 | None | 100 | 41 ± 2 | 10 ± 1 | 49 ± 1 | 0 ± 0 | 1.7 ± 0.0 |
| TT | 103 ± 3 | 61 ± 2 | 8 ± 1 | 31 ± 1 | 3 ± 1 | 3.1 ± 0.1 | |
| 9CTT | 36 ± 2 | 72 ± 1 | 10 ± 0 | 18 ± 1 | 54 ± 1 | 4.3 ± 0.1 | |
| U937 | None | 100 | 0 ± 0 | 1.4 ± 0.0 | |||
| TT | 72 ± 3 | ND | 18 ± 3 | 1.8 ± 0.0 | |||
| 9CTT | 41 ± 1 | 30 ± 3 | 2.1 ± 0.1 | ||||
| HL-60 | None | 100 | 0 ± 0 | 2.9 ± 0.3 | |||
| TT | 64 ± 4 | ND | 2 ± 1 | 1.8 ± 0.1 | |||
| 9CTT | 58 ± 6 | 6 ± 0 | 1.9 ± 0.0 | ||||
| Cell . | Treatment . | Growth (% of control) . | Cell Cycle (%) . | Morphological Differentiation-150 (%) . | CD11b (mean intensity) . | ||
|---|---|---|---|---|---|---|---|
| G1 . | G2/M . | S . | |||||
| NB4 | None | 100 | 38 ± 2 | 13 ± 1 | 49 ± 1 | 1 ± 0 | 1.7 ± 0.0 |
| TT | 85 ± 8 | 36 ± 1 | 16 ± 1 | 48 ± 1 | 2 ± 1 | 3.8 ± 0.0 | |
| 9CTT | 44 ± 5 | 58 ± 2 | 11 ± 1 | 30 ± 1 | 49 ± 8 | 4.5 ± 0.2 | |
| HT93 | None | 100 | 41 ± 2 | 10 ± 1 | 49 ± 1 | 0 ± 0 | 1.7 ± 0.0 |
| TT | 103 ± 3 | 61 ± 2 | 8 ± 1 | 31 ± 1 | 3 ± 1 | 3.1 ± 0.1 | |
| 9CTT | 36 ± 2 | 72 ± 1 | 10 ± 0 | 18 ± 1 | 54 ± 1 | 4.3 ± 0.1 | |
| U937 | None | 100 | 0 ± 0 | 1.4 ± 0.0 | |||
| TT | 72 ± 3 | ND | 18 ± 3 | 1.8 ± 0.0 | |||
| 9CTT | 41 ± 1 | 30 ± 3 | 2.1 ± 0.1 | ||||
| HL-60 | None | 100 | 0 ± 0 | 2.9 ± 0.3 | |||
| TT | 64 ± 4 | ND | 2 ± 1 | 1.8 ± 0.1 | |||
| 9CTT | 58 ± 6 | 6 ± 0 | 1.9 ± 0.0 | ||||
Cells (5 × 104 cells were incubated with or without 1.5 × 10−5 mol/L TT or 9CTT for 4 days. Values represent the means ± SD of three separate experiments.
Abbreviation: ND, not done.
Cells differentiating into myelocytes and more mature cells were counted.
Effects of TT and 9CTT on the NBT-reducing activity of myeloid leukemia cells. Effects of TT (○, ▵) and 9CTT (•, ▴) on APL-derived NB4 (○, •) and HT93 (▵, ▴) cells (A) are compared with those of ATRA (○, ▵) and 9CRA (•, ▴) on NB4 (○, •) and HT93 (▵, ▴) cells (B). Cells (5 × 104 cells/mL) were treated with TT, 9CTT, ATRA, or 9CRA for 4 days. Values represent the means ± SD of three separate experiments.
Effects of TT and 9CTT on the NBT-reducing activity of myeloid leukemia cells. Effects of TT (○, ▵) and 9CTT (•, ▴) on APL-derived NB4 (○, •) and HT93 (▵, ▴) cells (A) are compared with those of ATRA (○, ▵) and 9CRA (•, ▴) on NB4 (○, •) and HT93 (▵, ▴) cells (B). Cells (5 × 104 cells/mL) were treated with TT, 9CTT, ATRA, or 9CRA for 4 days. Values represent the means ± SD of three separate experiments.
Because TT and 9CTT are derivatives of ATRA and 9CRA, respectively, the effects of ATRA and 9CRA on the differentiation of APL-derived cells were compared. As previously reported,30 ATRA and 9CRA similarly induced NBT reduction in both cell lines, and a drastic difference in inducing this activity, as observed between TT and 9CTT, was not observed between ATRA and 9CRA (Fig 2B).
The effects of TT and 9CTT on other leukemia cells were examined. TT and 9CTT inhibited the proliferation of monoblastic leukemia U937 cells and induced morphologic differentiation into myelocytic cells without α-naphthyl acetate esterase activity or CD14 expression (Table 1 and data not shown). The expression of CD11b and NBT-reducing activity in U937 cells were only slightly induced by 9CTT (Table 1 and Fig 2C). HL-60 cells are promyelocytic leukemia cells that do not possess t(15;17) chromosomal rearrangement and are derived from FAB-M2.31 TT and 9CTT at 1.5 × 10−5mol/L minimally affected the differentiation of these cells (Table 1and Fig 2C). 9CTT only slightly induced the NBT-reducing activity of myeloblastic ML-1, monoblastic THP-1, P39/TSU, and P31/FUJ cells, and their induction was marginal, compared with that in APL-derived NB4 and HT93 cells (Fig 2C). Therefore, 9CTT was a potent and selective inducer of differentiation in APL-derived leukemia cells.
Effects of RAR antagonists on the differentiation of NB4 cells induced by 9CTT.
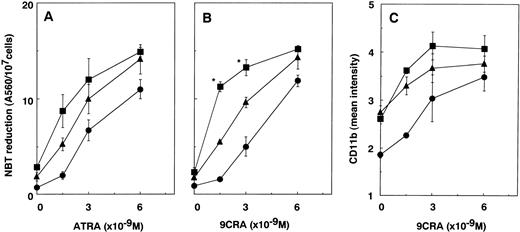
We examined the effect of LE540, which is an antagonist for RAR,32 on the differentiation of NB4 cells induced by 9CTT. LE540 inhibited the transactivation induced by ATRA through RAR-α and RAR-β on TK-TREpx2-LUC reporter (data not shown). As shown in Fig 3A and B, LE540 inhibited the NBT-reducing activity of NB4 cells induced by ATRA or 9CRA and slightly counteracted growth inhibition. LE540 effectively reversed the growth inhibition induced by 9CTT and completely inhibited the NBT-reducing activity induced by 9CTT (Fig 3C and D). The reversing action of LE540 on growth inhibition and NBT reduction in NB4 cells treated with 9CTT was more potent than that in cells treated with ATRA or 9CRA. 9CRA at 3 × 10−9 mol/L and 9CTT at 6 × 10−6 mol/L induced a similar mean intensity of CD11b expression in NB4 cells (3.0 U). LE540 also inhibited the CD11b expression (Fig 3E). The NBT-reducing activity and CD11b expression induced by 9CTT were also inhibited by another RAR antagonist, Ro41-525333 (data not shown). These findings suggest that the action of 9CTT in APL cells is mainly mediated by RAR.
Effects of the RAR antagonist LE540 on growth inhibition and differentiation in NB4 cells induced by ATRA, 9CRA, TT, or 9CTT. The effects of LE540 on growth inhibition (A) and NBT-reducing activity (B) in NB4 cells induced by ATRA (6 × 10−9 mol/L [○] or 6 × 10−8 mol/L [•]) or 9CRA (6 × 10−9 mol/L [▵] or 6 × 10−8 mol/L [▴]) were examined. Next, the effects of LE540 on growth inhibition (C) and NBT-reducing activity (D) induced by 9CTT at 6 × 10−6 mol/L (▿) or 9 × 10−6 mol/L (□) were examined. LE540 alone (•) did not affect growth or NBT reduction. *P < .05, compared with values in the absence of LE540 in (A) through (D). The effects of LE540 on CD11b expression that was induced equally by 3 × 10−9 mol/L 9CRA and 6 × 10−6 mol/L 9CTT are shown in (E). *P < .05, compared with 9CRA. Cells (5 × 104 cells/mL) were treated with ATRA, 9CRA, TT, or 9CTT in the absence or presence of LE540 for 4 days. Values represent the means ± SD of three separate experiments.
Effects of the RAR antagonist LE540 on growth inhibition and differentiation in NB4 cells induced by ATRA, 9CRA, TT, or 9CTT. The effects of LE540 on growth inhibition (A) and NBT-reducing activity (B) in NB4 cells induced by ATRA (6 × 10−9 mol/L [○] or 6 × 10−8 mol/L [•]) or 9CRA (6 × 10−9 mol/L [▵] or 6 × 10−8 mol/L [▴]) were examined. Next, the effects of LE540 on growth inhibition (C) and NBT-reducing activity (D) induced by 9CTT at 6 × 10−6 mol/L (▿) or 9 × 10−6 mol/L (□) were examined. LE540 alone (•) did not affect growth or NBT reduction. *P < .05, compared with values in the absence of LE540 in (A) through (D). The effects of LE540 on CD11b expression that was induced equally by 3 × 10−9 mol/L 9CRA and 6 × 10−6 mol/L 9CTT are shown in (E). *P < .05, compared with 9CRA. Cells (5 × 104 cells/mL) were treated with ATRA, 9CRA, TT, or 9CTT in the absence or presence of LE540 for 4 days. Values represent the means ± SD of three separate experiments.
Activation of retinoid receptors by 9CTT.
CV-1 cells were transfected with receptor plasmid and luciferase reporter plasmid to examine the luciferase-inducing activity of TT and 9CTT on the transactivation of retinoid receptors. TK-TREpx2-LUC and TK-mRARβx2-LUC respond to RAR1,2,25 and TK-CRBPII-LUC responds to RXR.26 We used ATRA and LGD106934as positive controls for RAR and RXR, respectively. As shown in Table 2, when using TK-TREpx2-LUC as a reporter, TT and 9CTT showed agonistic action to RAR-α, RAR-β, and RAR-γ. TT and 9CTT also stimulated the transactivating activity of RAR-α and RAR-β on the TK-mRARβx2-LUC reporter and that of endogenous RAR on the TK-βREx3-LUC reporter. The effect of 9CTT on the activation of RARs in these systems was similar to that of TT. We also examined the receptor selectivity of TT and 9CTT by using GAL4-chimeric receptor. Because the TK-GAL4-UASx4-LUC reporter does not respond to mammalian nuclear factors, the interaction between a ligand and a GAL4-chimeric receptor can be analyzed without interference.27 TT interacted with GAL4-RAR-α, GAL4-RAR-β, and GAL4-RAR-γ, whereas 9CTT activated GAL4-RAR-α and GAL4-RAR-β, but to a lesser extent than TT (Table 2). 9CTT did not effectively affect RXR-α under TK-CRBPII-LUC reporter or GAL4-RXRs under GAL4-UASx4-LUC reporter. Thus, although there are some differences among the receptor-reporter systems, TT and 9CTT are agonistic for RARs and not effective on RXRs. Next, we investigated the interaction of TT and 9CTT with PML/RAR-α fusion protein. TT and 9CTT activated the transactivating action of PML/RAR-α on the TK-TREpx2-LUC reporter, but less than that of RAR-α (Table 2). When PML/RAR-α was cotransfected with RAR-α or RAR-β, the fusion protein inhibited the transactivating function of RAR-α and RAR-β induced by TT and 9CTT. The PML/RAR-α protein also inhibited the transactivating activity of endogenous receptors stimulated by TT and 9CTT on the TK-βREx3-LUC reporter. Thus, as previously reported for ATRA,9 the fusion protein acts in a dominant negative manner on RARs in stimulation by TT and 9CTT. These findings indicate that the interaction with retinoid receptors is not involved in the predominance of 9CTT over TT in inducing the differentiation of APL-derived cells.
Transactivation of Retinoid Receptors by TT or 9CTT
| Receptor . | Reporter . | Luciferase Induction (ratio) . | |||
|---|---|---|---|---|---|
| TT . | 9CTT . | ATRA . | LGD1069 . | ||
| CMX | TK-TREpx2-LUC | 2.6 ± 0.1 | 2.4 ± 0.2 | 4.1 ± 0.3 | |
| RAR-α | 10.7 ± 1.8 | 14.0 ± 1.2 | 37.1 ± 3.9 | ||
| RAR-β | 7.1 ± 1.0 | 7.7 ± 1.0 | 11.8 ± 1.8 | ||
| RAR-γ | 7.9 ± 0.2 | 7.8 ± 0.6 | 12.7 ± 1.3 | ||
| PML/RAR-α | 3.5 ± 0.2 | 4.2 ± 0.3 | 13.3 ± 1.7 | ||
| RAR-α + PML/RAR-α | 4.0 ± 0.3 | 3.4 ± 0.5 | 13.3 ± 2.3 | ||
| RAR-β + PML/RAR-α | 2.6 ± 0.4 | 3.0 ± 0.2 | 10.4 ± 0.9 | ||
| CMX | TK-mRARβx2-LUC | 6.2 ± 1.0 | 5.5 ± 0.4 | 9.4 ± 0.5 | |
| RAR-α | 8.7 ± 0.9 | 9.0 ± 0.4 | 15.2 ± 0.4 | ||
| RAR-β | 7.9 ± 0.9 | 6.7 ± 0.2 | 10.2 ± 1.2 | ||
| RAR-γ | 3.8 ± 0.2 | 3.3 ± 0.3 | 5.5 ± 0.2 | ||
| PML/RAR-α | 2.3 ± 0.2 | 2.7 ± 0.2 | 9.0 ± 1.2 | ||
| CMX | TK-βREx3-LUC | 46.1 ± 3.2 | 47.4 ± 9.0 | 68.1 ± 22.2 | |
| PML/RAR-α | 10.6 ± 0.6 | 11.3 ± 0.4 | 38.7 ± 12.4 | ||
| GAL-RAR-α | TK-GAL4-UASx4-LUC | 94.5 ± 14.3 | 12.2 ± 2.4 | 2,648 ± 36 | |
| GAL-RAR-β | 42.6 ± 2.0 | 16.7 ± 0.7 | 119 ± 10 | ||
| GAL-RAR-γ | 15.1 ± 0.4 | 3.7 ± 0.8 | 24.0 ± 1.5 | ||
| CMX | TK-CRBPII-LUC | 1.2 ± 0.0 | 1.1 ± 0.2 | 1.4 ± 0.1 | |
| RXR-α | 1.7 ± 0.3 | 2.4 ± 0.4 | 5.9 ± 0.7 | ||
| GAL-RXR-α | TK-GAL4-UASx4-LUC | 2.1 ± 0.2 | 1.6 ± 0.1 | 70.5 ± 1.2 | |
| GAL-RXR-β | 1.7 ± 0.1 | 1.5 ± 0.1 | 59.8 ± 2.3 | ||
| GAL-RXR-γ | 2.0 ± 0.2 | 2.2 ± 0.1 | 9.4 ± 0.9 | ||
| Receptor . | Reporter . | Luciferase Induction (ratio) . | |||
|---|---|---|---|---|---|
| TT . | 9CTT . | ATRA . | LGD1069 . | ||
| CMX | TK-TREpx2-LUC | 2.6 ± 0.1 | 2.4 ± 0.2 | 4.1 ± 0.3 | |
| RAR-α | 10.7 ± 1.8 | 14.0 ± 1.2 | 37.1 ± 3.9 | ||
| RAR-β | 7.1 ± 1.0 | 7.7 ± 1.0 | 11.8 ± 1.8 | ||
| RAR-γ | 7.9 ± 0.2 | 7.8 ± 0.6 | 12.7 ± 1.3 | ||
| PML/RAR-α | 3.5 ± 0.2 | 4.2 ± 0.3 | 13.3 ± 1.7 | ||
| RAR-α + PML/RAR-α | 4.0 ± 0.3 | 3.4 ± 0.5 | 13.3 ± 2.3 | ||
| RAR-β + PML/RAR-α | 2.6 ± 0.4 | 3.0 ± 0.2 | 10.4 ± 0.9 | ||
| CMX | TK-mRARβx2-LUC | 6.2 ± 1.0 | 5.5 ± 0.4 | 9.4 ± 0.5 | |
| RAR-α | 8.7 ± 0.9 | 9.0 ± 0.4 | 15.2 ± 0.4 | ||
| RAR-β | 7.9 ± 0.9 | 6.7 ± 0.2 | 10.2 ± 1.2 | ||
| RAR-γ | 3.8 ± 0.2 | 3.3 ± 0.3 | 5.5 ± 0.2 | ||
| PML/RAR-α | 2.3 ± 0.2 | 2.7 ± 0.2 | 9.0 ± 1.2 | ||
| CMX | TK-βREx3-LUC | 46.1 ± 3.2 | 47.4 ± 9.0 | 68.1 ± 22.2 | |
| PML/RAR-α | 10.6 ± 0.6 | 11.3 ± 0.4 | 38.7 ± 12.4 | ||
| GAL-RAR-α | TK-GAL4-UASx4-LUC | 94.5 ± 14.3 | 12.2 ± 2.4 | 2,648 ± 36 | |
| GAL-RAR-β | 42.6 ± 2.0 | 16.7 ± 0.7 | 119 ± 10 | ||
| GAL-RAR-γ | 15.1 ± 0.4 | 3.7 ± 0.8 | 24.0 ± 1.5 | ||
| CMX | TK-CRBPII-LUC | 1.2 ± 0.0 | 1.1 ± 0.2 | 1.4 ± 0.1 | |
| RXR-α | 1.7 ± 0.3 | 2.4 ± 0.4 | 5.9 ± 0.7 | ||
| GAL-RXR-α | TK-GAL4-UASx4-LUC | 2.1 ± 0.2 | 1.6 ± 0.1 | 70.5 ± 1.2 | |
| GAL-RXR-β | 1.7 ± 0.1 | 1.5 ± 0.1 | 59.8 ± 2.3 | ||
| GAL-RXR-γ | 2.0 ± 0.2 | 2.2 ± 0.1 | 9.4 ± 0.9 | ||
The transactivating activity of TT and 9CTT for retinoid receptors was examined using several reporters. CV-1 cells that has been transfected with receptor plasmid and reporter plasmid were incubated with or without 10−5 mol/L TT or 9CTT for 24 hours. pCMX plasmid is an empty vector; the genes for retinoid receptors were introduced into the pCMX vector. ATRA (10−6 mol/L) and LGD1069 (10−6 mol/L) were used as positive controls for RARs and RXRs, respectively. Values are given relative to luciferase activity in unstimulated cells as 1 U and represent the means ± SD from duplicate or triplicate experiments.
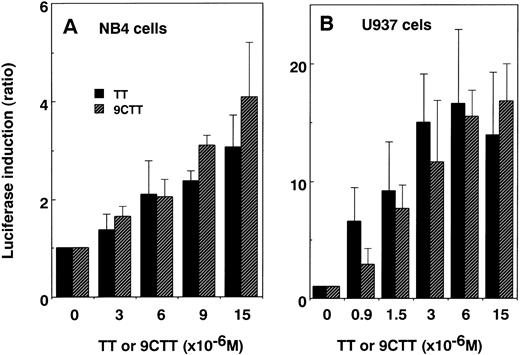
We examined the luciferase-inducing activity of TT and 9CTT on transactivation via endogenous retinoid receptors in leukemia cells. The TK-βREx3-LUC reporter was used because it is highly sensitive in response to the addition of a ligand via endogenous RARs without the cotransfection of expression plasmids for RARs.1 TT and 9CTT concentration-dependently induced luciferase activity in APL-derived NB4 and non-APL U937 cells (Fig4). In these cells, the effect of 9CTT on transactivation in the RAR-responsive reporter system was similar to that of TT.
Induction of the transactivation of endogenous RARs on an RAR-responsive element by TT or 9CTT in APL-derived NB4 (A) and non-APL U937 (B) cells. Cells were transfected with TK-βREx3-LUC reporter by an electroporation technique and treated with TT or 9CTT for 24 hours. Luciferase activities were adjusted for the efficiency of transfection by cotransfected β-galactosidase activities. ATRA and 9CTA at 1 × 10-6 mol/L induced the luciferase activity to 7.7 and 16.6 U, respectively, in NB4 cells and 24.8 and 54.8 U, respectively, in U937 cells. Values represent the means of three separate experiments.
Induction of the transactivation of endogenous RARs on an RAR-responsive element by TT or 9CTT in APL-derived NB4 (A) and non-APL U937 (B) cells. Cells were transfected with TK-βREx3-LUC reporter by an electroporation technique and treated with TT or 9CTT for 24 hours. Luciferase activities were adjusted for the efficiency of transfection by cotransfected β-galactosidase activities. ATRA and 9CTA at 1 × 10-6 mol/L induced the luciferase activity to 7.7 and 16.6 U, respectively, in NB4 cells and 24.8 and 54.8 U, respectively, in U937 cells. Values represent the means of three separate experiments.
Interaction of 9CTT with PML/RAR-α protein in transfected COS-7 cells and NB4 cells.
TT and 9CTT similarly stimulated transactivation via RARs in assay systems of transient transfection, but 9CTT induced the differentiation of APL-derived cells more effectively than TT. Recently, the PML/RAR-α protein was reported to be downregulated by treatment with ATRA, and this phenomenon was suggested to be one of the mechanisms for the action of ATRA on APL cells.28 29 COS-7 cells were transfected with PML/RAR-α expression vector and the interaction of the fusion protein with TT and 9CTT was examined. As shown in Fig 5A, 9CTT concentration-dependently downregulated the expression of the PML/RAR-α protein, whereas TT had only a slight effect. 9CTT at 3 × 10−6 mol/L obviously decreased the expression of PML/RAR-α protein and at higher concentrations of 1.2 to 1.5 × 10−5 mol/L markedly decreased it to faint levels. In contrast, the decrease induced by TT at 1.5 × 10−5 mol/L was slight. In NB4 cells, 9CTT also downregulated the PML/RAR-α protein more strongly than had TT (Fig 5B). Thus, the downregulation of the expression of the PML/RAR-α protein by 9CTT may lead to its selective induction of differentiation in APL cells.
Expression of PML/RAR-α protein inpml/RAR-α–introduced COS-7 cells (A) and NB4 cells (B) that had been treated with TT or 9CTT. (A) COS-7 cells were transfected with a pml/RAR-α expression vector, pCMX-PML-RAR-α, by lipofection and treated with various concentrations of TT or 9CTT for 2 days. Total cellular protein from 105 cells was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and blotted onto an Immobilon membrane. The PML/RAR-α protein was detected with a rabbit polyclonal antibody to RAR-α and visualized with a biotin-avidin-alkaline phosphatase system. The amount of the fusion protein was quantified with a densitometer and compared with that in the untreated cells. Two additional experiments showed similar results. (B) NB4 cells (2 × 105/mL) were treated with TT or 9CTT for 4 days and the crude nuclear extracts (20 μg) were separated on a gel. The fusion protein was detected with an anti-PML antibody.
Expression of PML/RAR-α protein inpml/RAR-α–introduced COS-7 cells (A) and NB4 cells (B) that had been treated with TT or 9CTT. (A) COS-7 cells were transfected with a pml/RAR-α expression vector, pCMX-PML-RAR-α, by lipofection and treated with various concentrations of TT or 9CTT for 2 days. Total cellular protein from 105 cells was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and blotted onto an Immobilon membrane. The PML/RAR-α protein was detected with a rabbit polyclonal antibody to RAR-α and visualized with a biotin-avidin-alkaline phosphatase system. The amount of the fusion protein was quantified with a densitometer and compared with that in the untreated cells. Two additional experiments showed similar results. (B) NB4 cells (2 × 105/mL) were treated with TT or 9CTT for 4 days and the crude nuclear extracts (20 μg) were separated on a gel. The fusion protein was detected with an anti-PML antibody.
Effects of 9CTT on the differentiation of NB4 cells induced by ATRA, 9CRA, and other retinoid analogues.
We examined the effects of 9CTT on the differentiation of NB4 cells treated with ATRA or 9CRA. TT slightly enhanced the differentiation of NB4 cells induced by ATRA, and the differentiation-enhancing effect of 9CTT was slightly greater than that of TT (Fig 6A). TT also enhanced the NBT-reducing activity of NB4 cells induced by 9CRA. Interestingly, 9CTT enhanced the activity induced by 9CRA significantly more than TT (Fig 6B). 9CRA-induced CD11b expression by NB4 cells was also augmented by 9CTT (Fig 6C).
Effects of TT and 9CTT on the differentiation induced by ATRA or 9CRA in NB4 cells. The enhancing effects of TT and 9CTT on the NBT reduction induced by ATRA (A) and 9CRA (B) and those on CD11b expression in the cells induced by 9CRA (C) were examined. Cells (5 × 104 cells/mL) were cultured with ATRA or 9CRA in the absence (•) or presence of 3 × 10−6 mol/L TT (▴) or 9CTT (▪) for 4 days. Values represent the means of three separate experiments. *P < .05, compared with the effects of TT.
Effects of TT and 9CTT on the differentiation induced by ATRA or 9CRA in NB4 cells. The enhancing effects of TT and 9CTT on the NBT reduction induced by ATRA (A) and 9CRA (B) and those on CD11b expression in the cells induced by 9CRA (C) were examined. Cells (5 × 104 cells/mL) were cultured with ATRA or 9CRA in the absence (•) or presence of 3 × 10−6 mol/L TT (▴) or 9CTT (▪) for 4 days. Values represent the means of three separate experiments. *P < .05, compared with the effects of TT.
Next, the effects of 9CTT in combination with synthetic retinoids on the differentiation of NB4 cells were examined. Am80 and Am580 are specific for RAR-α and RAR-β and Ch55 binds to RAR-α, RAR-β, and RAR-γ, but not to cytoplasmic retinoic acid binding protein (CRABP).18,35 Re80 is a very strong ligand for RAR-α, RAR-β, and RAR-γ and Am555s binds to RAR-α more effectively than to RAR-β.18 35 All of these synthetic retinoids inhibited the proliferation of NB4 cells and induced NBT-reducing activity and CD11b expression in these cells (Table 3). Combined with these retinoids at low concentrations, 9CTT at 3 × 10−6 mol/L, which alone had only marginal effects, augmented growth inhibition and differentiation as reflected by NBT reduction and CD11b expression (Table 3). Thus, 9CTT also enhanced the differentiation of NB4 cells induced by synthetic retinoids.
Effects of Combinations of 9CTT and Retinoid Analogues on Growth Inhibition, NBT Reduction, and CD11b Expression in NB4 Cells
| Compounds . | Growth (% of control) . | NBT Reduction (A560/107cells) . | CD11b Expression (mean intensity) . | |||
|---|---|---|---|---|---|---|
| −9CTT . | +9CTT . | −9CTT . | +9CTT . | −9CTT . | +9CTT . | |
| None | 100 | 109 ± 4 | 0.9 ± 0.1 | 2.1 ± 0.2 | 1.56 ± 0.03 | 2.26 ± 0.09 |
| Am80 | ||||||
| 3 × 10−10 mol/L | 113 ± 3 | 70 ± 10 | 1.3 ± 0.2 | 9.7 ± 2.3 | 2.21 ± 0.12 | 3.45 ± 0.15 |
| 6 × 10−10 mol/L | 89 ± 4 | 55 ± 3 | 5.6 ± 0.2 | 14.7 ± 1.3 | 3.06 ± 0.12 | 3.66 ± 0.12 |
| Am580 | ||||||
| 1 × 10−10 mol/L | 113 ± 3 | 84 ± 4 | 1.1 ± 0.0 | 8.6 ± 0.9 | 2.09 ± 0.05 | 3.17 ± 0.13 |
| 2 × 10−10 mol/L | 103 ± 8 | 67 ± 3 | 3.4 ± 0.6 | 13.3 ± 0.3 | 2.56 ± 0.21 | 3.39 ± 0.13 |
| Ch55 | ||||||
| 1.5 × 10−10 mol/L | 110 ± 3 | 82 ± 6 | 1.6 ± 0.2 | 7.2 ± 1.7 | 2.55 ± 0.29 | 3.68 ± 0.10 |
| 3 × 10−10 mol/L | 93 ± 1 | 66 ± 4 | 5.7 ± 0.4 | 11.8 ± 2.3 | 3.44 ± 0.41 | 4.36 ± 0.31 |
| Re80 | ||||||
| 1.5 × 10−10 mol/L | 111 ± 2 | 66 ± 7 | 1.4 ± 0.1 | 12.1 ± 0.8 | 2.76 ± 0.24 | 4.00 ± 0.27 |
| 3 × 10−10 mol/L | 85 ± 7 | 51 ± 3 | 5.7 ± 1.5 | 16.7 ± 0.6 | 3.67 ± 0.27 | 4.35 ± 0.17 |
| Am555s | ||||||
| 3 × 10−9 mol/L | 104 ± 4 | 71 ± 5 | 3.9 ± 0.7 | 12.9 ± 0.5 | 3.05 ± 0.15 | 4.30 ± 0.29 |
| 6 × 10−9 mol/L | 83 ± 5 | 56 ± 3 | 10.7 ± 1.0 | 16.5 ± 1.2 | 3.93 ± 0.14 | 4.61 ± 0.07 |
| Compounds . | Growth (% of control) . | NBT Reduction (A560/107cells) . | CD11b Expression (mean intensity) . | |||
|---|---|---|---|---|---|---|
| −9CTT . | +9CTT . | −9CTT . | +9CTT . | −9CTT . | +9CTT . | |
| None | 100 | 109 ± 4 | 0.9 ± 0.1 | 2.1 ± 0.2 | 1.56 ± 0.03 | 2.26 ± 0.09 |
| Am80 | ||||||
| 3 × 10−10 mol/L | 113 ± 3 | 70 ± 10 | 1.3 ± 0.2 | 9.7 ± 2.3 | 2.21 ± 0.12 | 3.45 ± 0.15 |
| 6 × 10−10 mol/L | 89 ± 4 | 55 ± 3 | 5.6 ± 0.2 | 14.7 ± 1.3 | 3.06 ± 0.12 | 3.66 ± 0.12 |
| Am580 | ||||||
| 1 × 10−10 mol/L | 113 ± 3 | 84 ± 4 | 1.1 ± 0.0 | 8.6 ± 0.9 | 2.09 ± 0.05 | 3.17 ± 0.13 |
| 2 × 10−10 mol/L | 103 ± 8 | 67 ± 3 | 3.4 ± 0.6 | 13.3 ± 0.3 | 2.56 ± 0.21 | 3.39 ± 0.13 |
| Ch55 | ||||||
| 1.5 × 10−10 mol/L | 110 ± 3 | 82 ± 6 | 1.6 ± 0.2 | 7.2 ± 1.7 | 2.55 ± 0.29 | 3.68 ± 0.10 |
| 3 × 10−10 mol/L | 93 ± 1 | 66 ± 4 | 5.7 ± 0.4 | 11.8 ± 2.3 | 3.44 ± 0.41 | 4.36 ± 0.31 |
| Re80 | ||||||
| 1.5 × 10−10 mol/L | 111 ± 2 | 66 ± 7 | 1.4 ± 0.1 | 12.1 ± 0.8 | 2.76 ± 0.24 | 4.00 ± 0.27 |
| 3 × 10−10 mol/L | 85 ± 7 | 51 ± 3 | 5.7 ± 1.5 | 16.7 ± 0.6 | 3.67 ± 0.27 | 4.35 ± 0.17 |
| Am555s | ||||||
| 3 × 10−9 mol/L | 104 ± 4 | 71 ± 5 | 3.9 ± 0.7 | 12.9 ± 0.5 | 3.05 ± 0.15 | 4.30 ± 0.29 |
| 6 × 10−9 mol/L | 83 ± 5 | 56 ± 3 | 10.7 ± 1.0 | 16.5 ± 1.2 | 3.93 ± 0.14 | 4.61 ± 0.07 |
Cells (5 × 104 cells/mL) were cultured with retinoid analogues in the absence or presence of 3 × 10−6 mol/L 9CTT for 4 days. Values represent the means ± SD of three separate experiments.
Effects of the combination of 9CTT and VD3 on the differentiation of leukemia cells.
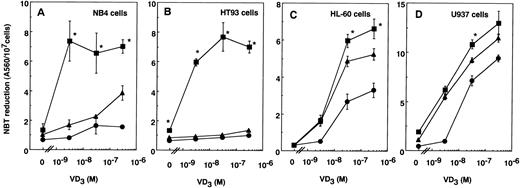
Because TT enhanced the differentiation of several myelomonocytic leukemia cells induced by VD3,20 the effects of 9CTT plus VD3 on the differentiation of leukemia cells were examined. VD3 at concentrations from 3 × 10−9 to 3 × 10−7 mol/L was combined with 3 × 10−6 mol/L TT or 9CTT. TT and 9CTT at this concentration barely induced NBT-reducing activity in NB4, HT93, U937, and HL-60 cells (Fig 7). VD3 at up to 3 × 10−7 mol/L was not effective in inducing NBT-reducing activity in NB4 cells (Fig 7A). TT slightly induced this activity in NB4 cells in combination with 3 × 10−7 mol/L VD3. On the other hand, 9CTT effectively induced this activity in cells treated with VD3 even at 3 × 10−9 mol/L (Fig7A). TT and 9CTT at 3 × 10−6 mol/L did not inhibit the proliferation of NB4 cells, and VD3 at 3 × 10−7 mol/L inhibited proliferation to 73% of that in the control. TT plus VD3 inhibited proliferation to 40% and 9CTT plus VD3 inhibited proliferation to 19% (Table 4). TT and 9CTT did not induce lysozyme activity, which is a marker of monocytic differentiation, in NB4 cells, but 9CTT enhanced the activity induced by VD3more effectively than TT. VD3 at 3 × 10−7 mol/L increased CD11b expression, but 9CTT decreased the CD11b expression induced by VD3. 9CTT plus VD3 effectively increased CD14 expression in NB4 cells (Table 4). In cells treated with 9CTT plus VD3, the nucleus was slightly indented, nucleoli disappeared, and cytoplasm became abundant and less basophilic and contained some vacuoles. The activity of α-naphthyl acetate esterase was increased in NB4 cells that had been treated with 9CTT plus VD3 (Table 4). These findings indicated that 9CTT plus VD3 induced monocytic differentiation of NB4 cells more effectively than TT plus VD3. 9CTT plus VD3 also effectively induced NBT-reducing activity in HT93 cells, whereas TT plus VD3did not (Fig 7B). TT enhanced the NBT-reducing activity of HL-60 and U937 cells induced by VD3, as previously reported,20 and 9CTT enhanced the activity by VD3 slightly more effectively than TT (Fig 7C and D). The enhancing effect of 9CTT on the differentiation induced by VD3, compared with that of TT, was more prominent in NB4 and HT93 cells than in U937 and HL-60 cells. Therefore, in combination with VD3, 9CTT also selectively induced the differentiation of APL-derived cells.
Effects of the combination of TT or 9CTT with VD3 on the NBT-reducing activity of APL-derived NB4 (A) and HT93 cells (B) and non-APL-derived HL-60 (C) and U937 cells (D). Cells (5 × 104 cells/mL) were treated with VD3 in the absence (•) or presence of 3 × 10−6 mol/L TT (▴) or 9CTT (▪) for 4 days. Values represent the means ± SD of three separate experiments. *P < .05, where the enhancing effects of 9CTT on VD3 were compared with those of TT.
Effects of the combination of TT or 9CTT with VD3 on the NBT-reducing activity of APL-derived NB4 (A) and HT93 cells (B) and non-APL-derived HL-60 (C) and U937 cells (D). Cells (5 × 104 cells/mL) were treated with VD3 in the absence (•) or presence of 3 × 10−6 mol/L TT (▴) or 9CTT (▪) for 4 days. Values represent the means ± SD of three separate experiments. *P < .05, where the enhancing effects of 9CTT on VD3 were compared with those of TT.
Effects of TT and 9CTT in Combination With VD3 on Growth Inhibition and Differentiation of Human Promyelocytic Leukemia NB4 Cells
| Treatment . | Growth Inhibition (% of control) . | Lysozyme (U/106 cells) . | CD11b (%) . | CD14 (%) . | Esterase3-150 (%) . |
|---|---|---|---|---|---|
| None | 100 | 0.20 ± 0.01 | 46 ± 5 | 30 ± 4 | 2 ± 1 |
| TT | 113 ± 2 | 0.06 ± 0.01 | 59 ± 1 | 28 ± 4 | 2 ± 1 |
| 9CTT | 122 ± 4 | 0.06 ± 0.01 | 62 ± 6 | 30 ± 4 | 3 ± 1 |
| VD3 | 73 ± 4 | 0.33 ± 0.08 | 83 ± 1 | 33 ± 4 | 15 ± 3 |
| VD3 + TT | 40 ± 3 | 0.85 ± 0.14 | 82 ± 1 | 38 ± 4 | 16 ± 1 |
| VD3 + 9CTT | 19 ± 3 | 2.65 ± 0.42 | 72 ± 2 | 53 ± 4 | 48 ± 2 |
| Treatment . | Growth Inhibition (% of control) . | Lysozyme (U/106 cells) . | CD11b (%) . | CD14 (%) . | Esterase3-150 (%) . |
|---|---|---|---|---|---|
| None | 100 | 0.20 ± 0.01 | 46 ± 5 | 30 ± 4 | 2 ± 1 |
| TT | 113 ± 2 | 0.06 ± 0.01 | 59 ± 1 | 28 ± 4 | 2 ± 1 |
| 9CTT | 122 ± 4 | 0.06 ± 0.01 | 62 ± 6 | 30 ± 4 | 3 ± 1 |
| VD3 | 73 ± 4 | 0.33 ± 0.08 | 83 ± 1 | 33 ± 4 | 15 ± 3 |
| VD3 + TT | 40 ± 3 | 0.85 ± 0.14 | 82 ± 1 | 38 ± 4 | 16 ± 1 |
| VD3 + 9CTT | 19 ± 3 | 2.65 ± 0.42 | 72 ± 2 | 53 ± 4 | 48 ± 2 |
Cells (5 × 104 cells were incubated with 3 × 10−6 mol/L TT or 9CTT in the absence or presence of 3 × 10−7 mol/L VD3 for 4 days. Values represent the means ± SD of three separate experiments.
α-Naphthyl acetate esterase positive cells were counted.
DISCUSSION
We previously reported the effects of TT on the differentiation of myelomonocytic leukemia cells,20 and in this study we investigated the effects of its Z-isomer, 9CTT. TT and 9CTT are α-tocopherol esters of ATRA and 9CRA, respectively. Although TT has been reported to be stable in vivo and in in vitro treatment with esterase,36 it is still unclear whether 9CTT acts on leukemia cells by being catabolized to 9CRA. Zhu et al30showed that ATRA and 9CRA similarly inhibit the proliferation of APL cells and induce differentiation, with 9CRA being only slightly more potent than ATRA,30 and we confirmed this similarity between ATRA and 9CRA in NB4 and HT93 cells, as shown in Fig 2. On the other hand, 9CTT effectively induced the differentiation of APL-derived cells, whereas TT had little or no effect. In the presence of VD3, a difference was also observed between TT and 9CTT in inducing the differentiation of APL cells. 9CTT enhanced the differentiation induced by 9CRA more effectively than TT on 9CRA or 9CTT on ATRA. These findings suggest that 9CTT acts on leukemia cells differently than 9CRA, although we cannot completely rule out the possibility that a very small amount of 9CTT is converted to 9CRA in the cells. TT increases the migration of guinea pig peritoneal macrophages, whereas ATRA does not, and TT stimulates, whereas ATRA inhibits, the proliferation of human skin fibroblasts.36Thus, α-tocopherol esterification of retinoic acids may produce different biological effects.
Because RAR antagonists inhibited the differentiation of NB4 cells induced by 9CTT, 9CTT may act on the cells through RARs. 9CTT and TT similarly activated RARs when TK-TREpx2-LUC or TK-mRARβx2-LUC was used as a reporter. 9CTT also activated GAL4-RAR chimeric receptors, but less effectively than TT. Because members of the nuclear hormone receptor family, including RARs, interact with several cofactors,37,38 the interaction between a ligand and the receptor complex may change in the GAL4 chimera system. These transient expression experiments showed that 9CTT is an agonist for RARs, but the predominance of 9CTT over TT with regard to their effects on RARs was not observed. TT did not activate RXRs and 9CTT had only a marginal effect on RXRs. α-Tocopherol esterification of 9CRA leads to the loss of its agonistic action on RXRs. The synthetic arotinoids Ro 13-7410 and St80 are potent activators of RARs, but the corresponding Z-isomers (9-cis isomers), Ro 18-8093 and St88, respectively, are less active on RARs and not active at all on RXRs.39Z-Isomerization of RAR agonists may not simply correlate with the acquisition of a response to RXRs. Recently, geranyl geranoic acid and its derivatives were shown to activate RARs and RXRs.40 The relationship between the structure of retinoids and their selectivity for receptors is too complex to be elucidated.
APL cells have a specific fusion protein, PML/RAR-α, which retains the ligand-binding and DNA-binding domains of RAR-α.1,2TT and 9CTT similarly induced the transactivating activity of PML/RAR-α on TK-TREpx2-LUC and TK-mRARβx2-LUC reporters (Table 2). On the other hand, PML/RAR-α exhibited a dominant negative effect on RAR-α under stimulation with ATRA,1,2 and transfection of the pml/RAR-α gene to HL-60 cells makes the cells resistant to ATRA.9 PML/RAR-α inhibited the transactivation induced by TT and 9CTT via RAR-α and RAR-β on TK-TREpx2-LUC and TK-mRARβx2-LUC and via endogenous receptors on TK-βREx3-LUC. Recently, Gianni et al41 reported that Am580 has selective differentiating effects on APL cells and induces the transactivating activity of PML/RAR-α more effectively than ATRA. However, Am580 also induces the differentiation of non-APL HL-60 cells more potently than ATRA.18 In testing 43 retinoids, including ATRA and Am580, for their differentiation-inducing activities in HL-60 and NB4 cells, a good linear correlation was found for these cells, and selectivity of Am580 for NB4 cells was not observed.42 Grignani et al43 showed that, in an experiment using deletion mutants of PML/RAR-α, transactivating activity does not necessarily correlate with the capacity to block differentiation. The fusion protein shows both positive and negative results with regard to transactivation depending on the assay system, including cells, receptors, and reporters. Thus, the induction of transactivation through PML/RAR-α by a ligand, which may appear to be a possible mechanism, is simply not considered to lead to selectivity for APL cells.
9CTT induced transactivation on RAR-responsive reporter as effectively as TT in a transient expression system in CV-1 cells cotransfected with the pml/RAR-α gene and in APL-derived NB4 cells. Recently, PML/RAR-α protein was reported to be downregulated in treatment with ATRA, and it suggests that this is a mechanism for the induction of differentiation in APL cells by ATRA.28,29 We investigated the interaction of 9CTT with PML/RAR-α protein and found that 9CTT diminished the expression of PML/RAR-α protein more effectively than TT. These findings suggest that the destruction of PML/RAR-α protein is important in the selectivity for the induction of differentiation. Esterification of 9CRA with α-tocopherol may disturb its fixing into a binding pocket in the receptor, as suggested from the chemical structure of 9CTT, and could contribute to making PML/RAR-α protein susceptible to degradation. The differentiation of NB4 cells induced by 9CTT was inhibited by RAR antagonists and enhanced by potent RAR agonists. Recently, Chen et al44 reported that arsenic trioxide downregulates PML/RAR-α protein and induces apoptosis of APL cells, but the differentiation of these cells is only partial. These findings suggest that both downregulation of PML/RAR-α protein and RAR activation are necessary to induce the differentiation of APL cells. PML/RAR-α forms a complex with itself, PML, or RXR, and, in addition to the dominant negative effect on RAR, blocks VDR function by sequestering RXR in the cytoplasm.45 The downregulation of PML/RAR-α protein by 9CTT may contribute to its potent enhancement of differentiation induced by VD3 in NB4 and HT93 cells. The interaction of a ligand with a nuclear receptor is very complicated. For example, an RXR ligand influences the function of LXR in the RXR/LXR heterodimer without liganding to LXR, and an RXR antagonist behaves as a phantom ligand for RAR in RXR/RAR.38 46Further studies are required to elucidate the functions of the PML/RAR-α complex and its interaction with various drugs. Although 9CTT activated the transactivation of RAR-responsive promoter in U937 cells as well as in NB4, its effects on differentiation in non-APL cells were much less than those in APL-derived cells. This may be because leukemogenesis in APL cells is mainly due to the abnormal protein PML/RAR-α, whereas that in non-APL cells is due to other mechanisms.
α-Tocopherol esterification of ATRA reduces retinoid-related toxicities, including teratogenicity.36 α-Tocopherol has been reported to ameliorate the toxicity of 13-cis retinoic acid in a trial against myelodysplastic syndrome.47 These findings suggest that the retinoid-related toxicities of 9CTT are weaker than those of ATRA and 9CRA and should be further examined in animal models. U937 cells into which the pml/RAR-α gene was introduced and APL-derived NB4 cells are reportedly resistant to VD3, and this sensitivity is restored in combined treatment with ATRA.48 APL-derived NB4 and HT93 cells are more resistant to VD3 than HL-60 and U937 cells, and 9CTT drastically restored the sensitivity to VD3 in APL cells, whereas TT had little or no additional effect. Furthermore, 9CTT enhanced the differentiation of APL cells induced by other retinoids, such as ATRA, 9CRA, and synthetic retinoids, as shown in Fig 6 and Table 3. 9CTT enhanced the differentiation induced by ATRA and 9CRA more effectively than TT. ATRA is successfully used in the treatment of APL,3,13 and 9CRA has pharmacologic advantages over ATRA because it is ineffective in inducing its own catabolism and it also induces a complete remission in APL patients.49 Am80 and Am580 are strong agonists for RAR-α and RAR-β and induce granulocytic differentiation of HL-60 cells more effectively than ATRA.18,35 Am80 reportedly induced a complete remission in an APL patient relapsing after ATRA treatment and was less toxic on skin than ATRA because it does not interact with RAR-γ.50Am580 is reportedly selective for APL cells,41 and the enhancing effect of 9CTT on the differentiation of NB4 cells by Am580 is interesting. The different mechanisms of Am580, which preferentially activates PML/RAR-α,41 and 9CTT, which downregulates PML/RAR-α protein, may contribute to their synergy in inducing differentiation in APL cells. Ch55 binds to RAR-α, RAR-β, and RAR-γ but not to CRABP, which regulates the serum concentration of ATRA, and induces the differentiation of HL-60 and NB4 cells more effectively than Am80.18,42 Re80 is also a very potent inducer of the differentiation of HL-60 and NB4 cells.18,42Am555s activates RAR-α more effectively than RAR-β and, in addition to its antileukemic activity, was recently reported to inhibit the liver metastasis of intrasplenically transplanted human gastric cancer in nude mice.51 Because these synthetic retinobenzoic acids have strong agonistic activity for RARs, the enhancing effects of 9CTT may not be due to the same agonistic activity for RARs but to other mechanisms such as the downregulation of PML/RAR-α protein. Therefore, 9CTT, alone or in combination with VD3 or other retinoids, may be useful for the treatment of APL.
ACKNOWLEDGMENT
The authors thank Drs Kohei Inomata, Toshihiro Takahashi, and Takao Kishie (Pharmaceutical Research Center, Nisshin Flour Milling Co, Saitama, Japan) for synthesizing and kindly providing TT and 9CTT and Dr Shin-ichi Hayashi (Department of Biochemistry, Saitama Cancer Center Research Institute) for his helpful discussions regarding the electroporation technique.
Supported in part by Grants for Cancer Research from the Ministry of Education, Science, Sports and Culture and from the Ministry of Health and Welfare, Japan.
Address reprint requests to Yoshio Honma, PhD, Department of Chemotherapy, Saitama Cancer Center Research Institute, 818 Komuro, Ina-machi, Kita-adachi, Saitama 362, Japan; e-mail:honma@saitama-cc.go.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 3. Effects of the RAR antagonist LE540 on growth inhibition and differentiation in NB4 cells induced by ATRA, 9CRA, TT, or 9CTT. The effects of LE540 on growth inhibition (A) and NBT-reducing activity (B) in NB4 cells induced by ATRA (6 × 10−9 mol/L [○] or 6 × 10−8 mol/L [•]) or 9CRA (6 × 10−9 mol/L [▵] or 6 × 10−8 mol/L [▴]) were examined. Next, the effects of LE540 on growth inhibition (C) and NBT-reducing activity (D) induced by 9CTT at 6 × 10−6 mol/L (▿) or 9 × 10−6 mol/L (□) were examined. LE540 alone (•) did not affect growth or NBT reduction. *P < .05, compared with values in the absence of LE540 in (A) through (D). The effects of LE540 on CD11b expression that was induced equally by 3 × 10−9 mol/L 9CRA and 6 × 10−6 mol/L 9CTT are shown in (E). *P < .05, compared with 9CRA. Cells (5 × 104 cells/mL) were treated with ATRA, 9CRA, TT, or 9CTT in the absence or presence of LE540 for 4 days. Values represent the means ± SD of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4715/4/m_blod41207003ax.jpeg?Expires=1769175797&Signature=Dzhu-a3zEzwJ6N5vNnWPnYoXMty8B35Xuw5F3WIq~2nAZEDzVyKTS6~6GVrrsnU0rBn1tI99qxPUbeLKKJTLlUCGVPs5ZEA08-6L-6I-96kjC3D9CpdPQxAXYJku3pKBSRQfalvsYj2wdOH9~8TX24MVkjqS7enwOy2yqPWRUPcZ15fR1rkMqd4fbS7EEur87vQRTMcVpoews4q~yTWNkzItg9fgc7TUNTurmxQ80N7ZdM3CpVYXPmHS0HkL2wWuv3mLm9sjYhhuwzunXszlzOIgvWw0j6Hm-sFlHXhGKJtDsIwqCxcDuXZl9a7E-9imBTIuKRlEmhqXxz~DAv-NHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of the RAR antagonist LE540 on growth inhibition and differentiation in NB4 cells induced by ATRA, 9CRA, TT, or 9CTT. The effects of LE540 on growth inhibition (A) and NBT-reducing activity (B) in NB4 cells induced by ATRA (6 × 10−9 mol/L [○] or 6 × 10−8 mol/L [•]) or 9CRA (6 × 10−9 mol/L [▵] or 6 × 10−8 mol/L [▴]) were examined. Next, the effects of LE540 on growth inhibition (C) and NBT-reducing activity (D) induced by 9CTT at 6 × 10−6 mol/L (▿) or 9 × 10−6 mol/L (□) were examined. LE540 alone (•) did not affect growth or NBT reduction. *P < .05, compared with values in the absence of LE540 in (A) through (D). The effects of LE540 on CD11b expression that was induced equally by 3 × 10−9 mol/L 9CRA and 6 × 10−6 mol/L 9CTT are shown in (E). *P < .05, compared with 9CRA. Cells (5 × 104 cells/mL) were treated with ATRA, 9CRA, TT, or 9CTT in the absence or presence of LE540 for 4 days. Values represent the means ± SD of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4715/4/m_blod41207003cx.jpeg?Expires=1769175797&Signature=zAF0xb8k9L1Q2N-fE-8yIiLxPK6OQfzcXO6d8YGdZaYXHC-0yEkMzhBL13z1hCpDl-~AO1JXIWyEAfFehtX3tIBezafIOYDrNqSW7sNR~vLSAmA4xs4YkbhYoPMv8t7bSpSK5HbQOiQTYFOPNRubkWzJ0PAwziUomvmjLtkCT~IaN2NxsiNNQMjR8qLRLXNXbXiqzcvQBk8cDE5cg35CNoGvlNYCfKHnbCBCSedyTPeP682qV7io3rShRnPymuSYu-A56PdPNq3nuL3Lo-UoJRLvbo0mZuHJC9nhrutbYbKlIH8OxhRbptWfD6kqge9P1N3wDkHUgamdHhzv~Xu-TQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of the RAR antagonist LE540 on growth inhibition and differentiation in NB4 cells induced by ATRA, 9CRA, TT, or 9CTT. The effects of LE540 on growth inhibition (A) and NBT-reducing activity (B) in NB4 cells induced by ATRA (6 × 10−9 mol/L [○] or 6 × 10−8 mol/L [•]) or 9CRA (6 × 10−9 mol/L [▵] or 6 × 10−8 mol/L [▴]) were examined. Next, the effects of LE540 on growth inhibition (C) and NBT-reducing activity (D) induced by 9CTT at 6 × 10−6 mol/L (▿) or 9 × 10−6 mol/L (□) were examined. LE540 alone (•) did not affect growth or NBT reduction. *P < .05, compared with values in the absence of LE540 in (A) through (D). The effects of LE540 on CD11b expression that was induced equally by 3 × 10−9 mol/L 9CRA and 6 × 10−6 mol/L 9CTT are shown in (E). *P < .05, compared with 9CRA. Cells (5 × 104 cells/mL) were treated with ATRA, 9CRA, TT, or 9CTT in the absence or presence of LE540 for 4 days. Values represent the means ± SD of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4715/4/m_blod41207003ex.jpeg?Expires=1769175797&Signature=e7qyinbH2dvvYRUwJfdB18xybFOcO5S0icSxQ6Ah8O-a72RsNGf26~Olp-7RK29lRunkkzCs5~hyuqv67tKwoSU7jaFZiVa4iosriWVIFuykAKnKyTrLLNdoShkvqnufIcwwoyeSEQ0IP6hHAau4Ww1coVf4ypGtfAMl8BtYJwnuqHlNi8qPn61wqhDrExEJ3XJD5NcBiUX4~mUZxfr~maNU9Fh2spNM9oSernIjQZYrJ~7DTuLmmiU8~7tjIXiYZFZ9Y9rbTjcjci8jP0yq3f9kuvtX8pgDexyXRyNTvmohwR8yanKgYFEMtmObZZLmvRmLM0v8lB3cP1b81NBeiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal