Abstract
Follicular lymphomas are thought to arise from the follicle center B cells and are characterized by follicular structures that recapitulate many features of normal secondary lymphoid follicles. The neoplastic B cells of follicular lymphoma reside not only in follicles but also in the interfollicular zone in which they form a diffuse infiltrate. We have investigated the frequency, extent, and biological characteristics of this interfollicular component in 30 cases of follicular lymphoma. An interfollicular B-cell infiltrate of variable extent (minimal, moderate, or prominent) was present in all cases. Morphologically interfollicular neoplastic B cells were small centrocyte-like cells with lower grade cytology and lower proliferation fraction compared with the neoplastic follicles. The neoplastic phenotype of these cells (CD20+, light chain restricted) was confirmed in 18 cases. Clonal identity between the follicular and interfollicular components was shown in five cases using microdissection and PCR amplification of immunoglobulin heavy chain genes. Analysis of Ig heavy chain gene sequences showed identical variants of tumor subclones in both follicular and interfollicular compartments, indicating active tumor cell traffic between the two. In six cases in which frozen tissue was available, the immunophenotype of follicular and interfollicular tumor cells were compared using immunohistochemistry. Activation markers such as CD10, CD38, and CD95 and T-cell costimulatory molecules CD80 and CD86, which were expressed by neoplastic follicles, were either downregulated or absent in the interfollicular component in most of the cases. The low-grade cytological features, low proliferation fraction, and downregulation of activation markers in the interfollicular neoplastic B cells suggests that these are resting cells analogous to memory B cells of normal lymphoid tissues. The presence of such a resting tumor cell subpopulation in the majority of follicular lymphomas may partly account for the remarkable resistance to therapy of this disease.
FOLLICULAR LYMPHOMA is the most common variety of non-Hodgkin's lymphoma in the West, accounting for 40% to 50% of all cases in the United States1-4 and 35% in England and Wales.5 Although histologically low grade and clinically indolent, follicular lymphoma is incurable and most patients die of the disease within 7 to 10 years from the time of diagnosis.6-8 Recent attempts to devise successful therapies for follicular lymphoma have focused on ablation of the malignant B-cell clone using both radiotherapy and chemotherapy followed by bone marrow transplantation,7,8 or biological therapy based on antibodies to tumor immunoglobulin or B-cell antigens.9 In either case an understanding of the cell biology of the disease is essential because it is necessary to identify the target (ie, which cells need to be killed). In this context it has long been assumed that the neoplastic cells of follicular lymphoma constitute a homogeneous population of follicle center cells (centrocytes and centroblasts) that reside in neoplastic follicle centers. It is, therefore, these cells that comprise the neoplastic follicles that constitute the therapeutic “target.”
In follicular lymphomas, the presence of a separate interfollicular neoplastic B-cell population that infiltrate in a diffuse pattern between the follicles was first noted by Rappaport.10 The neoplastic nature of these cells was confirmed by Harris et al11,12 using immunohistochemistry to show light chain restriction in cryostat sections. These interfollicular neoplastic B cells should not be confused with the diffuse component that has often been described complicating follicular lymphoma, which is essentially an expression of focal high-grade transformation.13Although this interfollicular population has been recognized for some time, it has never been characterized in any detail. This could be an important omission because it is conceivable that these interfollicular cells may be relevant to the therapeutic resistance of follicular lymphoma. We have, therefore, investigated the frequency, extent, and biological characteristics of the interfollicular component of follicular lymphoma.
MATERIALS AND METHODS
Tissues.
Paraffin-embedded blocks from 30 cases of classical follicular lymphoma were retrieved from the archives of the Department of Histopathology, UCL Medical School, London, UK. Frozen tissue blocks were available in 6 of the cases studied.
Histology.
Routine hematoxylin and eosin (H&E) stained sections were prepared in each case. The morphological features of follicular lymphoma were confirmed and the cytological features of any interfollicular component noted.
Immunohistochemistry.
Immunohistochemistry was performed on both paraffin sections and frozen sections using an avidin-biotin-peroxidase technique preceded by heat retrieval of antigenicity where required.14 The list of antibodies used for immunostaining is shown in Table 1. A sequential double immunostaining technique was used to show proliferating T (Ki67/CD3) and B (Ki67/CD20) cells in frozen sections.15
The Details of the Antibodies Used in Study
| Clone . | Specificity . | Source . | Tissue . |
|---|---|---|---|
| Polyclonal | CD3 | Dako Ltd, High Wycombe, UK | P |
| UCHT1 | CD3 | Dako Ltd | F |
| L26 | CD20 | Dako Ltd | P/F |
| 1F8 | CD21 | Dako Ltd | P/F |
| MIB-1 | Ki-67 antigen | Coulter Electronics Ltd, Luton, UK | P |
| Ki67 | Ki67 antigen | Dako Ltd | F |
| M90 | CD10 | Dako Ltd | F |
| EA-5 | CD38 | Serotec Ltd, Oxford, UK | F |
| BB1 | CD80 | Serotec Ltd | F |
| BU63 | CD86 | Serotec Ltd | F |
| APO-1 | CD95 | Pharmingen, San Diego, CA | F |
| 124 | bcl-2 | Dako Ltd | P |
| R10-21-F3 | Ig κ light chain | Dako Ltd | F |
| N10/2 | Ig λ light chain | Dako Ltd | F |
| Polyclonal | Ig κ light chain | Dako Ltd | P |
| Polyclonal | Ig λ light chain | Dako Ltd | P |
| IgD26 | Ig δ heavy chain | Dako Ltd | F |
| Polyclonal | Ig δ heavy chain | Dako Ltd | P |
| Polyclonal | Ig μ heavy chain | Dako Ltd | P/F |
| Polyclonal | Ig γ heavy chain | Dako Ltd | P/F |
| Polyclonal | Ig α heavy chain | Dako Ltd | P/F |
| Clone . | Specificity . | Source . | Tissue . |
|---|---|---|---|
| Polyclonal | CD3 | Dako Ltd, High Wycombe, UK | P |
| UCHT1 | CD3 | Dako Ltd | F |
| L26 | CD20 | Dako Ltd | P/F |
| 1F8 | CD21 | Dako Ltd | P/F |
| MIB-1 | Ki-67 antigen | Coulter Electronics Ltd, Luton, UK | P |
| Ki67 | Ki67 antigen | Dako Ltd | F |
| M90 | CD10 | Dako Ltd | F |
| EA-5 | CD38 | Serotec Ltd, Oxford, UK | F |
| BB1 | CD80 | Serotec Ltd | F |
| BU63 | CD86 | Serotec Ltd | F |
| APO-1 | CD95 | Pharmingen, San Diego, CA | F |
| 124 | bcl-2 | Dako Ltd | P |
| R10-21-F3 | Ig κ light chain | Dako Ltd | F |
| N10/2 | Ig λ light chain | Dako Ltd | F |
| Polyclonal | Ig κ light chain | Dako Ltd | P |
| Polyclonal | Ig λ light chain | Dako Ltd | P |
| IgD26 | Ig δ heavy chain | Dako Ltd | F |
| Polyclonal | Ig δ heavy chain | Dako Ltd | P |
| Polyclonal | Ig μ heavy chain | Dako Ltd | P/F |
| Polyclonal | Ig γ heavy chain | Dako Ltd | P/F |
| Polyclonal | Ig α heavy chain | Dako Ltd | P/F |
Abbreviations: P, paraffin sections; F, frozen sections; Ig, immunoglobulin.
The extent of the interfollicular neoplastic (CD20+, IgD−) B-cell component was estimated in serial paraffin sections and confirmed where possible in serial sections stained for immunoglobulin (Ig) light chains. In two cases in which the tumor cells expressed IgD, the extent of the neoplastic interfollicular component was estimated in sections stained for Ig light chains. The interfollicular component was estimated as minimal (+) when less than 10% of all neoplastic B cells were in the interfollicular zone, moderate (++) when between 10% to 30% of all neoplastic B cells were in the interfollicular zone, and prominent (+++) when more than 30% of all neoplastic B cells were in the interfollicular zone.
The proliferation fraction of follicular and interfollicular components was determined semiquantitatively by estimating the percentage of cells expressing the Ki67 antigen in respective compartments as follows: (−), if there is no expression of Ki67; (+/−), if 0% to 5% of the cells express Ki67; (+), if 5% to 15% of the cells express Ki67; (++), if 15% to 30% of the cells express Ki67; and (+++), if more than 30% of the cells express Ki67.
Molecular genetics.
The Ig genes were polymerase chain reaction (PCR) amplified from framework (Fr)2 or Fr3 to the joining (Jh) region with a seminested PCR protocol16 using DNA extracted from microdissected follicles and interfollicular tissue identified by appropriate immunostaining of cryostat sections with either CD10, CD21, or both. Microdissection was performed using the method of Pan et al.17 All PCR reactions were performed using a hot start procedure18 and appropriate positive (a B-cell lymphoma) and negative controls (without template DNA) were included in each experiment. All samples were analyzed in duplicate.
PCR products were purified on Sephacryl S-400 MicroSpin columns (Pharmacia, St Albans, UK), ligated to TA cloning vector pCR II, and transfected into One Shot competent cells (INVaF') according to the manufacturer's protocol (Invitrogen, Leek, The Netherlands). The transfected cells were plated onto LB-ampicillin agar plates containing X-gal. White colonies were transferred to a fresh LB-ampicillin agar plate containing X-gal and grown overnight for secondary selection. Confirmed white colonies were then transferred into 150 μL of LB medium containing 50 g/mL ampicillin and cultured at 37°C for 4 hours. The cultures (100 μL) were centrifuged, resuspended in 20 μL of water, and heated at 98°C for 10 minutes. After centrifugation, the resulting supernatants were used for PCR with vector primers Sp6 and T7.
The PCR products showing the expected insert size were sequenced using an ABI sequencer with dye terminators (Perkin Elmer, Warrington, UK). In each case, at least six PCR clones from each follicular or interfollicular cell population were sequenced. All sequencing was performed in both orientations.
The identification of Vh and Jh germline sequences was performed by sequence comparison with the V BASE, which is a comprehensive database of human immunoglobulin germline gene sequences compiled from the published sequences by Tomlinson,19 using online DNAPLOT. Mutations in Vhwere identified by comparing the tumor sequence with the closest published germlines, whereas mutations in the CDR3 region were recorded according to the most closely related PCR clone.
RESULTS
The majority of follicular lymphomas contain an Ig light chain restricted neoplastic B-cell population in the interfollicular zone.
Examination of the H&E stained sections from 30 cases of follicular lymphoma showed that all cases contained varying degrees of interfollicular tissue between the neoplastic follicles (Fig 1A, C, and E). Cytologically, this tissue comprised small lymphocytes and a variable population of slightly larger cells with dense euchromatic nuclei showing slight to moderate irregularity of their nuclear outline (Fig 1G and H). These cells resembled the intrafollicular small centrocytes (small cleaved cells) but were, nevertheless, cytologically distinctive. Only occasional centroblasts (large noncleaved cells) were present.
Interfollicular neoplastic B cells in follicular lymphoma. (A-F) Three cases of follicular lymphoma with minimal (case 8; A, B), moderate (case 13; C, D), or prominent (case 2; E, F) CD20+ interfollicular neoplastic B-cell component. (G, H) High-power view of follicular (G) and interfollicular (H) components showing lower-grade cytology of interfollicular cells in case 13. (A, C, E, G, and H, H&E; B, D, and F, immunoperoxidase)
Interfollicular neoplastic B cells in follicular lymphoma. (A-F) Three cases of follicular lymphoma with minimal (case 8; A, B), moderate (case 13; C, D), or prominent (case 2; E, F) CD20+ interfollicular neoplastic B-cell component. (G, H) High-power view of follicular (G) and interfollicular (H) components showing lower-grade cytology of interfollicular cells in case 13. (A, C, E, G, and H, H&E; B, D, and F, immunoperoxidase)
A significant proportion of the interfollicular cells were CD20+ (Table 2 and Fig 1B, D, and F) including a minor component of IgD+ small lymphocytes (mantle zone cells) around follicles and scattered in the interfollicular zone (Fig 2A and B). Immunostaining for Ig light chains showed identical light chain restriction in the follicular and interfollicular compartments in 18 cases (68%) including 2 cases in which the neoplastic cells were IgD+ (Fig 2C and D). The extent of interfollicular B-cell component varied between the cases (Table 2). It was minimal in 3 cases (Fig 1A and B), moderate in 15 cases (Fig 1C and D), and prominent in 12 cases (Fig 1E and F).
Results of Paraffin Immunohistochemistry
| Case . | BCL-2* . | CD20† . | Light Chain Restriction‡ . | Ki671-153 . | ||
|---|---|---|---|---|---|---|
| F . | IF . | F . | IF . | |||
| 1 | + | +++ | κ | κ | +++ | + |
| 2 | + | +++ | λ | λ | +++ | +/− |
| 3 | + | +++ | κ | κ | ++ | +/− |
| 4 | + | ++ | λ | λ | ++ | +/− |
| 5 | + | ++ | λ | λ | ++ | +/− |
| 6 | + | +++ | λ | λ | ++ | +/− |
| 7 | + | ++ | λ | κ, λ | ++ | − |
| 8 | + | + | κ | − | + | − |
| 9 | + | ++ | us | us | us | us |
| 10 | + | +++ | λ | κ, λ | ++ | + |
| 11 | + | +++ | λ | κ, λ | ++ | + |
| 12 | + | +++ | − | − | +++ | +/− |
| 13 | + | ++ | κ | κ | ++ | +/− |
| 14 | + | +++ | κ | κ | +++ | +/− |
| 15 | + | ++ | λ | κ, λ | +++ | +/− |
| 16 | + | +++ | κ | κ | ++ | +/− |
| 17 | + | ++ | κ | κ | ++ | ++ |
| 18 | + | +++ | κ | κ | ++ | +/− |
| 19 | + | +++ | κ | κ | ++ | +/− |
| 20 | + | ++ | κ | κ | +++ | + |
| 21 | + | ++ | κ | κ | ++ | + |
| 22 | + | +++ | us | us | +++ | + |
| 23 | + | ++ | κ | κ | ++ | + |
| 24 | + | + | κ | − | + | − |
| 25 | + | ++ | λ | PCλ | us | us |
| 26 | + | ++ | λ | λ | + | − |
| 27 | + | ++ | us | us | ++ | + |
| 28 | + | ++ | κ | κ | ++ | +/− |
| 29 | + | ++ | κ | κ | ++ | +/− |
| 30 | + | + | κ | − | ++ | + |
| Case . | BCL-2* . | CD20† . | Light Chain Restriction‡ . | Ki671-153 . | ||
|---|---|---|---|---|---|---|
| F . | IF . | F . | IF . | |||
| 1 | + | +++ | κ | κ | +++ | + |
| 2 | + | +++ | λ | λ | +++ | +/− |
| 3 | + | +++ | κ | κ | ++ | +/− |
| 4 | + | ++ | λ | λ | ++ | +/− |
| 5 | + | ++ | λ | λ | ++ | +/− |
| 6 | + | +++ | λ | λ | ++ | +/− |
| 7 | + | ++ | λ | κ, λ | ++ | − |
| 8 | + | + | κ | − | + | − |
| 9 | + | ++ | us | us | us | us |
| 10 | + | +++ | λ | κ, λ | ++ | + |
| 11 | + | +++ | λ | κ, λ | ++ | + |
| 12 | + | +++ | − | − | +++ | +/− |
| 13 | + | ++ | κ | κ | ++ | +/− |
| 14 | + | +++ | κ | κ | +++ | +/− |
| 15 | + | ++ | λ | κ, λ | +++ | +/− |
| 16 | + | +++ | κ | κ | ++ | +/− |
| 17 | + | ++ | κ | κ | ++ | ++ |
| 18 | + | +++ | κ | κ | ++ | +/− |
| 19 | + | +++ | κ | κ | ++ | +/− |
| 20 | + | ++ | κ | κ | +++ | + |
| 21 | + | ++ | κ | κ | ++ | + |
| 22 | + | +++ | us | us | +++ | + |
| 23 | + | ++ | κ | κ | ++ | + |
| 24 | + | + | κ | − | + | − |
| 25 | + | ++ | λ | PCλ | us | us |
| 26 | + | ++ | λ | λ | + | − |
| 27 | + | ++ | us | us | ++ | + |
| 28 | + | ++ | κ | κ | ++ | +/− |
| 29 | + | ++ | κ | κ | ++ | +/− |
| 30 | + | + | κ | − | ++ | + |
See Materials and Methods for details of semiquantitative assessment of immunohistochemical reactivity.
Abbreviations: F, follicular component; IF, interfollicular component; us, immunohistochemical staining was unsatisfactory.
Bcl-2 immunostaining, (+), tumor expresses bcl-2; (−), tumor is bcl-2 negative.
CD20 immunostaining, the extent of CD20 positive interfollicular B cells; (+) minimal, (++) moderate, (+++) prominent.
Light chain restriction, κ, Ig κ light chain restriction; λ, Ig λ light chain restriction; κ and λ, no light chain restriction, polyclonal; (−) no light chain staining; PCλ, plasma cells show λ light chain restriction.
MIB-1 immunostaining, +++, ++, +, +/−, − indicate the number of cells expressing Ki67 antigen in follicular and interfollicular zones.
Immunophenotype of interfollicular neoplastic B cells in paraffin sections of follicular lymphoma. (A-F) Serial sections of a follicular lymphoma (case 13) immunostained for CD20 (A), IgD (B), Ig κ light chain (C), Ig λ light chain (D), Ki67 (E), and bcl-2 protein (F). There are numerous CD20+ B cells both in the follicles and the interfollicular zone. IgD+ cells are concentrated in a narrow follicular mantle and scattered in the interfollicular area. There is Ig κ light chain restriction both in the follicular and interfollicular B cells showing the neoplastic nature of both cell populations. Immunostaining for Ki67 antigen shows that follicular neoplastic B cells have a much higher proliferation fraction than the interfollicular component (E) and Bcl-2 immunostaining shows that bcl-2 is expressed by both follicular and interfollicular neoplastic B cells (F). (A-F, immunoperoxidase)
Immunophenotype of interfollicular neoplastic B cells in paraffin sections of follicular lymphoma. (A-F) Serial sections of a follicular lymphoma (case 13) immunostained for CD20 (A), IgD (B), Ig κ light chain (C), Ig λ light chain (D), Ki67 (E), and bcl-2 protein (F). There are numerous CD20+ B cells both in the follicles and the interfollicular zone. IgD+ cells are concentrated in a narrow follicular mantle and scattered in the interfollicular area. There is Ig κ light chain restriction both in the follicular and interfollicular B cells showing the neoplastic nature of both cell populations. Immunostaining for Ki67 antigen shows that follicular neoplastic B cells have a much higher proliferation fraction than the interfollicular component (E) and Bcl-2 immunostaining shows that bcl-2 is expressed by both follicular and interfollicular neoplastic B cells (F). (A-F, immunoperoxidase)
Immunostaining also showed numerous CD3+ small lymphocytes (T cells) in the interfollicular zone with a CD4:CD8 ratio of approximately 4 to 3:1. Both T-cell subsets were also present within neoplastic follicle centers in which CD4+ cells predominated.
The proliferation fraction determined with the antibody MIB-1 was significantly higher in the follicles, although lower than expected for reactive follicles, than in the interfollicular compartment (Table 2and Fig 2E). Double immunohistochemical staining with CD20 or CD3 and MIB-1 showed that in contrast to the follicles, the majority of cells expressing the Ki67 antigen in the interfollicular compartment were T cells. In all cases both follicular and interfollicular components showed bcl-2 expression (Table 2 and Fig 2F).
The neoplastic B cells in the interfollicular zone have a resting phenotype compared with the activated phenotype of follicular neoplastic B cells.
The expression of cell surface molecules that are known to be induced in normal follicle center B cells were examined in frozen sections of six cases, all of which contained a light chain restricted interfollicular neoplastic B-cell component (Table 3 and Fig 3A, C and D). CD10 was expressed by the follicular component in all six cases but was either downregulated or absent in the interfollicular component in four of the six cases (Table 3 and Fig 3B). CD38 was expressed by the neoplastic follicles in all six cases and downregulated in the interfollicular B-cell component in five (Table 3 and Fig 3E). T cells both in the interfollicular and follicular compartments were also labelled with the CD38 antibody. Staining for CD95 (fas antigen) showed weak expression in neoplastic follicles in all cases. The interfollicular B cells were CD95 negative in 4 cases but stained with similar intensity to the follicles in the remaining 2 cases (Table 3 and Fig 3F). The T-cell costimulatory molecules CD80 and CD86 were both expressed, albeit weakly, in the neoplastic follicles in all cases. In the interfollicular component the expression of CD80 was either downregulated or absent in 5 cases whereas CD86 was downregulated in all cases (Table 3 and Fig 3G and H). In some cases the dendritic cells in the interfollicular zone expressed CD80 and CD86.
Differences in the Intensity of Staining in Interfollicular Component Compared With Follicular Component in Six Cases in Which Frozen Tissue Was Available
| Case . | Tumor Ig . | CD10 . | CD38 . | CD95 . | CD80 . | CD86 . |
|---|---|---|---|---|---|---|
| 1 | IgG/κ | ↓ | ↓ | ↓ | ↓ | ↓ |
| 2 | IgG/λ | = | ↓ | ↓ | ↓ | ↓ |
| 3 | IgM, IgD/κ | = | ↑ | = | ↓ | ↓ |
| 4 | IgM, IgD/λ | ↓ | ↓ | = | ↓ | ↓ |
| 5 | IgG/λ | ↓ | ↓ | ↓ | ↓ | ↓ |
| 6 | IgG/λ | ↓ | ↓ | ↓ | = | ↓ |
| Case . | Tumor Ig . | CD10 . | CD38 . | CD95 . | CD80 . | CD86 . |
|---|---|---|---|---|---|---|
| 1 | IgG/κ | ↓ | ↓ | ↓ | ↓ | ↓ |
| 2 | IgG/λ | = | ↓ | ↓ | ↓ | ↓ |
| 3 | IgM, IgD/κ | = | ↑ | = | ↓ | ↓ |
| 4 | IgM, IgD/λ | ↓ | ↓ | = | ↓ | ↓ |
| 5 | IgG/λ | ↓ | ↓ | ↓ | ↓ | ↓ |
| 6 | IgG/λ | ↓ | ↓ | ↓ | = | ↓ |
Abbreviations: Ig, immunoglobulin; ↓, expression downregulated; =, no difference; ↑, expression upregulated.
Immunophenotype of interfollicular neoplastic B cells in frozen sections of follicular lymphoma. (A-F) Serial sections of case 6 immunostained for CD20 (A), CD10 (B), Ig κ light chain (C), Ig λ light chain (D), CD38 (E), and CD95 (F). The interfollicular zone contains an Ig λ light chain restricted B-cell population. CD10, CD38, and CD95 are expressed by the neoplastic follicle but not by the neoplastic B cells of the interfollicular zone. (G,H) Sections of case 1 immunostained for CD80 (G) and case 2 for CD86 (H) show that CD80 and CD86 are expressed in the follicles but are downregulated in the interfollicular zone. (A-H, immunoperoxidase)
Immunophenotype of interfollicular neoplastic B cells in frozen sections of follicular lymphoma. (A-F) Serial sections of case 6 immunostained for CD20 (A), CD10 (B), Ig κ light chain (C), Ig λ light chain (D), CD38 (E), and CD95 (F). The interfollicular zone contains an Ig λ light chain restricted B-cell population. CD10, CD38, and CD95 are expressed by the neoplastic follicle but not by the neoplastic B cells of the interfollicular zone. (G,H) Sections of case 1 immunostained for CD80 (G) and case 2 for CD86 (H) show that CD80 and CD86 are expressed in the follicles but are downregulated in the interfollicular zone. (A-H, immunoperoxidase)
Analysis of rearranged Ig heavy chain genes confirms the clonal identity of interfollicular and follicular components.
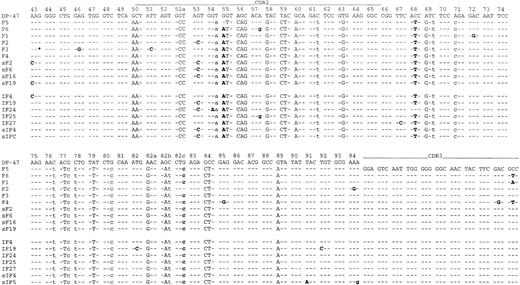
The clonal identity of follicular and interfollicular components was confirmed by analysis of rearranged Ig heavy chain genes from Fr2 to Jh with PCR amplification in 5 of the 6 cases in which frozen material was available. PCR amplification of microdissected fragments from follicular and interfollicular areas showed identical-sized dominant bands from either site in each of these cases. In case 5, the rearranged Ig heavy chain gene could not be amplified with either Fr2 or Fr3 primers. The PCR products from cases 1, 2, and 3 were cloned and sequenced. In each case the dominant clone from either site had the same CDR3 sequence (Fig 4).
Intraclonal Ig sequence variations in case 1. Identity with the germline sequence is shown by dashes, replacement mutations are shown by uppercase letters, and silent mutations are shown by lowercase letters. Sequence variations are highlighted in bold. The primer sequences are not included. F, follicular neoplastic B cells; IF, interfollicular neoplastic B cells. s prefix indicates clones analyzed from a separate tissue block; and * indicates nucleotide deletions.
Intraclonal Ig sequence variations in case 1. Identity with the germline sequence is shown by dashes, replacement mutations are shown by uppercase letters, and silent mutations are shown by lowercase letters. Sequence variations are highlighted in bold. The primer sequences are not included. F, follicular neoplastic B cells; IF, interfollicular neoplastic B cells. s prefix indicates clones analyzed from a separate tissue block; and * indicates nucleotide deletions.
Mutation analysis indicates active traffic between follicular and interfollicular compartments.
In each of the three cases sequenced, cell populations of both follicular and interfollicular compartments showed frequent intraclonal Ig gene sequence variations (Fig 4, Table4), which were well above the PCR error rate (<0.2%) in the system used. There was no significant difference in the frequency of intraclonal variations between the two neoplastic cell populations (Table 4). In addition, identical variants of the tumor subclones were observed between the two compartments in each case, such as follicular clones sF6, sF16, and interfollicular clone sIF4, as well as follicular clone sF19 and interfollicular clone IF4 in case 1 (Fig 4 ). Similar results were observed when cell populations of follicular and interfollicular compartments from independent blocks were examined (performed in case 1; Fig 4).
Frequency of Intraclonal Sequence Variations in Follicular (F) and Interfollicular (IF) Compartments
| Case . | F . | IF . |
|---|---|---|
| 1 | 2.27% (10) | 1.80% (7) |
| 2 | 0.79% (4) | 0.97% (6) |
| 3 | 2.48% (6) | 2.82% (6) |
| Case . | F . | IF . |
|---|---|---|
| 1 | 2.27% (10) | 1.80% (7) |
| 2 | 0.79% (4) | 0.97% (6) |
| 3 | 2.48% (6) | 2.82% (6) |
The number in brackets indicates the total tumor clones analyzed.
DISCUSSION
This study shows that significant numbers of B cells are present between the follicles in most cases of follicular lymphoma. Because these cells express the same light chain and harbor the same rearranged Ig heavy chain gene as the neoplastic follicle center cells, they are clearly part of the same neoplastic clone. These interfollicular tumor cells are dispersed diffusely between the neoplastic follicles, without disturbing the overall follicular architecture of the tumor, and often constitute a substantial component of the lymphoma. This together with the observation of identical variants of tumor subclones in both follicular and interfollicular compartments, indicates that there is tumor cell traffic between the two compartments. Because there is no difference in the degree of intraclonal sequence variation between the two compartments and somatic mutations of the Ig gene are introduced only in the follicle,20 the tumor cell traffic must be active. Thus, it appears that the tumor cells, which originate from the follicle center B cells, constantly travel from the follicle to the interfollicular region, then return back to the follicle. Similar events are also observed in other low-grade B-cell lymphomas such as the lymphomas of mucosa-associated lymphoid tissue (MALT).21 22 The neoplastic B cells of MALT lymphomas normally diffusely infiltrate between the reactive follicles characteristically present in this disease and have the phenotypic and genotypic features of postfollicular B cells. However, they may selectively colonize reactive follicle centers and can closely resemble follicle center B cells. This interaction between the two compartments in both follicular lymphoma and MALT lymphoma may represent an important mechanism underlying the tumor progression.
Despite the presence of active traffic between the follicular and interfollicular compartments, the phenotypic features remain distinct between the follicular and interfollicular neoplastic cell populations. The interfollicular tumor cells are small- to medium-sized cells that resemble the follicle center centrocytes, but are cytologically of lower grade. Importantly, this population is not related to the diffuse component of so-called mixed follicular and diffuse lymphoma in which the diffuse component is usually composed of cytologically higher-grade cells.13
When compared with the cells comprising the neoplastic follicles, the immunophenotype of the interfollicular cells is consistent with their lower cytological grade and, in keeping with this, significantly fewer of these cells are in cell cycle. The immunophenotype of the neoplastic interfollicular cells is similar to that of a subpopulation of postfollicular B cells observed outside the follicles in normal lymphoid tissue.8 23-25 Like the neoplastic interfollicular cells these have downregulated expression of follicle center activation markers such as CD10, CD38, and CD95. They are thought to be memory B cells that have differentiated from the follicle center B cells. It is possible, therefore, that the interfollicular neoplastic B cells in follicular lymphoma are an analogous population that is the result of differentiation of neoplastic follicle center cells. It appears that differentiation towards a cell with a more mature phenotype and a lower proliferation fraction is a normal occurrence in follicular lymphoma.
Distinct phenotypic differences between the follicular and interfollicular components suggests that responses of these two cell populations to various treatment regimes could be different. The interfollicular neoplastic B cells are likely to be less sensitive to chemotherapy and radiotherapy than the intrafollicular cells with their larger nuclei and higher proliferation fraction.26 Based on clinical observations, Longo27 has suggested that there are two tumor cell populations in follicular lymphoma with different sensitivities to chemotherapy and radiotherapy. Our study supports this notion but suggests that the two populations are comprised of follicular and interfollicular cells rather than small and large intrafollicular cells (centrocytes and centroblasts) as proposed by Longo.
Downregulation of expression of T-cell costimulatory molecules, CD80 and CD86, in tumor cells has been proposed as a possible mechanism developed by follicular lymphomas to escape immune surveillance.25 Expression of CD80 and CD86 is even lower in the interfollicular component than the follicular component, which suggests that the interfollicular tumor cells are likely to be even more resistant to natural or induced T-cell mediated antitumor immunity than their follicular counterparts.
Given that there is a two-way traffic of neoplastic B cells between follicular and interfollicular compartments as shown by the analysis of Ig heavy chain genes, then it is likely that the interfollicular tumor cells have the capacity to acquire an activated phenotype when the conditions are right. Thus, the interfollicular component could provide a reservoir of tumor cells for the progression of the follicular lymphoma after treatment, which may account for the remarkable therapeutic resistance of this disease. Conceivably, it is these cells that constitute the optimum target for the treatment of follicular lymphoma.
Supported by the Cancer Research Campaign and the Leukaemia Research Fund.
Address correspondence to Professor Peter G. Isaacson, Department of Histopathology, UCL Medical School, Rockefeller Building, University St, London WC1E 6JJ, UK; e-mail: p.isaacson@ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal