Abstract
Recent studies showed that gallium and iron uptake are decreased in gallium-resistant (R) CCRF-CEM cells; however, the mechanisms involved were not fully elucidated. In the present study, we compared the cellular uptake of 59Fe-transferrin (Tf) and59Fe-pyridoxal isonicotinoyl hydrazone (PIH) to determine whether the decrease in iron uptake by R cells is caused by changes in Tf receptor (TfR)-dependent or TfR-independent iron uptake. We found that both 59Fe-Tf and 59Fe-PIH uptake were decreased in R cells. The uptake of 59Fe-Tf but not59Fe-PIH could be blocked by an anti-TfR monoclonal antibody. After 59Fe-Tf uptake, R cells released greater amounts of 59Fe than gallium-sensitive (S) cells. However, after 59Fe-PIH uptake 59Fe release from S and R cells was similar. 125I-Tf exocytosis was greater in R cells. At confluency, S and R cells expressed equivalent amounts of TfR; however, at 24 and 48 hours in culture, TfR expression was lower in R cells. Our study suggests that the decrease in Tf-Fe uptake by R cells is caused by a combination of enhanced iron efflux from cells and decreased TfR-mediated iron transport into cells. Furthermore, because TfR-dependent and -independent iron uptake is decreased in R cells, both uptake systems may be controlled at some level by similar regulatory signal(s).
GALLIUM NITRATE IS A group-IIIa metal salt in clinical use for the treatment of hypercalcemia and certain malignancies.1,2 As an antineoplastic agent, gallium has significant activity against bladder cancer and lymphoma.3-8 Recent investigations have shown that the mechanism of cytotoxicity of gallium includes perturbation of iron-dependent cell proliferation, including inhibition of ribonucleotide reductase, an iron-containing enzyme responsible for deoxyribonucleotide synthesis.9-11
Malignant lymphoid cells in vitro and in animal tumor models are uniformly sensitive to growth inhibition by gallium.2However, clinical studies have shown that 40% to 50% of patients with relapsed lymphoma respond to treatment with gallium nitrate whereas the remainder have disease that is resistant to gallium.8 In an attempt to understand why certain lymphomas and other malignancies are relatively resistant to the cytotoxicity of gallium, investigation in our laboratory has focused on elucidating the biological changes that tumor cells undergo during the development of drug resistance to gallium. Recently, we reported that human lymphoid leukemic CCRF-CEM cells with acquired resistance to gallium (R cells) have a decrease in their uptake of gallium, suggesting that drug resistance to gallium involves a downregulation of gallium transport into cells.12
Gallium binds avidly to the iron transport protein transferrin (Tf)13 and the cellular uptake of gallium closely parallels that of iron.14-17 The uptake of iron and gallium by cells occurs by Tf receptor (TfR)-mediated endocytosis of Tf-Fe or Tf-Ga. Inside the cell, the TfR-ligand complex translocates to an acidic endosome where iron/gallium dissociates from Tf and trafficks out of the endosome. The receptor-apoTf (metal-free) complex then recycles back to the cell surface where Tf is released to the exterior.18-20 In addition to TfR-mediated uptake, certain cells can acquire iron and gallium (as low molecular weight chelates) through a Tf-independent uptake system.21-25
Our recent investigation showed that in addition to the decrease in gallium uptake, R cells also have a decrease in their uptake of iron.12 However, the mechanisms responsible for this decrease in gallium/iron uptake remained to be determined. In the present study, we have investigated the steps involved in the transport of iron into R cells to determine whether the previously observed downregulation of iron uptake by these cells is caused by changes in TfR-dependent or -independent iron uptake pathways. We show that both Tf-Fe and non-Tf iron uptake are downregulated in R cells and that this is associated with changes in TfR synthesis and cycling and the egress of iron from cells.
MATERIALS AND METHODS
Gallium nitrate was obtained from Alpha Aesar (Ward Hill, MA). Human Tf (substantially iron free), pronase and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co (St Louis, MO). [35S]methionine was obtained from Dupont (Wilmington, DE). 59FeCl3 and 125I-Na were obtained from Amersham (Arlington Heights, IL). 59Fe-Tf was prepared as described by Bates and Schlabach,26 whereas125I-Tf was prepared by the Chloramine T method.27 Pyridoxal isonicotinoyl hydrazone (PIH) and59Fe-PIH were prepared as described by Ponka.28Monoclonal antibody (MoAb) 42/6 and rabbit antiserum against the human TfR were generously provided by Ian Trowbridge (The Salk Institute) and Caroline Enns (Oregon Health Sciences University).
Cells.
Human T lymphoblastic leukemic CCRF-CEM cells (gallium-sensitive or S cells) were obtained from American Type Culture Collection (Rockville, MD) and were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; complete medium) in an atmosphere of 6% CO2. A gallium-resistant CCRF-CEM cell line (R cells) was developed from the parent line through a process of continuous exposure of cells to increasing concentrations of gallium nitrate over a course of several months. R cells were grown either in medium containing 150 μmol/L gallium nitrate (R1 cells) or in medium without gallium nitrate (R2 cells). R2 cells displayed a stable gallium-resistant phenotype even in the absence of gallium.
Cell growth experiments.
Cell growth in the presence and absence of gallium was determined by MTT assay as previously described by Mosmann29 or by counting cells directly with a hemocytometer. For the MTT assay, cells grown to confluency were plated at an initial density of 2 × 105 cells/mL in 96-well microwell plates and incubated for 72 hours in the presence of 0 to 1,000 μmol/L gallium nitrate. At the end of the incubation, 10 μL of MTT (5 mg/mL stock solution) was added to each well and the cells were incubated at 37°C for an additional 4 hours. Cells were then solubilized by the addition of 100 μL of 0.04 N HCl in isopropanol to each well, and the absorbance of each well was determined spectrophotometrically at dual wave length 570/630 nm by using an EL 310 microplate auto-reader (Biotech Instruments, Winooski, VT). The absorbance of the wells containing gallium nitrate was compared with that of wells in which the drug was omitted. The growth rate of S, R1, and R2 cells in the absence of gallium nitrate was also compared by counting cells after 24, 48, and 72 hours of growth.
Uptake of 59Fe by cells.
59Fe uptake studies were performed by using either59Fe-Tf or 59Fe-PIH. S, R1, and R2 cells in growth phase (after 24 or 48 hours of incubation in fresh medium) or in confluent/stationary phase (after 72 hours of incubation in medium) were washed twice with medium and replated (0.5 × 106cells/mL) in 1-mL 24-well plates in complete medium or serum-free medium. 59Fe-Tf or 59Fe-PIH was added to each well as specified in the figure legends and incubation continued for 3 to 24 hours. Because of potential loss of cell viability in serum-free medium, uptake times for studies in this medium did not exceed 24 hours. In certain experiments, 59Fe uptake was performed in the presence of 10 μg/mL of MoAb 42/6. At specified times, cells were removed from the wells, washed twice by centrifugation with ice-cold phosphate-buffered saline (PBS) and 59Fe cpm in the cell pellet was determined using a Wallac Compugamma gamma counter (Wallac Inc, Gaithersburg, MD).
Release of 59Fe from cells.
S, R1, and R2 cells (106 cells/mL) were incubated in complete medium with 59Fe-Tf (4 μg/mL Tf, 5.9 ng Fe/mL, 28,000 59Fe cpm/mL) for 1 or 3 hours at 37°C in a CO2 incubator. At the end of the incubation, an aliquot of cell suspension was removed and centrifuged, and the amount of59Fe taken up by cells was determined. The remaining cells were washed twice by centrifugation with ice-cold PBS to remove unincorporated 59Fe-Tf and suspended in the original volume of fresh complete medium without 59Fe-Tf (release medium). These cells were then reincubated in tissue culture flasks at 37°C. At specified times, aliquots of cell suspension were removed and centrifuged. The radioactivity in the cell pellet and supernatant (medium) was counted to determine the fraction of 59Fe released from cells. In additional experiments, 59Fe uptake and release conditions were similar except that after the59Fe uptake step, cells were incubated with pronase (150 μg/mL) for 20 minutes at 4°C to remove surface-bound59Fe before reincubation in fresh medium. 59Fe release by cells after uptake of 59Fe-PIH was also examined. The experimental conditions were similar to those described for 59Fe-Tf except that 59Fe-PIH uptake was performed in serum-free medium over 3 hours, whereas the release medium was supplemented with 1% FCS.
125I-Tf binding.
Cellular TfR expression in cells was determined by an125I-Tf binding assay as previously described.30 S and R1 cells were harvested after incubation in medium for 24, 48, and 72 hours in the absence of gallium. Cells were washed with PBS containing 0.1% bovine serum albumin and assayed for 125I-Tf binding at 4°C. Maximum Tf binding was determined according to the method of Scatchard.31
TfR synthesis.
Cellular TfR synthesis was examined as described by Rutledge.32 S, R1, and R2 cells (5 × 105/mL) were incubated for 3 or 20 hours with 10 μC/mL [35S]methionine in methionine-free RPMI 1640 medium supplemented with 5% FCS. Cells were washed with PBS and lysed in 10 mmol/L Tris pH 7.4/150 mmol/L NaCl/5 mmol/L EDTA buffer containing 1% Triton X-100. Cell lysates were preadsorbed with 50 μLStaphylococcus aureus cells (Pansorbin cells; Calbiochem, La Jolla, CA) at 4°C for 1 hour. Pansorbin cells were then removed by centrifugation and the supernatant containing the radiolabeled TfR was immunoprecipitated by incubation with 1.4 μL of anti-TfR antiserum and 25 μL fresh Pansorbin cells. Pansorbin cells with receptor-antibody complexes bound to it were washed extensively and finally resuspended in 2× Laemmli sample buffer.33The sample was heated in a boiling waterbath, it was centrifuged to remove the Pansorbin cells, and the supernatant was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Autoradiography of the gel was performed by exposing the dried gel to XAR-5 film (Eastman Kodak, Co, Rochester, NY) with intensifying screens at −70°C for 24 to 48 hours.
125I-Tf internalization and release.
The kinetics of internalization of cell surface TfR-bound125I-Tf and the release of internalized 125I-Tf was examined by using a modification of a previously described method.18 For the Tf internalization experiments, 107 cells were harvested at confluency, washed with PBS-BSA, and incubated at 4°C for 60 minutes in 100 μL of the same buffer with 138 ng 125I-Tf (approximately 6,200 cpm/ng Tf) to allow for ligand binding to cell surface TfR. Cells were then washed by centrifugation with ice-cold PBS-BSA and resuspended in 1 mL serum-free medium prewarmed to 37°C. The cell suspension was maintained at 37°C in a water bath. One hundred-microliter aliquots were removed at 2.5-minute intervals, added to 1 mL of ice-cold 10 mmol/L acetic acid/150 mmol/L NaCl pH 3 buffer (acid wash) to remove125I-Tf on the cell surface, and centrifuged in a microfuge centrifuge for 1 minute at full speed. The supernatant was carefully removed and the radioactivity in the cell pellet and supernatant counted to determine the fraction of 125I-Tf internalized by cells (acid-resistant cpm). For the 125I-Tf release studies, pulse-chase experiments were performed in which 107 cells were first incubated at 37°C in 500 μL serum-free medium containing 0.1% BSA with 125I-Tf to allow for uptake of the radiolabeled ligand (pulse). After 30 minutes of incubation, cells were washed twice with ice-cold serum-free medium to remove unincorporated 125I-Tf and resuspended in 1 mL of serum-free medium containing 100 μg/mL of nonradioactive Tf-iron at 37°C (chase). The cell suspension was maintained at 37°C in a water bath and 100-μL aliquots were removed at 2.5-minute intervals and centrifuged. Radioactivity in the pellet and supernatant was counted to determine the percent of 125I-Tf released from cells over time.
RESULTS
Gallium-resistant CCRF-CEM cells display a stable drug-resistant phenotype.
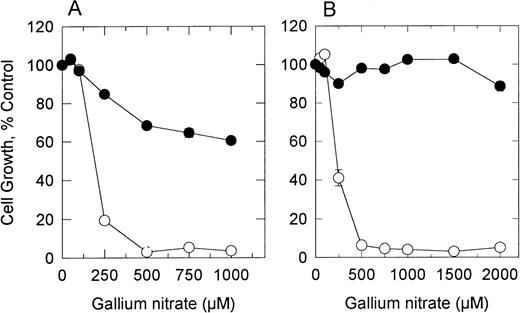
The effect of gallium nitrate on the growth of S and R1 cells is shown in Fig 1A. To determine whether R cells would revert to a gallium-sensitive phenotype in the absence of gallium, they were propogated in complete medium without gallium for 10 weeks and then analyzed for sensitivity to gallium. As shown in Fig 1B, these cells (R2 cells) remained resistant to gallium, indicating that continued exposure to gallium was not necessary to maintain a stable gallium-resistant phenotype. Subsequent experiments were performed by using both R1 and R2 cells. S, R1, and R2 cells displayed similar growth rates.
Effect of gallium nitrate on the growth of gallium-sensitive (S) and -resistant (R1 and R2) CCRF-CEM cells. Cells were plated at 2 × 105 cells/mL in the presence of increasing concentrations of gallium nitrate, and growth was determined by MTT assay after a 72-hour incubation. (A) gallium-resistant cells (•) that had been maintained continuously in medium containing 150 μmol/L gallium nitrate (R1 cells). (B) gallium-resistant cells (•) that had been grown without gallium in the medium for 10 weeks (R2 cells). (○) gallium-sensitive cells.
Effect of gallium nitrate on the growth of gallium-sensitive (S) and -resistant (R1 and R2) CCRF-CEM cells. Cells were plated at 2 × 105 cells/mL in the presence of increasing concentrations of gallium nitrate, and growth was determined by MTT assay after a 72-hour incubation. (A) gallium-resistant cells (•) that had been maintained continuously in medium containing 150 μmol/L gallium nitrate (R1 cells). (B) gallium-resistant cells (•) that had been grown without gallium in the medium for 10 weeks (R2 cells). (○) gallium-sensitive cells.
Iron uptake by cells.
Recently, we showed that the development of drug resistance to gallium nitrate in CCRF-CEM cells is related to a decrease in their uptake of gallium and that this is accompanied by a parallel decrease in iron uptake.12 To confirm that these differences in iron uptake between S and R cells were consistent, 59Fe-Tf uptake was examined by using cells that were actively proliferating (after 24 or 48 hours of growth in culture) or were confluent (after 72/0 hours of growth in culture). Cells that had been previously grown in medium for the specified times were reincubated in fresh medium containing59Fe-Tf and the amount of 59Fe taken up by cells was measured after 3, 6, and 24 hours of incubation. As shown in Fig 2, 59Fe uptake by R1 cells was significantly less than S cells regardless of whether they were initially confluent or actively proliferating.
59Fe-Tf uptake by S and R1 CCRF-CEM cells at different times of proliferation. CCRF-CEM cells were grown for 0 to 72 hours in fresh medium and then used for 59Fe uptake studies. Cells were plated at 2 × 105 cells/mL in complete medium containing 59Fe-Tf (228 pmole59Fe/mL), and 59Fe uptake by cells was determined at the times shown. (A) 59Fe uptake by confluent, 0/72-hour cells; (B) 59Fe uptake by cells previously grown for 24 hours in fresh medium; and (C) 59Fe uptake by cells previously grown for 48 hours in fresh medium. (•) S cells; (○) R cells. Values are means ± standard error (SE) of a representative experiment performed in triplicate.
59Fe-Tf uptake by S and R1 CCRF-CEM cells at different times of proliferation. CCRF-CEM cells were grown for 0 to 72 hours in fresh medium and then used for 59Fe uptake studies. Cells were plated at 2 × 105 cells/mL in complete medium containing 59Fe-Tf (228 pmole59Fe/mL), and 59Fe uptake by cells was determined at the times shown. (A) 59Fe uptake by confluent, 0/72-hour cells; (B) 59Fe uptake by cells previously grown for 24 hours in fresh medium; and (C) 59Fe uptake by cells previously grown for 48 hours in fresh medium. (•) S cells; (○) R cells. Values are means ± standard error (SE) of a representative experiment performed in triplicate.
It is known that the cellular uptake of 59Fe-Tf is mediated by the TfR, whereas the uptake of 59Fe-PIH occurs independent of Tf and its receptor.28 Therefore, the uptakes of 59FeTf and 59Fe-PIH were compared to further define the pathway(s) involved in the decrease of iron transport into R cells. As shown in Fig 3A and B,59Fe uptake by R1 and R2 cells was significantly lower than S cells regardless of whether iron was delivered to cells as59Fe-Tf or 59Fe-PIH. After a 6-hour incubation in complete medium, 59Fe-Tf uptake by R1 and R2 cells was 56% and 60% that of S cells, whereas 59Fe-PIH uptake by R1 and R2 cells was 75% that of S cells (Fig 3A). After a 24-hour incubation, 59Fe-Tf uptake by R1 and R2 cells was 48% and 60% that of S cells, whereas 59Fe-PIH uptake was 51% and 56% that of S cells (Fig 3B). Figure 3A and B also illustrate that the cellular uptake of radioiron from 59Fe-Tf was several times greater than from 59Fe-PIH.
59Fe-Tf and 59Fe-PIH uptake by S, R1, and R2 cells. Cells were plated in medium containing equivalent amounts of 59Fe (106 pmole Fe/mL) as either59Fe-Tf or 59Fe-PIH and incubated for 6 to 24 hours. (A) 59Fe uptake by cells over 6 hours in complete medium; (B) 59Fe uptake by cells over 24 hours in complete medium; (C) 59Fe-PIH uptake over 24 hours in complete medium; (D) 59Fe-PIH uptake over 24 hours in serum-free medium. Values shown represent means ± SE of an experiment performed in triplicate. Similar results were obtained in two additional experiments.
59Fe-Tf and 59Fe-PIH uptake by S, R1, and R2 cells. Cells were plated in medium containing equivalent amounts of 59Fe (106 pmole Fe/mL) as either59Fe-Tf or 59Fe-PIH and incubated for 6 to 24 hours. (A) 59Fe uptake by cells over 6 hours in complete medium; (B) 59Fe uptake by cells over 24 hours in complete medium; (C) 59Fe-PIH uptake over 24 hours in complete medium; (D) 59Fe-PIH uptake over 24 hours in serum-free medium. Values shown represent means ± SE of an experiment performed in triplicate. Similar results were obtained in two additional experiments.
Because the 59Fe-PIH uptake experiments were performed in serum-supplemented medium, the possibility existed that Tf (present in bovine serum) may have influenced iron uptake. To confirm that cells incorporated 59Fe from 59Fe-PIH independent of the TfR pathway, cellular 59Fe-PIH uptake studies were also performed in serum-free, Tf-free medium. Under these conditions,59Fe uptake by R1 and R2 cells was 44% and 66% of S cells, respectively (Fig 3D), and was comparable with 59Fe uptake in serum-supplemented medium (Fig 3C). To exclude the possibility that the decreased 59Fe-PIH uptake by R cells was not a function of the amount of 59Fe-PIH in the medium,59Fe-PIH uptake by S and R cells was measured over a fivefold range of 59Fe-PIH concentrations. As shown in Fig 4, 59Fe uptake by S and R cells increased progressively with increasing concentrations of59Fe-PIH; however, the amount of 59Fe uptake by R cells was markedly less than that of S cells at all concentrations of Fe-PIH examined and reached approximately 80% of saturation levels with 500 pmole of Fe-PIH in the medium.
Uptake of 59Fe-PIH by cells. Cells were incubated in serum-free medium with increasing concentrations of59Fe-PIH. 59Fe uptake was determined after 20 hours of incubation. (•) S cells; (○) R cells. Values represent the means of a duplicate experiment.
Uptake of 59Fe-PIH by cells. Cells were incubated in serum-free medium with increasing concentrations of59Fe-PIH. 59Fe uptake was determined after 20 hours of incubation. (•) S cells; (○) R cells. Values represent the means of a duplicate experiment.
To further verify that the decrease in 59Fe-PIH uptake by R1 and R2 cells was truly independent of the TfR, additional59Fe uptake studies were performed in the presence of 42/6, a MoAb that blocks internalization of the TfR.34 As expected, 42/6 blocked the uptake of 59Fe-Tf by S, R1, and R2 cells (Fig 5A); however, it had no effect on the uptake of 59Fe-PIH (Fig 5B). Collectively, these experiments indicate that 59Fe-PIH delivers iron to CCRF-CEM cells independent of the TfR and that TfR-independent iron uptake is decreased in R1 and R2 cells.
Effect of anti-TfR MoAb 42/6 on 59FeTf and59Fe-PIH uptake. Cells were plated in serum-free medium containing either 59Fe-Tf (109 pmole Fe/mL) or59Fe-PIH (245 pmole Fe/mL) with (+) or without (−) 10 μg/mL 42/6. 59Fe uptake by cells was determined after a 24-hour incubation. (A) 59Fe-Tf uptake; (B)59Fe-PIH uptake. Values represent means ± SE (n = 3).
Effect of anti-TfR MoAb 42/6 on 59FeTf and59Fe-PIH uptake. Cells were plated in serum-free medium containing either 59Fe-Tf (109 pmole Fe/mL) or59Fe-PIH (245 pmole Fe/mL) with (+) or without (−) 10 μg/mL 42/6. 59Fe uptake by cells was determined after a 24-hour incubation. (A) 59Fe-Tf uptake; (B)59Fe-PIH uptake. Values represent means ± SE (n = 3).
Iron release from cells.
To determine whether the efflux of iron from cells could play a role in the decrease in iron uptake by R1 and R2 cells, cells that had incorporated 59Fe-Tf were reincubated in fresh medium to determine whether they would release 59Fe to the external environment. These studies showed that after their initial uptake of59Fe-Tf, R1 and R2 cells released significantly greater amounts of 59Fe than S cells. As shown in Fig 6A, after a 1-hour uptake of 59Fe-Tf, R1 and R2 cells released approximately 1.7-fold and 2.1-fold more 59Fe to the medium than S cells over the subsequent 75 minutes of reincubation. A similar pattern of59Fe release was seen when cells were allowed to incorporate 59Fe-Tf over 3 hours and then were reincubated in fresh medium for 20 hours (Fig 6B). After 3 hours of reincubation, R1 and R2 cells released 2.9-fold and 2.3-fold more 59Fe to the medium than S cells. Even after 20 hours of reincubation, both R1 and R2 cells continued to release significantly greater amounts of59Fe than S cells (Fig 6B). To determine whether59Fe released from cells after the 1- or 3-hour uptake represented 59Fe released from the cell surface (external) or 59Fe released from inside the cell, cells were treated with Pronase (to remove 59Fe on the cell) before reincubation in fresh medium. Under these conditions, results similar to those shown in Fig 6 were obtained, thus indicating that the59Fe released from cells represented the efflux of intracellular 59Fe.
59Fe release after 59Fe-Tf uptake. S, R1, and R2 cells were allowed to incorporate59Fe-Tf over 1 or 3 hours and then washed and reincubated in fresh medium. At the specified times, aliquots of cell suspension were harvested and the 59Fe in the medium and cells was counted to determine the percent of 59Fe released from cells to the medium. (A) 59Fe released by cells after a 1-hour uptake. Insert figure shows the amount of 59Fe (pmole Fe/106 cells) taken up by cells over the 1-hour incubation before release. Data represent means ± SE (n = 3). (B) Experimental conditions were similar to (A) except that59Fe release was examined after a 3-hour uptake of59Fe-Tf and the percent 59Fe released was measured over 20 hours. Data represent means ± SE (n = 3). Insert figure shows the amount of 59Fe (pmole Fe/106cells) taken up by cells over the 3-hour incubation before release.
59Fe release after 59Fe-Tf uptake. S, R1, and R2 cells were allowed to incorporate59Fe-Tf over 1 or 3 hours and then washed and reincubated in fresh medium. At the specified times, aliquots of cell suspension were harvested and the 59Fe in the medium and cells was counted to determine the percent of 59Fe released from cells to the medium. (A) 59Fe released by cells after a 1-hour uptake. Insert figure shows the amount of 59Fe (pmole Fe/106 cells) taken up by cells over the 1-hour incubation before release. Data represent means ± SE (n = 3). (B) Experimental conditions were similar to (A) except that59Fe release was examined after a 3-hour uptake of59Fe-Tf and the percent 59Fe released was measured over 20 hours. Data represent means ± SE (n = 3). Insert figure shows the amount of 59Fe (pmole Fe/106cells) taken up by cells over the 3-hour incubation before release.
In contrast to 59Fe-Tf, significant differences in the release of 59Fe from S, R1, and R2 cells were not seen after they had incorporated 59Fe-PIH (Fig7).
59Fe release after 59Fe-PIH uptake. Experimental conditions were similar to that described in Fig 6except that cells were incubated in serum-free medium with59Fe-PIH for 3 hours and then washed and reincubated in fresh medium supplemented with 1% FCS. Data represent means ± SE (n = 4).
59Fe release after 59Fe-PIH uptake. Experimental conditions were similar to that described in Fig 6except that cells were incubated in serum-free medium with59Fe-PIH for 3 hours and then washed and reincubated in fresh medium supplemented with 1% FCS. Data represent means ± SE (n = 4).
TfR expression and synthesis.
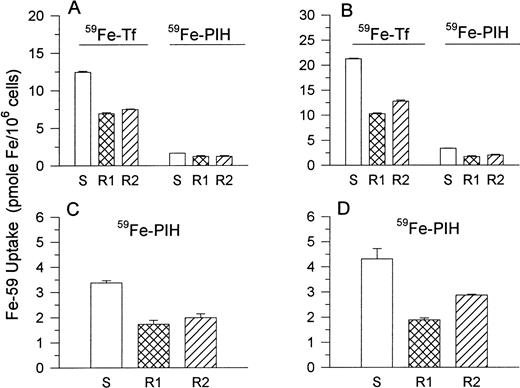
Because the TfR plays a central role in the uptake of Tf-Fe,125I-Tf binding and TfR synthesis were examined. At confluency (0 and 72 hours) in culture, R1 and S cells displayed equivalent cell surface 125I-Tf binding. In contrast, after 24 and 48 hours of growth in fresh medium, maximal 125I-Tf binding to R1 cells was lower than that to S cells by approximately 29% and 23%, respectively (Fig 8A). Measurement of TfR synthesis at the 24-hour time point by using a 3-hour [35S]methionine pulse-label showed that the synthesis of new TfR at this time point was decreased in R1 and R2 cells (Fig 8B). Interestingly, [35S]methionine pulse-labeling of cells over a longer period (20 hours) during the first 20 hours of incubation in fresh medium did not show differences in TfR synthesis. With the 3-hour [35S]methionine pulse, the reduced TfR was identified on SDS-PAGE analysis as two bands corresponding to 86 kD and 90 kD, consistent with different glycosylation states of the TfR.35 However, with the 20-hour pulse, the major band was 90 kD, consistent with the size of the mature TfR.
(A) 125I-Tf binding studies.125I-Tf binding to cells was measured after growth of cells in fresh medium for the times shown. Open columns, S cells; hatched columns, R1 cells. Data shown represent the means ± SE (n = 3). (B) TfR synthesis. Newly synthesized TfRs were labeled with [35S]methionine over a 3-hour or 20-hour pulse as described in the text. The 3-hour pulse was performed on cells after they had been incubated in fresh medium for 24 hours. The 20-hour pulse was performed immediately after the initial plating of confluent cells in fresh medium (0 to 20 hours). The autoradiograph shown is representative of three separate experiments.
(A) 125I-Tf binding studies.125I-Tf binding to cells was measured after growth of cells in fresh medium for the times shown. Open columns, S cells; hatched columns, R1 cells. Data shown represent the means ± SE (n = 3). (B) TfR synthesis. Newly synthesized TfRs were labeled with [35S]methionine over a 3-hour or 20-hour pulse as described in the text. The 3-hour pulse was performed on cells after they had been incubated in fresh medium for 24 hours. The 20-hour pulse was performed immediately after the initial plating of confluent cells in fresh medium (0 to 20 hours). The autoradiograph shown is representative of three separate experiments.
Tf cycling.
To determine whether S, R1, and R2 cells differ with regard to TfR function, the kinetics of internalization and release of receptor-bound Tf were examined by using cells that were in the same growth phase as those used for the iron release studies. Cells were allowed to internalize surface receptor-bound 125I-Tf at 37°C and the amount of 125I-Tf within the cell (acid-resistant cpm) determined. Figure9A shows that the rates of 125I-Tf internalization by S, R1, and R2 cells were similar during the first 10 to 15 minutes. However, the amount of acid-resistant 125I-Tf in R1 and R2 cells peaked at the 20-minute time point and decreased thereafter, indicating that after this time a fraction of 125I-Tf had cycled out of these cells. In contrast, the amount of acid-resistant125I-Tf in S cells peaked at the 30-minute time point and decreased only slightly thereafter.
(A) Internalization of 125I-Tf by cells. Cells were incubated with 125I-Tf at 4°C to allow for ligand binding to cell surface TfRs. Cells were washed to remove unbound 125I-Tf and incubated at 37°C to allow for internalization of 125I-Tf. At the specified times, aliquots of cells were removed and centrifuged through an acidic buffer to determine the fraction of 125I-Tf internalized. Data shown are representative of three separate experiments. (•) S; (00) R1; (▵) R2 cells. (B) Release of 125I-Tf from cells. Cells were allowed to incorporate 125I-Tf at 37°C, washed to remove unincorporated radioactivity, and then incubated in serum-free medium containing 100 μg/mL Tf-Fe (nonradioactive). The amount of 125I-Tf released by cells at the specified times was determined as described the text. (•) S; (00) R1 cells. Data represent means ± SE (n = 3). Differences between S and R1 cells after the 5-minute time point are significant (P < .004).
(A) Internalization of 125I-Tf by cells. Cells were incubated with 125I-Tf at 4°C to allow for ligand binding to cell surface TfRs. Cells were washed to remove unbound 125I-Tf and incubated at 37°C to allow for internalization of 125I-Tf. At the specified times, aliquots of cells were removed and centrifuged through an acidic buffer to determine the fraction of 125I-Tf internalized. Data shown are representative of three separate experiments. (•) S; (00) R1; (▵) R2 cells. (B) Release of 125I-Tf from cells. Cells were allowed to incorporate 125I-Tf at 37°C, washed to remove unincorporated radioactivity, and then incubated in serum-free medium containing 100 μg/mL Tf-Fe (nonradioactive). The amount of 125I-Tf released by cells at the specified times was determined as described the text. (•) S; (00) R1 cells. Data represent means ± SE (n = 3). Differences between S and R1 cells after the 5-minute time point are significant (P < .004).
Because these results suggested that the exocytosis of internalized Tf was greater in R cells than in S cells, an additional experiment was performed in which cells were pulsed with 125I-Tf for 30 minutes and then chased with nonradioactive Tf. As shown in Fig 9B, these studies showed the egress of 125I-Tf from R1 cells to be significantly greater than from S cells.
DISCUSSION
In an earlier investigation, we showed that gallium-resistant CCRF-CEM cells have a decrease in their uptake of both gallium and iron.12 Whereas the decrease in gallium uptake protects these cells from the cytotoxicity of gallium, the decrease in iron uptake could potentially threaten cellular iron-dependent processes. However, because the growth of R cells is similar to S cells, it is clear that R cells still acquire a critical amount of iron needed for viability and proliferation. The decrease in iron uptake by R cells raised important questions regarding the relative roles of TfR-dependent and -independent iron transport into these cells, and therefore, we sought to determine whether this decrease was caused by changes in TfR-dependent or TfR-independent iron transport. TfR expression and the uptake of Tf-Fe by nonerythroid cells is closely linked to the need for iron to maintain cell viability and DNA synthesis.30,36,37 The TfR-independent iron uptake system in contrast, may serve to remove potentially toxic low molecular weight iron complexes from the circulation and may represent a transport system shared by several metals.23,38-40 Because Fe-PIH has been shown to support the growth of cells independent of Tf,41 this iron complex was used to examine TfR-independent iron uptake.
Comparison of 59Fe uptake by using 59Fe-Tf or59Fe-PIH as the source of iron showed that, whereas TfR-dependent iron uptake was always greater than TfR-independent iron uptake, both pathways were significantly downregulated in R cells. Evidence that the PIH-mediated uptake of iron was independent of the TfR was provided by performing the uptake studies in serum-free, Tf-free medium and by showing that 59Fe-PIH uptake was unaffected by blockade of the TfR with MoAb 42/6. The finding that both TfR-dependent and -independent iron uptake was decreased in R cells was unexpected because these two iron uptake pathways are generally perceived as separate, independent systems. One possible explanation for the parallel decrease in TfR-dependent and -independent iron uptake is that both iron uptake pathways may be controlled at some level by the same mechanism and that this regulatory mechanism is affected during the development of drug resistance to gallium.
TfR-mediated uptake of iron is generally in balance with the amount of iron needed to support cellular iron-dependent processes. When iron is incorporated in excess of cellular requirements, it is sequestered in ferritin, and hence, very little of it effluxes from cells under normal conditions. However, R cells released significantly greater amounts of incorporated iron to the exterior than S cells, indicating that, with respect to Tf-Fe, an increased efflux of iron contributes to the decrease in iron uptake. The basis for the increase in iron release from R cells remains to be determined. Under normal conditions, the intracellular release of iron from Tf occurs through a process of endosomal acidification involving an adenosine triphosphate (ATP)-dependent proton pump.20 The movement of iron out of the endosome is poorly understood and may include the activity of low or high molecular weight intermediates or even direct interaction between organelles.20 Perturbation of any of these processes could lead to a block in the unloading of iron from Tf and a subsequent release of iron from cells.
The decrease in Tf-Fe uptake and the increase in iron release from R cells could be caused by changes in the synthesis, posttranslational modification, or cycling of the TfR. Earlier studies did not show differences in the affinity of the TfR for Tf in R cells.12In the present study, differences in the size of the TfR were not seen on SDS-PAGE analysis, making it unlikely that the TfR in R cells is structurally altered. Although TfR expression in R cells was comparable with S cells when measured at confluency (0 and 72 hours), TfR expression was lower in R cells only after 24 and 48 hours of subculture. Hence, the decrease in Tf-Fe uptake by R cells over the 24- and 48-hour period of incubation can be explained in part by the decrease in TfR number. In contrast, experiments showing an increase in the release of iron from R cells were performed over the initial 1 and 3 hours of subculture of cells in fresh medium, time points at which TfR expression in S and R cells was similar. Pulse-chase experiments performed at these time points showed that the cycling of125I-Tf out of R cells was also increased. Based on these results, we conclude that the decrease in Tf-Fe uptake by R cells is caused by a combination of enhanced efflux of iron from cells and decreased TfR-mediated iron transport into cells. The former mechanism appears to dominate during the initial period of subculture of cells in fresh medium, whereas the latter mechanism comes into play later on.
Although our studies shed light on the mechanisms involved in the decrease in TfR-dependent iron uptake by R cells, the basis for the decrease in TfR-independent iron uptake remains to be determined. Differences in the release of iron from S and R cells were not seen after the uptake of 59Fe-PIH, suggesting that the primary mechanism for the decrease in TfR-independent iron uptake involves a quantitative or qualitative decrease in a non-Tf iron transport system. It remains to be determined whether these differences in the handling of TfR-dependent and -independent Fe by R cells are the result of changes in single or multiple mechanisms involved in the regulation of iron uptake during the development of gallium resistance.
To our knowledge, there have been no previously reported examples in which the downregulation of uptake of one metal as a protective adaptation by a tumor cell results in a decrease in iron transport as well. Because of the interaction between gallium and iron proteins, R cells may serve as a unique model system to gain insights into adaptive changes in cellular iron transport. It is hoped that further investigation of iron metabolism in these cells will yield new information regarding regulatory mechanisms responsible for the uptake and intracellular trafficking of iron and iron proteins.
Supported by US Public Health Service Grant No. RO1 CA68028.
Address reprint requests to Christopher R. Chitambar, MD, Division of Hematology/Oncology, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee, WI, 53226.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






![Fig. 8. (A) 125I-Tf binding studies.125I-Tf binding to cells was measured after growth of cells in fresh medium for the times shown. Open columns, S cells; hatched columns, R1 cells. Data shown represent the means ± SE (n = 3). (B) TfR synthesis. Newly synthesized TfRs were labeled with [35S]methionine over a 3-hour or 20-hour pulse as described in the text. The 3-hour pulse was performed on cells after they had been incubated in fresh medium for 24 hours. The 20-hour pulse was performed immediately after the initial plating of confluent cells in fresh medium (0 to 20 hours). The autoradiograph shown is representative of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4686/4/m_blod41215008w.jpeg?Expires=1765895144&Signature=CGQ2AixkVldOcY32NX-8MHf09mZz6DzP7RgVss-4ylYlRgoIKELm~-wr0tdnLVMnjhlcng~MsA4BMUBpTGLYkYl8QZhhao9NmRO7x9zIA9sdUboxXfyw0U9lGc4VUE4dYWddq3NcYxfVEB18lK14F1pAeZqDbOFyYrjZEdxNO96WGFjgo06gRuF28yOR2EAfX01Srh4wbXe~ZQocF48Dx4jpZwBQ5L7KxYLwImjXH4dLapcDqK2-gFQSrsXrwgABZYszkJJpDwdqpqgBLJr5ba5nfq~~IYonsn4vkO1SaA9JV9iKnZEp91lnw0FD6-8tJ0e-y6mQBKojjGdHZ16G-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal