Abstract
A new strategy for the treatment of autoimmune diseases in chimeric resistant MRL/lpr mice is established. The strategy includes injection of cyclophosphamide (CY), fractionated irradiation (5 Gy × 2), bone grafts (to recruit stromal cells), and two transplantations of whole bone marrow cells (WBMCs) from allogeneic normal C57BL/6 mice (CY/2X/Bone/2BMT). MRL/lpr mice, thus treated, survived more than 40 weeks (1 mouse survived for >40 weeks, 7 for >50 weeks, and 4 for >60 weeks after these treatments). Immunohistological studies showed that the mice were completely free from both lymphadenopathy and autoimmune diseases such as systemic lupus erythematosis and rheumatoid arthritis. The levels of autoantibodies (IgM/IgG rheumatoid factors and IgM/IgG anti-ssDNA antibodies [Abs]) in the treated mice decreased to those in the normal mice. In addition, successful cooperation among T cells, B cells, and antigen-presenting cells (APCs) was observed. Abnormal T cells with immunophenotypes of B220+/Thy-1+/CD3+/CD4−/CD8−present in untreated MRL/lpr mice disappeared, and the hematolymphoid cells of the treated mice were of donor origin. However, the mice that had been irradiated with 8.5 Gy and then reconstituted with T-cell–depleted BMCs plus bone grafts died within 2 weeks due to the side effect of irradiation. The depletion of CD8+ cells (not CD4+ cells) from WBMCs resulted in graft failure; 60% of the recipient mice, thus treated, died within 2 weeks, and all recipients died by 15 weeks. Furthermore, limiting dilution assays showed that approximately more than 0.5% of T cells contained in the BMCs are necessary not only for engraftment of BMCs but also for long-term disease-free survival of the recipients. In contrast, recipients that had received CD4-depleted BMCs with CY plus fractionated irradiation (5Gy × 2) survived for more than 40 weeks without showing graft-versus-host reaction (GVHR). This indicates that CD8+cells in the BMCs are essential for the successful engraftment of the donor-type hematolymphoid cells.
MRL/MP-lpr/lpr (MRL/lpr) MICE spontaneously develop massive lymphadenopathy with the accumulation of abnormal T cells with immunophenotypes of B220+/Thy-1+/CD3+/CD4−/CD8−,1which might be attributed to the mutation of the Fas gene encoding a membrane receptor transducing apoptotic signals.2 The MRL/lpr mouse is also known as an animal model for systemic lupus erythematosis (SLE) and rheumatoid arthritis (RA). The mouse shows high levels of autoantibodies (rheumatoid factors and anti-DNA antibodies [Abs]).3-6 In our previous report, we have shown that the combination of bone marrow transplantation (BMT) with bone grafts has completely preventative effects on autoimmune diseases in this strain; we have found that there is a requirement for donor-derived stromal cells for successful allogeneic BMT.7,8 However, we have also found that BMT with bone grafts has no effect on the treatment of autoimmune diseases in MRL/lpr mice. MRL/lpr mice seem to become more radiosensitive after the onset of autoimmune disease (lupus nephritis), because they become susceptible to uremic enterocolitis. To determine the optimal radiation dose to treat autoimmune MRL/lpr mice, we have irradiated MRL/lpr mice with 6 to 9.5 Gy in combination with BMT plus bone grafts. Almost all MRL/lpr mice (after the onset) suffered from intestinal death (due to infection from the intestine) if the doses were greater than 8.5 Gy. Because irradiation doses less than 8.5Gy cannot kill abnormal hematopoietic stem cells (HSCs) of MRL/lpr mice, as we previously described,9 we have performed fractionated total body irradiation (TBI) in the present study.
It has been shown that long-term injections of Abs against T cells (anti-Thy1.2 or anti-CD4 Ab) to MRL/lpr mice retard both lymphadenopathy and the progression of autoimmune diseases, although autoimmune diseases recur unless the treatment is continued.10,11 Furthermore, various nonspecific anti-inflammatory and immunosuppressive agents have been used to treat SLE.12 13 However, the long-term administration of these drugs is required, which results in cumulative side effects. In the present study, we show a new method for treating autoimmune diseases in chimeric resistant MRL/lpr mice.
MATERIALS AND METHODS
Mice.
MRL/lpr, C57BL/6 (B6), DBA/2, and C3H/HeN mice were obtained from SLC (Shizuoka, Japan) and maintained in our animal facility under specific pathogen-free conditions.
Antibodies and surface marker analyses.
Purified rat monoclonal antibodies (MoAbs) against Thy-1 (30-H-12), CD4 (GK1.5), and CD8 (53-6.72) were purchased from PharMingen (San Diego, CA). These MoAbs were used to deplete CD4+, CD8+, or T cells in combination with magnetic beads conjugated with sheep antirat IgG Ab (Dynabeads M-450; Dynal A.S., Oslo, Norway). MoAbs against B cells (B220, RA3-6B2), macrophages (Mac-1, M1/70), granulocytes (Gr-1, RB6), and erythroid-lineage cells (TER119) were also from PharMingen. These were used to enrich T cells from the peripheral blood. Fluorescein isothiocyanate (FITC)-coupled anti-H-2Kb and phycoerythrin (PE)-coupled anti-H-2Kk MoAb from PharMingen were used for H-2 typing. FITC- or PE-coupled MoAbs against Thy-1, B220, CD4, and CD8 were also from PharMingen to analyze the cell surface phenotype. Lymph node and spleen cells were prepared from recipient mice, and the cells were stained with appropriate FITC- or PE-conjugated MoAbs to detect abnormal T cells with the immunophenotypes of B220+/Thy-1+/CD3+/CD4−/CD8−or donor-derived cells. Cells were analyzed by a FACScan (Becton Dickinson & Co, Mountain View, CA).
Depletion of CD4+, CD8+, or whole T cells.
Bone marrow cells (BMCs) were treated with appropriately diluted MoAbs against CD4, CD8, or Thy-1.2, followed by antirat IgG-conjugated magnetic beads to deplete the cells bearing the respective marker. Residual CD4+, CD8+, or T cells after the treatment were less than 0.05% when stained with FITC- or PE-anti-CD4, CD8, or Thy-1.2, and examined by a FACScan.
Enrichment of T cells from peripheral blood mononuclear cells.
Peripheral blood was collected, and mononuclear cells were enriched by centrifugation on a cushion of Lympholyte-M (Cedarlane Laboratories Ltd, Hornby, Ontario, Canada). Mononuclear cells were treated with a mixture of MoAbs (anti-B220, antimacrophage [Mac-1], anti-granulocyte [Gr-1], and erythroid-lineage cells) and then incubated with antirat IgG-conjugated magnetic beads. After depleting cells with these markers, more than 98% of the residual cells were positive for Thy-1.2 and used as highly enriched T cells.
Treatment of MRL/lpr mice.
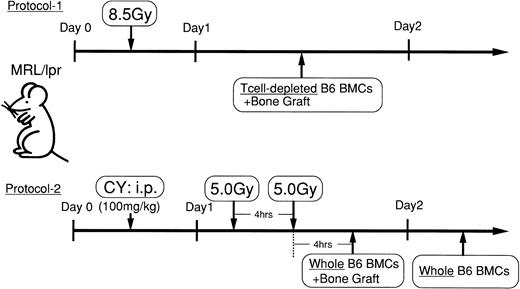
Experimetal procedures are depicted in Fig1. The onset of autoimmune diseases in MRL/lpr mice was monitored by proteinuria (>2.5+) and lymphadenopathy. Female MRL/lpr mice (3 to 4 months of age) with autoimmune diseases were intraperitoneally injected with 100 mg/kg cyclophosphamide (CY) and, 1 day later, lethally irradiated (fractionated radiation: 5 Gy × 2 = 10 Gy; 4-hour interval). Four hours after the second irradiation, the bones of B6 mice, from which BMCs had been flushed out, were engrafted under the subcutis of the MRL/lpr mice (2 femurs and 2 tibias per mouse), and the mice received 5 × 107 whole BMCs from B6 mice through a tail vein. One day after the first BMT plus bone graft, the mice were further transplanted with B6 whole BMCs (WBMCs; 5 × 107 cells; experimental protocol 2 in Fig 1). MRL/lpr mice that had been irradiated (8.5 Gy) and transplanted with T-cell–depleted BMCs plus bone graft of B6 mice were also prepared. This protocol was previously used for the prevention of autoimmune diseases in MRL/lpr (experimental protocol 1 in Fig 1).7 8Furthermore, the following groups were prepared: (1) CY/2X/Bone/BMT (without first BMT); (2) 2X/Bone/2BMT (without CY injection); (3) CY/2X/Bone/2BMT(−CD4) (CD4-depleted BMCs were used for both BMT), (4) CY/2X/Bone/2BMT(−CD8) (CD8-depleted BMCs were used for both BMT), and (5) CY/2X/Bone/2BMT (−T cell) (T-cell–depleted BMCs were used for both BMTs). In some experiments, various percentages (0.05, 0.1, 0.5, and 1.0) of mature T cells enriched from the peripheral blood (the purity of T cells was >98%) were added to T-cell–depleted BMCs (5 × 107 cells) and transplanted to MRL/lpr mice to provide quantitative information as to the number of T cells being coadministered to conditioned recipients.
Experimental protocols. Experimental protocol 1: Female MRL/lpr mice (3 to 4 months of age) with autoimmune diseases were irradiated (8.5 Gy) and transplanted with T-cell–depleted BMCs plus bone graft of B6 mice. Experimental protocol 2: Female MRL/lpr mice with autoimmune diseases were intraperitoneally injected with 100 mg/kg CY and then lethally irradiated (5 Gy × 2 = 10 Gy for 4-hour interval) 1 day later. Four hours after the second irradiation, the bones of the B6 mice, from which the BMCs had been flushed out, were engrafted under the subcutis of the MRL/lpr mice, and the mice received 5 × 107 WBMCs from B6 mice. One day after the first BMT plus bone graft, the mice were further transplanted with B6 WBMCs (5 × 107 cells).
Experimental protocols. Experimental protocol 1: Female MRL/lpr mice (3 to 4 months of age) with autoimmune diseases were irradiated (8.5 Gy) and transplanted with T-cell–depleted BMCs plus bone graft of B6 mice. Experimental protocol 2: Female MRL/lpr mice with autoimmune diseases were intraperitoneally injected with 100 mg/kg CY and then lethally irradiated (5 Gy × 2 = 10 Gy for 4-hour interval) 1 day later. Four hours after the second irradiation, the bones of the B6 mice, from which the BMCs had been flushed out, were engrafted under the subcutis of the MRL/lpr mice, and the mice received 5 × 107 WBMCs from B6 mice. One day after the first BMT plus bone graft, the mice were further transplanted with B6 WBMCs (5 × 107 cells).
Immunological assays.
Recipient mice were killed, and their spleens were removed. The immunological functions of the mice were assayed as follows: (1) antibody production against sheep red blood cells (SRBCs) and (2) mixed leukocyte reaction (MLR). In the anti-SRBC antibody response, 4 × 106 spleen cells were cultured with the same number of SRBCs in 24-well flat-bottom culture plates for 5 days, and anti-SRBC antibody production was measured by the modified Jerne's plaque-forming cell (PFC) assay, as previously described.14 MLR was performed as follows. Triplicate cultures were set up in 96-well round-bottom microwell trays (Corning Glass Works, Corning, NY). Each well contained 2 × 105 responder T cells and 3 × 105irradiated (15 Gy) spleen cells in a total of 0.2 mL of RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, and 50 μmol/L 2-mercaptoethanol (2-ME; Wako, Osaka, Japan). The culture was incubated for 72 hours and pulsed with 0.5 μCi of3[H]-thymidine for the last 16 hours of the culturing period.
Proteinuria.
Proteinuria was measured using testing papers (Albustix; Miles-Sankyo Ltd Co, Tokyo, Japan).
Measurement of autoantibodies.
RF (IgG and IgM) and anti-ssDNA Abs (IgG and IgM) in the sera of the recipient mice were determined by a standard enzyme-linked immunosorbent assay (ELISA), based on the method described by Izui and Eisenberg.15 Relative quantities of autoantibodies were measured by absorbance of OD405, which is the maximum absorbance using phosphatase substrate tablet (Sigma 104; Sigma Diagnostics, St Louis, MO).
Pathological findings.
The kidneys or joints of the recipient mice were removed and fixed in 10% phosphate-buffered formalin. For the immunofluorescence study, the specimens were frozen in dry-ice/acetone, as previously described.16 Briefly, 3-μm sections were incubated at room temperature with FITC-conjugated rabbit antimouse IgG or FITC-conjugated antimouse C3 (Medical and Biological Laboratories, Nagoya, Japan) and then washed three times with phosphate-buffered saline. The samples were also embedded in paraffin, sectioned, and then stained with hematoxilin and eosin (H-E).
Statistical analyses.
Statistical analyses in the survival rate of recipient mice were performed using a logrank test.
RESULTS
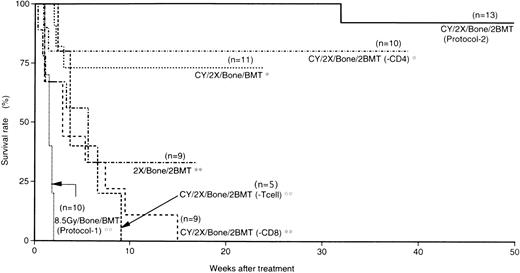
Prolonged survival of MRL/lpr mice treated with CY/2X/Bone/2BMT.
In our previous report, MRL/lpr mice (before the onset of diseases) treated with BMT (T-cell–depleted BMCs) plus bone grafts after 8.5 Gy irradiation survived more than 1 year without showing any symptoms of autoimmune diseases, indicating that this protocol is useful for the prevention of the diseases. However, MRL/lpr mice at the age of 3 to 4 months (after the onset of the diseases) treated with this protocol died within 2 weeks due to the side effects of radiation (8.5 Gy) or graft rejection (Fig 2). Therefore, we have devised a new method that reduces the side effects of radiation and prevents graft rejection. As shown in Fig 1, MRL/lpr mice that had shown symptoms of autoimmune diseases (proteinuria and massive lymphadenopathy) were treated with CY and fractionated radiation (5 Gy × 2), followed by two transplantations of WBMCs plus bone grafts from normal B6 mice (CY/2X/Bone/2BMT). MRL/lpr mice that had received such treatments survived more than 40 weeks after the treatment (1 mouse survived for >40 weeks, 7 for >50 weeks, and 4 for >60 weeks; Fig 2). Furthermore, more than 70% of the recipients of CY/2X/Bone/BMT (without the first BMT) survived more than 25 weeks. However, 67% of the mice treated with 2X/Bone/2BMT (without CY injection) died within 6 weeks. Furthermore, all the recipients that had received T-cell–depleted BMCs [CY/2X/Bone/2BMT(−T cell)] died within 10 weeks without the reconstitution of donor-derived cells, indicating that transplantation with T-cell–containing BMCs is essential to the engraftment and treatment. The mice treated with CY/2X/Bone/2BMT(−CD8) (CD8-depleted BMCs were used for both BMT) died within 15 weeks due to graft rejection and lupus nephritis; cells recovered from these mice had H-2 of the recipient type when examined by a FACScan (H-2k+ cells were 97.5% ± 0.5%). Furthermore, recipients treated with 2X/Bone/2BMT (without CY injection), CY/2X/Bone/2BMT(−T cell), or CY/2X/Bone/2BMT(−CD8) showed proteinuria; histological examinations showed the presence of lupus nephritis. In contrast, 80% of the MRL/lpr mice treated with CY/2X/Bone/2BMT(−CD4) (CD4-depleted BMCs were used for both BMT) survived more than 40 weeks (8 of 10 mice), suggesting that CD8+cells in the BMCs are neccessary for the engraftment of donor cells. As mentioned above, the presence of T cells in the transplanted BMCs is a critical factor in the long-term, disease-free survival of the recipient mice (mouse BMCs usually contain approximately 1% of T cells). Therefore, various ratios (0.05%, 0.1%, 0.5%, and 1.0%) of mature T cells enriched from the peripheral blood (the purity of T cells was >98% as determined by a FACScan) were added to T-cell–depleted BMCs (5 × 107) to provide the quantitative information as to the number of mature T cells necessary for the treatment. As shown in Table 1, the recipients that received BMCs containing 0.05% and 0.1% of mature T cells showed hematolymphoid cells with the recipient H-2 phenotype, and no donor-derived cells were detected in these recipients when tested 6 weeks after the treatment as observed in those treated with CY/2X/Bone/2BMT (−T cell). However, the hematolymphoid cells of the recipients that had received BMCs containing greater than 0.5% of T cells were almost all of donor origin (>98%). Thus, the presence of small numbers of T cells in BMCs (∼0.5% of BMCs that contain 0.3% of CD8+ T cells) seems to be necessary for the engraftment of donor BMCs and for long-term survival.
Survival rate in 6 groups. Numbers in parentheses are numbers of mice in each group. Treatment of mice is shown in the figure. Statistical analyses were performed by a logrank test, and asterisks (**) represent the P values of treated (CY/2X/Bone/2BMT) versus other groups; **P < .01, *statistical insignificance.
Survival rate in 6 groups. Numbers in parentheses are numbers of mice in each group. Treatment of mice is shown in the figure. Statistical analyses were performed by a logrank test, and asterisks (**) represent the P values of treated (CY/2X/Bone/2BMT) versus other groups; **P < .01, *statistical insignificance.
H-2 Typing of MRL/lpr Mice Transplanted With BMCs Containing Various Percentages of T Cells
| % of T Cells Added* . | Survival Rate . | No. of Mice Bearing-151 Donor Phenotype (H-2Kb) . | No. of Mice Bearing Recipient Phenotype (H-2Kk) . |
|---|---|---|---|
| 0.05 | 4/5-152 | 0 | 4 |
| 0.1 | 3/5 | 0 | 3 |
| 0.5 | 4/5 | 3 | 1 |
| 1.0 | 5/5 | 5 | 0 |
| % of T Cells Added* . | Survival Rate . | No. of Mice Bearing-151 Donor Phenotype (H-2Kb) . | No. of Mice Bearing Recipient Phenotype (H-2Kk) . |
|---|---|---|---|
| 0.05 | 4/5-152 | 0 | 4 |
| 0.1 | 3/5 | 0 | 3 |
| 0.5 | 4/5 | 3 | 1 |
| 1.0 | 5/5 | 5 | 0 |
*T cells from the peripheral blood of donor mice were added to 5 × 107 T cell-depleted BMCs, and injected into recipients that had been treated with CY/2X/Bone.
Peripheral blood mononuclear cells were collected 6 weeks after treatment, and stained with FITC-labeled anti-H-2Kk and PE-labeled anti-H-2Kb to detect donor- and recipient-derived cells, respectively.
Numbers represent live/total recipients 6 weeks after the treatment.
Serum autoantibody levels of MRL/lpr mice treated with CY/2X/Bone/2BMT.
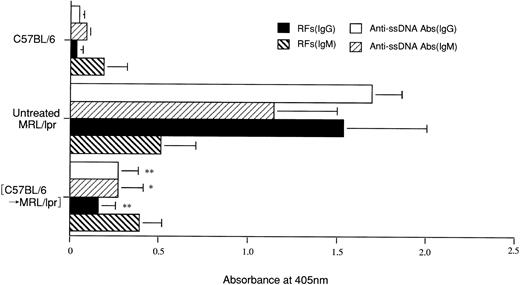
As previously reported, untreated MRL/lpr mice and MRL/lpr mice reconstituted with syngeneic BMCs showed increased RFs (IgM and IgG) and anti-ssDNA Abs (IgM and IgG) at the age of 18 weeks, whereas MRL/lpr mice treated with CY/2X/Bone/2BMT showed normal levels in these parameters even 48 weeks after the treatment, with the levels being comparable with those of 20-week-old B6 mice (Fig 3).
Autoantibodies in MRL/lpr mice treated with CY/2X/Bone/2BMT (Experimental protocol 2). RFs and anti-ss DNA Abs were measured at 48 weeks after the treatment (64 weeks of age). Autoantibodies in normal C57BL/6 and untreated MRL/lpr mice were measured at 18 weeks of age. The results are expressed as the mean ± SD at 405 nm from 5 mice. Asterisks (* and **) represent Pvalues of treated versus untreated MRL/lpr mice; *P < .005 and **P < .001.
Autoantibodies in MRL/lpr mice treated with CY/2X/Bone/2BMT (Experimental protocol 2). RFs and anti-ss DNA Abs were measured at 48 weeks after the treatment (64 weeks of age). Autoantibodies in normal C57BL/6 and untreated MRL/lpr mice were measured at 18 weeks of age. The results are expressed as the mean ± SD at 405 nm from 5 mice. Asterisks (* and **) represent Pvalues of treated versus untreated MRL/lpr mice; *P < .005 and **P < .001.
Immunological findings of MRL/lpr mice treated with CY/2X/Bone/2BMT.
Untreated MRL/lpr mice showed an extremely low anti-SRBC response (number of PFC/culture, 130 ± 8), whereas MRL/lpr mice treated with CY/2X/Bone/2BMT showed a high anti-SRBC response even 48 weeks after the treatment (number of PFC/culture, 440 ± 29), although the level was not completely restored to the normal level as seen in B6 or DBA/2 mice (numbers of PFC/culture, 737 ± 46 and 980 ± 141, respectively). These findings indicate that cooperation is achieved among the T cells, B cells, and antigen-presenting cells (APCs) of the treated MRL/lpr mice.
Disappearance of abnormal T cells in MRL/lpr mice treated with CY/2X/Bone/2BMT.
It has been shown that the numbers of abnormal T cells with immunophenotypes of B220+/Thy-1+/CD3+/CD4−/CD8−increase in the spleen and lymph nodes of MRL/lpr mice with age (Table 2).1 We have found that this is due to the presence of abnormal HSCs of MRL/lpr mice.9 However, as shown in Table 2, these abnormal T cells did not appear in the MRL/lpr mice treated with CY/2X/Bone/2BMT at 48 weeks (32 weeks after the treatment) and 72 weeks (56 weeks after the treatment). It should be noted that almost all the cells in the spleen and lymph nodes are of donor origin (H-2b but not H-2k; Table 2), indicating that the hematolymphoid cells are completely reconstituted with donor cells. In MLR, newly developed T cells were tolerant to both host (MRL/lpr)-type and donor (B6)-type MHC determinants, but they showed a normal responsiveness to the third party (DBA/2) cells (data not shown). It should also be noted that the hematolymphoid cells in the mice treated with 2X/Bone/2BMT (without CY treatment) are of MRL/lpr origin (data not shown), indicating that CY injection is essential for the engraftment of donor hematolymphoid cells; CY seems to eliminate host-derived activated T cells, as previously reported.17
Analyses of Cell Surface Antigens in the Spleen and Lymph Nodes of Treated and Untreated MRL/lpr Mice
| Cell Source . | Cell Surface Antigen (%)* . | H-2 Typing (%) . | |||||
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | Thy1.2 . | B220 . | B220/Thy1.2 . | H-2Kk . | H-2Kb . | |
| Untreated MRL/lpr† | |||||||
| Spleen | 17.4 ± 2.1 | 12.4 ± 0.3 | 34.0 ± 9.0 | 16.9 ± 2.3 | 28.2 ± 19.4 | 98.6 ± 0.7 | 0.0 ± 0.0 |
| Lymph nodes | 6.8 ± 0.4 | 4.2 ± 0.2 | 8.2 ± 0.2 | 12.6 ± 2.6 | 78.3 ± 2.5 | 98.3 ± 1.5 | 0.0 ± 0.0 |
| Treated MRL/lpr (protocol 2) | |||||||
| Spleen | 13.4 ± 4.2 | 11.6 ± 4.3 | 26.0 ± 9.7 | 49.0 ± 17.5 | 0.7 ± 0.3 | 0.3 ± 0.2 | 94.2 ± 2.1 |
| Lymph nodes | 25.9 ± 4.8 | 27.4 ± 9.7 | 53.1 ± 15.2 | 34.6 ± 14.3 | 0.8 ± 0.4 | 0.4 ± 0.2 | 97.1 ± 1.2 |
| Cell Source . | Cell Surface Antigen (%)* . | H-2 Typing (%) . | |||||
|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | Thy1.2 . | B220 . | B220/Thy1.2 . | H-2Kk . | H-2Kb . | |
| Untreated MRL/lpr† | |||||||
| Spleen | 17.4 ± 2.1 | 12.4 ± 0.3 | 34.0 ± 9.0 | 16.9 ± 2.3 | 28.2 ± 19.4 | 98.6 ± 0.7 | 0.0 ± 0.0 |
| Lymph nodes | 6.8 ± 0.4 | 4.2 ± 0.2 | 8.2 ± 0.2 | 12.6 ± 2.6 | 78.3 ± 2.5 | 98.3 ± 1.5 | 0.0 ± 0.0 |
| Treated MRL/lpr (protocol 2) | |||||||
| Spleen | 13.4 ± 4.2 | 11.6 ± 4.3 | 26.0 ± 9.7 | 49.0 ± 17.5 | 0.7 ± 0.3 | 0.3 ± 0.2 | 94.2 ± 2.1 |
| Lymph nodes | 25.9 ± 4.8 | 27.4 ± 9.7 | 53.1 ± 15.2 | 34.6 ± 14.3 | 0.8 ± 0.4 | 0.4 ± 0.2 | 97.1 ± 1.2 |
*Cells from the spleen and lymph nodes (inguinal and axillar regions) were stained with FITC- or PE-conjugated MoAbs, and analyzed using a FACScan.
Numbers represent means ± SDs of 6 mice in each group.
Histopathological findings of MRL/lpr mice treated with CY/2X/Bone/2BMT.
Neither lymphadenopathy nor autoimmune diseases such as lupus nephritis (Fig 4B) and RA (Fig 5B) were observed in the MRL/lpr mice treated with CY/2X/Bone/2BMT, even at 72 weeks of age, whereas untreated MRL/lpr mice clearly showed typical lupus nephritis (Fig 4A) and RA-like lesions (Fig 5A) with the infiltration of lymphocytes and pannus formation at 18 weeks of age. It is noted that MRL/lpr mice, thus treated, did not show the recurrence of autoimmune manifestations. This was the case when the recipients were reconstituted with CD4-depleted BMCs [CY/2X/Bone/2BMT(−CD4)]; these mice survived more than 40 weeks without any evidence of graft-versus-host disease (GVHD), as shown in Fig 6.
Immunofluorescence microscopical findings of glomerular IgG deposits in the kidneys of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The glomerulus of an untreated MRL/lpr mouse shows the IgG deposit, whereas the glomerulus of the treated mouse shows no IgG deposit.
Immunofluorescence microscopical findings of glomerular IgG deposits in the kidneys of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The glomerulus of an untreated MRL/lpr mouse shows the IgG deposit, whereas the glomerulus of the treated mouse shows no IgG deposit.
Histopathologic findings in the hindpaw joint of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The joint of the untreated MRL/lpr mouse shows marked lymphoid cell infiltration and pannus formation, whereas the joint of the treated MRL/lpr mouse shows neither lymphoid cell infiltration nor pannus formation.
Histopathologic findings in the hindpaw joint of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The joint of the untreated MRL/lpr mouse shows marked lymphoid cell infiltration and pannus formation, whereas the joint of the treated MRL/lpr mouse shows neither lymphoid cell infiltration nor pannus formation.
Histopathologic findings in the spleen (A), bone marrow (B), liver (C), colon (D), skin (E), and lung (F) of mice reconstituted with CD4-depleted BMCs [CY/2X/Bone/2BMT(−CD4)]. Histopathologic examination was performed 40 weeks after the treatment. The spleen and bone marrow show normal architecture with normal hematopoiesis, and there is no remarkable lymphocyte infiltration in the liver, colon, skin, or lung, indicating no GVHD.
Histopathologic findings in the spleen (A), bone marrow (B), liver (C), colon (D), skin (E), and lung (F) of mice reconstituted with CD4-depleted BMCs [CY/2X/Bone/2BMT(−CD4)]. Histopathologic examination was performed 40 weeks after the treatment. The spleen and bone marrow show normal architecture with normal hematopoiesis, and there is no remarkable lymphocyte infiltration in the liver, colon, skin, or lung, indicating no GVHD.
DISCUSSION
We have previously found that allogeneic BMT plus bone grafts (to recruit donor stomal cells) has completely preventative effects on autoimmune diseases in MRL/lpr mice.7 However, this strategy was found to have no effect on the treatment of autoimmune diseases in MRL/lpr mice after the onset of the diseases (Fig 2; 8.5Gy/Bone/BMT), because MRL/lpr mice are radiosensitive (8.5 Gy is the maximum) and MRL/lpr mice become more sensitive to radiation due to renal failure after the onset of autoimmune diseases and are resistant to allogeneic engraftment when irradiated at lower doses.9
Chu et al18 have reported that the massive upregulation of Fas-ligand is observed on T cells (particularly on CD4/CD8 double-negative T cells) from enlarged lymph nodes of old (4 to 5 months) MRL/lpr mice. The GVH-like reaction is thought to be due to the increased production of Fas-ligand in MRL/lpr donor cells, which may induce apoptosis of MRL/+ recipient cells in [MRL/lpr → MRL/+] chimeras.18 19 In our system, the excessive production of Fas-ligand in old MRL/lpr mice probably leads to the Fas/Fas-ligand-induced death of the donor cells, resulting in the resistance to allogeneic engraftment.
We performed fractionated radiation (5Gy × 2) to reduce the acute radiation injury, and CY was used to eliminate host-derived activated T cells.17,20,21 The mechanisms underlying tolerance induction by CY have been reported: CY induces tolerance by (1) clonal deletion of reactive T cells,22 (2) clonal anergy in MHC class I and/or class II disparate combinations,23(3) the involvement of donor-derived veto cells in the recipient mice after the injection of CY in MHC class I disparate transplantation,24 and (4) the induction of suppressor T cells in CY-induced tolerance to the non–H-2-encoded alloantigens.25 In the present study, the newly developed T cells were tolerant to both host (MRL/lpr)-type and donor (B6)-type MHC determinants, but they showed a normal responsiveness to the third party (DBA/2) cells (Fig 5). Furthermore, the hematolymphoid cells in the mice without CY treatment were of MRL/lpr origin. Therefore, the tolerant state in the recipient mice may be maintained by the combination of these mechanisms after the injection of CY.
To facilitate the engraftment of donor hematopoietic cells, we performed BMT twice. Although there was no statistically significant difference between the groups treated with CY/2X/Bone/2BMT and CY/2X/Bone/BMT, the former group had a higher survival rate, particularly at 3 to 4 weeks after the treatment (100% v 70% to 80%; Fig 2). To further prevent graft rejection, WBMCs (not T-cell–depleted BMCs) were used. MRL/lpr mice, thus treated (CY/2X/Bone/2BMT), showed a good survival rate (>90%) for 50 weeks after the treatment without showing any symptoms of autoimmune diseases through their whole life (Fig 2) without a reappearance of abnormal T cells.
To elucidate which T-cell subsets (CD4 or CD8) are necessary for the engraftment, we compared the effects of CD4- or CD8-depleted BMCs on the survival rates. As shown in Fig 2, MRL/lpr mice with CD4-depleted BMT [CY/2X/Bone/2BMT (−CD4)] showed a good survival rate (80%) for more than 40 weeks after the treatment, whereas MRL/lpr mice with CD8-depleted BMT [CY/2x/Bone/2BMT (−CD8)] died within 20 weeks of the treatment. Thus, it seems likely that donor-derived CD8+ T cells are necessary for the engraftment. This was in accordance with the report by Martin26: the addition of a small number of donor CD8+ T cells (not CD4+ T cells) to T-cell–depleted donor BMCs was capable of reconstituting recipients with donor hematopoietic cells. The graft-enhancing effect of CD8+ T cells in the BM might be attributed to their cytotoxic or suppressive activity against host CD8+and/or CD4+ T cells responsible for causing graft rejection.25
In the last few decades, remarkable advances have been made in treating autoimuune diseases, which include anti-inflammatory drugs (such as steroid hormones), immunosuppressants, and cytotoxic drugs. However, long-term administration is necessary in these treatments, which results in cumulative side effects. Recently, human data have also accumulated indicating that allogeneic BMT27 (not autologous BMT28) can be used to treat various autoimmune diseases; it has been reported that no autoimmune diseases have been seen to recur after allogeneic BMT in 13 patients with autoimmune diseases plus leukemia or aplastic anemia during long-term observations (range, 7 to 20 years).29 However, there have recently been reports on the rapid recurrence or persistence of autoimmune diseases after autologous BMT,28 as we9,16 and Karussis et al30 have previously reported in MRL/lpr mice. Based on these findings, we would like to propose here a new safe method for allogeneic BMT using chimeric resistant MRL/lpr mice: (1) fractionated radiation (5 Gy × 2) rather than one shot of a high dose of radiation (8.5 Gy), (2) CY for the induction of tolerance, and (3) two injections of WBMCs or CD4-depleted BMCs.
In conclusion, we believe that chimeric resistant mice such as MRL/lpr mice provide a useful tool for not only discovering a new safe method for allogeneic BMT but also analyzing the mechanism behind tolerance induction.
ACKNOWLEDGMENT
The authors thank Fujio Ishida and Eiichi Ohtsuki (Research Center of Kansai Medical University) for flow cytometry studies and Keiko Ando for preparing the manuscript.
K.T. and M.I. contributed equally to this work.
Supported by a grant for Experimental Models for Intractable Diseases from the Ministry of Health and Welfare of Japan and a grant from the Japanese Private School Promotion Foundation.
Address reprint requests to Susumu Ikehara, MD, PhD, First Department of Pathology, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi City, Osaka 570, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Immunofluorescence microscopical findings of glomerular IgG deposits in the kidneys of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The glomerulus of an untreated MRL/lpr mouse shows the IgG deposit, whereas the glomerulus of the treated mouse shows no IgG deposit.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4616/4/m_blod412tak04z.jpeg?Expires=1767966833&Signature=ycRSod03r5wdpGKRliEtH~id9N7FLl9ZM6smZ3DjroRm0PMOKk0jSISH95DtSiUTaU2Og1ZILmnrj4urkBghhIgMWnU1VK38gqpSIDXgIqM9XexHKX8TyhRg2GE-7Y4waevrRwwTgQZ1aeREkBuwpIVyv~JADeGuE~4cRk2mfGZlCTgw5um7bEf~loaYsVOhQ3IwokL4UbWH4Bnh8YOnMgqB0UUeTfe0g8G8~9bMr1el7UWOkcfk1ioOl55obtop2ppm~8aPqK5qZ8-CeH1rWCptSmsITUOO-qmPOIZp1x6gJUcWrq6lu-snD4K2xKtSRZoD7xAyVqbvLr0La7aOHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Histopathologic findings in the hindpaw joint of (A) untreated MRL/lpr at 18 weeks of age and (B) MRL/lpr mice treated with CY/2X/Bone/2BMT (56 weeks after the treatment [72 weeks of age]). The joint of the untreated MRL/lpr mouse shows marked lymphoid cell infiltration and pannus formation, whereas the joint of the treated MRL/lpr mouse shows neither lymphoid cell infiltration nor pannus formation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4616/4/m_blod412tak05z.jpeg?Expires=1767966833&Signature=1s6mjNk~aNNbG84BX4bdBNSszP38GCmd6304R0TK7jOHIskJ~Vt4hsKXOEGYe8yenbLwM-8dM0vHepZvmU6UUbgge41ozZcdxk9gZ48shvGmptHlJpgdBiESb28rYNoj2V4ZYNkRTzmravNZ6G4Ws~N-b~nUQbyDE9IXwe62lo-O2mxu9r9lCAL~jDmoeN~JMnD~ohVxP5RPVyLdEyeqWa5ZD8trjWDmo~3ad1VgVPPWGeMkTVm1ozq27dSuqV~0LeN8jtshEmeFnl7PKzfVYIFSr77~pYC0xhWAg6meW1UNzOW4UVnXMUBUpZYsfV3C1hJX2m-C70cRMhvq26VwpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Histopathologic findings in the spleen (A), bone marrow (B), liver (C), colon (D), skin (E), and lung (F) of mice reconstituted with CD4-depleted BMCs [CY/2X/Bone/2BMT(−CD4)]. Histopathologic examination was performed 40 weeks after the treatment. The spleen and bone marrow show normal architecture with normal hematopoiesis, and there is no remarkable lymphocyte infiltration in the liver, colon, skin, or lung, indicating no GVHD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4616/4/m_blod412tak06z.jpeg?Expires=1767966833&Signature=T7O~5pa-u2fyMfY~0UILAg6wgL8hJ1QMb4o7xENGgqMfltQsxEuVk9v-zk0-H2WNkLO-PONmkWGrunsatS-g7zyTyo4qOLeYnPexKfvQ5Whc0mqrXyT0NVOSqzstznUID0iOKqPZu4lrDjH9FtrYR1RR2rwqSZ2MYkIauTVvjuWtmTVGZOYL-pLGbseK7I9uVkuV03ikwmyukZWX7IAncB9GEWMw1-Jws5qVD4N38ca5Ajs4GwH-czGmKytlwdUPGLvJgzDtTQznB4cDCIVVSiZALlzJg~kQDrf6KiaCE8pJUeajRSvSckXcjNx0ql132420BYeR60iyAXZlrzaf5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal