Abstract
Factor V (FV) is a central regulator of hemostasis, serving both as a critical cofactor for the prothrombinase activity of factor Xa and the target for proteolytic inactivation by the anticoagulant, activated protein C (APC). To examine the evolutionary conservation of FV procoagulant activity and functional inactivation by APC, we cloned and sequenced the coding region of murine FV cDNA and generated recombinant wild-type and mutant murine FV proteins. The murine FV cDNA encodes a 2,183-amino acid protein. Sequence comparison shows that the A1-A3 and C1-C2 domains of FV are highly conserved, demonstrating greater than 84% sequence identity between murine and human, and 60% overall amino acid identity among human, bovine, and murine FV sequences. In contrast, only 35% identity among all three species is observed for the poorly conserved B domain. The arginines at all thrombin cleavage sites and the R305 and R504 APC cleavage sites (corresponding to amino acid residues R306 and R506 in human FV) are invariant in all three species. Point mutants were generated to substitute glutamine at R305, R504, or both (R305/R504). Wild-type and all three mutant FV recombinant proteins show equivalent FV procoagulant activity. Single mutations at R305 or R504 result in partial resistance of FV to APC inactivation, whereas recombinant murine FV carrying both mutations (R305Q/R504Q) is nearly completely APC resistant. Thus, the structure and function of FV and its interaction with APC are highly conserved across mammalian species.
HUMAN FACTOR V (FV) is synthesized as a single-chain precursor glycoprotein of 2,224 amino acids (aa), consisting of a 28-aa N-terminal signal peptide, followed by the 2,196-aa mature protein.1,2 Mature FV is composed of internally repeated homologous domains A (A1, A2, A3) and C (C1, C2), and a nonrepeated B domain, organized in the order A1, A2, B, A3, C1, and C2.1-3 FV is activated to FVa by thrombin cleavage at three residues (R709, R1018, and R1545), removing most of the B domain.4-7 The resulting noncovalent heterodimer is composed of a heavy-chain (residues 1-709) and a light-chain (1546-2196) held together by divalent cation-dependent interactions.1,5,8-11 The function of the B domain remains unclear, although it may be involved with facilitating FV activation by thrombin.12
FVa is a critical protein in the coagulation cascade. It is an essential cofactor for factor Xa (FXa), together forming the prothrombinase complex which, in the presence of calcium and a phospholipid surface, efficiently converts prothrombin to active thrombin.13 FVa is also a proteolytic target for activated protein C (APC).14,15 APC exerts its anticoagulant function through its inactivation of FVa and factor VIIIa (FVIIIa).16-20 In humans, APC inactivates FVa to FVi via proteolytic cleavage at R306, R506, and R679 in the FVa heavy chain.14,15,21,22 An initial rapid cleavage at R506 facilitates the otherwise slow cleavage reaction at R306. Cleavage at R306 is associated with the complete loss of FVa activity.23 Although cleavage at R506 does not result in complete loss of FVa activity, the 10-fold reduction in APC inactivation of the mutant form of FVa, R506Q, results in a higher risk for thrombotic disorders.24-26
APC resistance is a very frequent finding in patients with thrombotic disorders.27-29 The R506Q mutation in human FV accounts for nearly all individuals with APC resistance, with an allele frequency of 2% to 7% in European populations,30 making it one of the most common genetic risk factors for thrombosis.25,31Cosegregation of the R506Q mutation increases the penetrance of thrombosis in protein C–deficient32 and protein S–deficient33 patients.
Analysis of human1 and bovine34 FV cDNAs shows a high degree of sequence conservation. Sequence data has not yet been reported for any other mammalian species. To develop an animal model for the study of FV function in vivo and to examine the evolutionary conservation of FV procoagulant activity and functional inactivation by APC, we have cloned and sequenced the coding region of the murine FV cDNA and analyzed FV mutations at the putative APC cleavage sites. The murine FV cDNA shows a high degree of evolutionary conservation. Recombinant murine FV proteins carrying one or both of two mutations at the APC cleavage sites (R305Q and R504Q, homologous to R306Q and R506Q in human FV) maintain normal procoagulant function. However, mutant murine FV proteins carrying either R305Q or R504Q are partially resistant to APC inactivation, whereas the double-mutant is markedly resistant to APC.
MATERIALS AND METHODS
Chemicals and reagents.
The mouse C57BL/6J bone marrow (BM) cDNA library was a gift of J. Lowe (University of Michigan, Ann Arbor) and the mouse Sv129 genomic library was purchased from Stratagene (La Jolla, CA). The Muta-Gene in vivo mutagenesis kit and Affi-Gel 10 affinity matrix were purchased from Bio-Rad (Hercules, CA). OPTI MEM I media and Trizol for total RNA isolation were from GIBCO-BRL (Gaithersburg, MD). Normal and FV-deficient human plasmas were purchased from George King Bio-Medical, Inc (Overland Park, KS). Thromboplastin (with 25 mmol/L calcium) was obtained from Sigma (St Louis, MO). Human thrombin was purchased from Calbiochem (San Diego, CA). APC was from Enzyme Research Labs Inc (South Bend, IN), and phospholipid vesicles (Inosithin) were a gift of P.J. Fay (University of Rochester, Rochester, MD). FV dilution buffer is from Pharmacia Heper (Franklin, OH). Precharged Ni2+affinity purification matrix was bought from Invitrogen (Carlsbad, CA), as ProBond. Nitrocellulose membrane (BA85) was purchased from Schleicher & Schuell (Keene, NH).
Cloning of murine FV genomic DNA.
Two λ phage spanning exons 7 to 13 were cloned as described previously.35 The intron/exon junctions were determined by DNA sequencing using primers based on the murine FV cDNA sequence. Introns were amplified by polymerase chain reaction (PCR) and their size determined by comparison to a commercial 1-kb DNA ladder (GIBCO-BRL) separated by agarose gel electrophoresis. A 790-bp fragment of the murine FV cDNA containing the 3′ end of the coding sequence and including 13 bp beyond the termination codon was used as a probe to screen the same mouse genomic library. Two additional λ phage were isolated, and a portion of their sequence beyond the FV stop codon was determined (sequence identical for both clones).
Cloning of the murine FV cDNA.
A total of 5 × 106 clones from a C57BL/6J BM cDNA library, in plasmid pCDM8, was screened by standard methods36 using the human FV cDNA as probe. Seven unique cDNA clones were identified (mFV1-7, Fig1). Additional sequence corresponding to the B domain was obtained from a 4.7-kb genomic Nco I fragment (designated as “exon 13” in Fig 1). An additional 275 bp from the A2 domain was obtained by reverse transcriptase (RT)-PCR amplification of BM and liver RNA templates obtained from a C57BL/6J X DBA mouse. Total RNA was isolated from mouse liver and BM using Trizol, according to manufacturer's instructions. First-strand cDNA synthesis was performed with avian myeloblastosis virus (AMV) RT and a primer complementary to sequence in exon 13 (5′-CTGGAGAAAGGGACACC-3′) at 42°C for 1 hour, followed by PCR amplification using primers in exons 7 (5′-TCATAGCCGCAGAGGAGGTCA-3′) and 12 (5′-ATGGGGAACAGGGTCAAGGTG-3′). BM and liver experiments were performed independently; two BM- and three liver-derived clones were sequenced and found to be identical (clone labeled “PCR,” Fig 1). The overlapping clones are shown in Fig 1. The entire cDNA sequence was determined on both strands.
Schematic of murine FV cDNA and protein structure. The top bar represents the murine FV protein with the domain boundaries numbered above. The two putative APC cleavage sites at R305 and R504 in murine FV are indicated by arrows. FV domains are labeled as A1-A3, B, and C1-C2. The FV coding sequence (lower bar) was cloned from a C57BL/6J BM cDNA library using the human FV cDNA as probe. The seven unique cDNAs identified are shown as single lines and labeled mFV1-7. The clone labeled “PCR” indicates the DNA fragment obtained through RT-PCR, and “exon 13” is the segment obtained from a genomic library. The major restriction enzyme sites used in the assembly are labeled on the top of the bar [Sac I (781),Apa I (1258), Pst I (1834), Nde I (2073),Kpn I (3976)]. Cla I and Sal I cut the vector sequences adjacent to the FV cDNA.
Schematic of murine FV cDNA and protein structure. The top bar represents the murine FV protein with the domain boundaries numbered above. The two putative APC cleavage sites at R305 and R504 in murine FV are indicated by arrows. FV domains are labeled as A1-A3, B, and C1-C2. The FV coding sequence (lower bar) was cloned from a C57BL/6J BM cDNA library using the human FV cDNA as probe. The seven unique cDNAs identified are shown as single lines and labeled mFV1-7. The clone labeled “PCR” indicates the DNA fragment obtained through RT-PCR, and “exon 13” is the segment obtained from a genomic library. The major restriction enzyme sites used in the assembly are labeled on the top of the bar [Sac I (781),Apa I (1258), Pst I (1834), Nde I (2073),Kpn I (3976)]. Cla I and Sal I cut the vector sequences adjacent to the FV cDNA.
Site-directed mutagenesis.
A Sac I-Pst I fragment (nucleotides 781-1834, Fig 1) of the murine FV cDNA, containing both putative APC cleavage sites (R305 and R504), was cloned into pSELECT-1 (Promega, WI) and the mutations R305Q, R504Q, or both mutations in cis were introduced by site-directed mutagenesis following the manufacturer's instructions. The mutagenesis oligonucleotides were 5′-CCAAAGAAAACGCA(AG)GAGCCCCAAGACC-3′ (R305Q) and 5′-CCTGGACCAGCA(AG)GGGTGTACAG-3′ (R504Q), in which the underlined nucleotides represent the mutations, with the native sequence in parentheses. Presence of appropriate mutations was confirmed by DNA sequencing.
Assembly of the wild-type and mutant FV cDNAs.
The full-length FV cDNA was assembled in an eight-step procedure from five different clones into the expression vector pCMV537 as a Cla I-Sal I fragment (Fig 1). The plasmid vector pCMV5 contains the cytomegalovirus promoter, and the human growth hormone (hGH) polyadenylation signal. The 5′ end of the FV cDNA to theApa I site (1-1263) was derived from clone mFV1; the second fragment, Apa I-Pst I (1263-1839), is from clone “PCR”; the third fragment, Pst I-Nde I (1839-2078), is from clone mFV2; the fourth fragment, NdeI-Kpn I (2078-3981), is from the exon 13 genomic clone; and the last fragment, from Kpn I to the 3′ end (3981-6585), is taken from clone mFV3 (Fig 1). The mutations were first introduced into theSac I-Pst I fragment (786-1839, Fig 1), and both wild-type and mutant cDNAs were then assembled in parallel. The integrity of junction regions in each construct was confirmed by DNA sequencing.
Transient transfection of COS-1 cells.
COS-1 cells grown in a 100-mm plate were transfected with 10 μg of the appropriate wild-type or mutant construct by calcium phosphate precipitation,38 and grown for 24 hours before 3 mL serum-free OPTI-MEM I media were added and then grown for another 48 hours before harvesting.
FV clotting assay.
Conditioned media were procured, diluted in dilution buffer, and assayed for FV activity. Samples (50 μL) were mixed 1:1 with human FV-deficient plasma, and warmed at 37°C for 3 minutes. Prewarmed thromboplastin with 25 mmol/L CaCl2 (100 μL) was then added, and the time to clot formation was measured in a Medical Laboratory Automation (Pleasantville, NY) Electra 750 coagulation timer. A standard curve was generated using dilutions of pooled normal human plasma.
APC resistance assay.
Wild-type and mutant FV conditioned media were obtained as described above, and concentrated threefold to fivefold using Centricon-30 concentrators (Amicon, Beverly, MA) to yield preparations with 500 to 1,000 mU/mL FV activity and activated using thrombin (1 U/mL). Complete activation was usually obtained within 5 minutes, at which time (designated t = 0) 0.1 μg/mL human APC, 100 μg/mL phospholipid vesicles, and 5 mmol/L CaCl2 were added to initiate FVa inactivation by APC. Samples were taken at various time points, diluted 50-fold in 50 mmol/L Tris (pH = 7.3), 0.2% bovine serum albumin, and immediately assayed for FV activity. FV activity was determined as described above except that 100 μL of the sample was incubated with 100 μL of human FV-deficient plasma and 100 μL thromboplastin for 3 minutes, then 100 μL of 25 mmol/L CaCl2 was added and the time to form a clot was determined.
Generation of polyclonal rabbit antimurine FV heavy-chain antibodies.
Murine FV cDNA encoding amino acids −27 to 662 (includes the signal peptide and heavy chain) was amplified by PCR and cloned into the vector pGEX-KG (kindly provided by J.E. Dixon, University of Michigan, Ann Arbor).39 The corresponding Escherichia coli–expressed insoluble GST-fusion protein (GST-mFV-H) was first washed in 2 mol/L guanidine-HCl, 50 mmol/L Tris-Cl, pH 6.8, 5 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, and solubilized in the same solution containing 3 mol/L guanidine-HCl. GST-mFV-H was then precipitated by dilution to 0.3 mol/L guanidine-HCl, washed twice, and separated by electrophoresis on a 3%/8% sodium dodecyl sulfate (SDS)-polyacrylamide gel.40 The portion of gel containing GST-mFV-H was excised, lyophilized, and used as the immunogen for the generation of rabbit antisera by a commercial supplier (Rockland, Gilbertsville, PA). A second murine FV heavy-chain fusion protein (Thx-mFV-H) containing amino acids 12 to 662 was expressed in the vector pET-32a (Novagen, Madison, WI), solubilized in 8 mol/L urea, 500 mmol/L NaCl, 20 mmol/L Tris-Cl, pH 7.9, purified on Ni2+affinity matrix, and coupled to Affi-Gel 10 affinity purification matrix according to the manufacturer's instructions. Crude rabbit antiserum was affinity purified, eluted with 20 mmol/L citrate buffer, pH 2.5, and neutralized by NaOH.
Analysis of APC cleavage of murine FV.
Serum-free conditioned media from COS-1 cells transfected with wild-type or mutant murine FV expression plasmids were procured and treated first with thrombin (1 U/mL) for 5 minutes at 37°C, and then with APC (0.1 μg/mL) plus phospholipid vesicles (100 μg/mL) and 5 mmol/L CaCl2 for 25 minutes at 37°C. Samples were separated by electrophoresis on an 8% SDS-polyacrylamide gel. Western blotting was performed as previously described41 with the affinity-purified polyclonal antimurine FV heavy-chain antibody. Mouse anti-rabbit IgG conjugated to horseradish peroxidase was purchased from Accurate (Westbury, NY), and the ECL chemiluminescence kit from Amersham (Arlington Heights, IL).
RESULTS AND DISCUSSION
The intron-exon junction sequences in the murine FV gene.
The genomic structure of murine FV exons 7-13 was determined from two previously identified SV129 genomic clones.35 The intron-exon junction sequences have been deposited in GenBank (GenBank Accession No. AF040572-AF040577). Complete conservation of exon structure is observed in this region, compared with the human gene.42 As in the human, the entire mouse FV B domain is contained within a single exon (exon 13).
Analysis of the murine FV cDNA coding sequence.
The complete cDNA sequence for the murine FV coding region (6,552 bp) was determined on both strands (GenBank Accession No. U52925). The sequence in the vicinity of the initiation codon fits the consensus described by Kozak.43 A consensus polyadenylation signal (AATAAA) is found 39 bp following the termination codon (determined from the genomic sequence, and confirmed by RNA PCR).
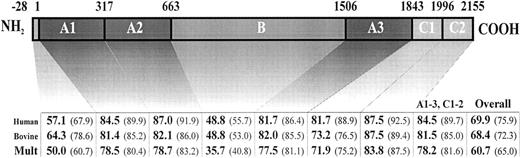
The murine FV cDNA encodes a 2,183-aa protein, which includes a 28-aa signal peptide, followed by the mature protein of 2,155 aa. Alignment of the predicted aa sequence for murine FV with the human1and bovine34 sequences shows that the functionally important A1-A3 and C1-C2 domains are highly conserved (>84% sequence identity between murine and human, and 60% overall amino acid identity among the human, bovine, and murine FV sequences) (Fig2). In contrast, only 35% identity among all three species is observed for the poorly conserved B domain. A similar high degree of interspecies divergence has been noted for the FVIII B domain, which is also poorly conserved in homology comparison between FV and FVIII. These data have suggested that the B domain may serve primarily a spacer function, with little selective pressure to conserve a specific aa sequence. Consistent with this hypothesis, human FV and FVIII lacking B domain sequences retain procoagulation activity.44 45
FV amino acid sequence comparison. The human FV amino acid sequence (“Human”) and the bovine FV amino acid sequences (“Bovine”) were compared pairwise against the murine FV sequence. A multiple comparison of all three sequences simultaneously was also done (“Mult”). All comparisons were performed by the MegAlign computer program (DNASTAR, Madison, WI), using the Clustal method. The numbers on the top of the bar indicate the domain boundaries in murine FV, deduced from sequence comparison with the human and bovine proteins. Values for each domain are the percentage amino acid identity, with the percentage amino acid similarity in parentheses.
FV amino acid sequence comparison. The human FV amino acid sequence (“Human”) and the bovine FV amino acid sequences (“Bovine”) were compared pairwise against the murine FV sequence. A multiple comparison of all three sequences simultaneously was also done (“Mult”). All comparisons were performed by the MegAlign computer program (DNASTAR, Madison, WI), using the Clustal method. The numbers on the top of the bar indicate the domain boundaries in murine FV, deduced from sequence comparison with the human and bovine proteins. Values for each domain are the percentage amino acid identity, with the percentage amino acid similarity in parentheses.
The murine FV protein is 41 aa shorter than human FV. This difference is due primarily to a relative deletion within the murine B domain. The human FV B domain contains two tandem repeats of a 17-aa sequence and 31 tandem repeats of a 9-aa motif. The murine sequence has only one 17-aa repeat, and is missing 9 aa corresponding to parts of the 26th and 27th 9-aa repeat, as well as 11 aa immediately preceding the first repeat. Differences in these repeat sequences account for nearly all of the variation in length (37 of 41 aa) between human and murine FV. Bovine FV contains only one 17-aa repeat and 29 copies of the 9-aa motif.34
A number of distinct structural features noted in the human FV B domain1 are also conserved in the mouse. Human FV contains 37 potential N-linked glycosylation sites, with 25 located in the B domain. By comparison, murine FV contains 27 potential N-glycosylation sites, with 17 located in the B domain similar to that of bovine FV (28 total and 18 in the B domain). Interspecies variation among the B domain 9-aa repeat motifs accounts for the greater number of potential N-linked glycosylation sites in human FV relative to mouse or bovine FV. Sixteen of the potential N-linked glycosylation sites in murine FV (9 in the B domain) are conserved in both human and bovine sequences. Thus, the B domain appears to be a consistent site for extensive N-linked glycosylation, a processing step that may be required for efficient secretion of FV.46
All cysteine residues predicted to form disulfide linkages in bovine FV are conserved in murine FV as well as in human FV, as are the three predicted free cysteines located one each in the A2, C1, and C2 domains.47,48 Murine FV, like bovine FV, lacks the single free cysteine in the human FV B domain.1 Based on the conservation between the species, this would predict disulfide linkages in murine FV between Cys138-Cys164, Cys219-Cys300, Cys470-Cys496, Cys573-Cys654, Cys1657-Cys1683, Cys1838-Cys1992, and Cys1997-Cys2152. Residues Cys537, Cys583, and Cys1919 are predicted to be free cysteines.
The arginine residues at all thrombin and APC cleavage sites are conserved between human, murine, and bovine FV, except at the R679 APC cleavage site (Fig 3). Bovine FV is cleaved instead at R662,49 where human FV has a proline at the corresponding position. Interestingly, murine FV has arginine at both positions (R656, corresponding to bovine R662, and two consecutive arginines, R678 and R679, corresponding to human R679). From our present data we are unable to precisely determine at which homologous position APC cleaves murine FV. The sequences surrounding the cleavage sites are also highly conserved between species. In addition, the sequence in the vicinity of the R506 APC cleavage site is well conserved in comparison between the homologous segments of FV and FVIII.
Cross species alignment of FV sequences in the vicinity of (A) human APC cleavage sites R306 and R506; (B) thrombin cleavage sites; and (C) the acidic region at the C-terminus of the heavy chain, including the human APC cleavage site R679. Cleavage sites are numbered according to either the human (hFV) or bovine (bFV) protein, as indicated in parentheses. The “↓” indicates the cleavage site in both (A) and (B) at the conserved underlined arginine residue. The third APC cleavage site (C) varies between the human and bovine proteins, as indicated. Only the sequence surrounding the FV APC cleavage site R506 is conserved between FV and FVIII; all other APC and thrombin cleavage sites fail to show significant homology across these two proteins (not shown).
Cross species alignment of FV sequences in the vicinity of (A) human APC cleavage sites R306 and R506; (B) thrombin cleavage sites; and (C) the acidic region at the C-terminus of the heavy chain, including the human APC cleavage site R679. Cleavage sites are numbered according to either the human (hFV) or bovine (bFV) protein, as indicated in parentheses. The “↓” indicates the cleavage site in both (A) and (B) at the conserved underlined arginine residue. The third APC cleavage site (C) varies between the human and bovine proteins, as indicated. Only the sequence surrounding the FV APC cleavage site R506 is conserved between FV and FVIII; all other APC and thrombin cleavage sites fail to show significant homology across these two proteins (not shown).
Three of the seven tyrosine sulfation sites predicted in human FV50 are conserved in murine FV. These are Tyr664, Tyr697, and Tyr1470, corresponding to Tyr665, Tyr698, and Tyr1510 of human FV.1 The same three tyrosine residues and surrounding acidic residues are also conserved in bovine FV.34 Tyrosine sulfation of FV appears to be required for efficient activation by thrombin.51 The serine phosphorylation site corresponding to Ser690 of bovine FV is not present in murine FV.52
Procoagulant activity of the wild-type and mutant FV proteins.
Plasmid expression vectors carrying wild-type murine FV cDNA, and mutants at the R305 and R504 APC cleavage sites (R305Q and R504Q) as well as the double mutant (R305Q/R504Q), were transiently transfected into COS-1 cells, and the FV procoagulant activity in the conditioned media measured by reconstitution of human FV-deficient plasma. Using normal human plasma as standard, the conditioned media from wild-type and all mutants contained similar FV clotting activities (ranging from 450 mU/mL to 650 mU/mL). Thus, introduction of these mutations does not appear to affect FV procoagulant function, consistent with observations for the corresponding human native and recombinant FV proteins.21 53-55 This observation also shows that murine FV efficiently complements human FV function in plasma.
APC resistance of wild-type and mutant FV.
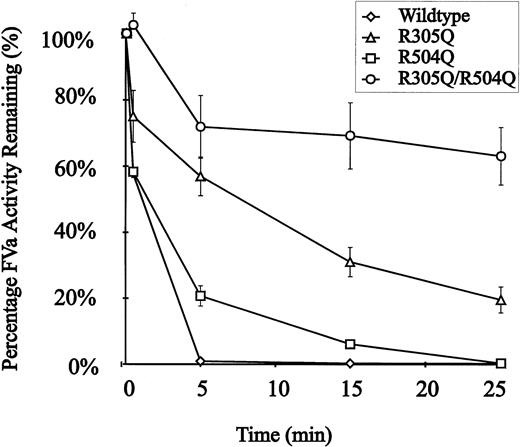
The recombinant murine FV proteins were assayed for susceptibility to APC inactivation using standard methods (see Materials and Methods). Conditioned media were concentrated and then activated by thrombin before addition of APC. Wild-type recombinant murine FV was completely inactivated by t = 5 minutes (Fig 4). In contrast, introduction of a single mutation at R305Q or R504Q results in partial resistance to APC. The double mutant (R305Q/R504Q) is markedly resistant to APC inactivation, retaining ∼70% of its initial peak activity at t = 25 minutes (Fig 4). The observed ∼30% decrease in activity in the R305Q/R504Q double-mutant could be explained by either cleavage at the COOH terminus of the FVa heavy chain similar to the R679 cleavage in humans22,23,53-56 or by inhibition of FV activation due to binding of APC to the APC-resistant mutant.57
APC resistance of recombinant wild-type and mutant murine FV. Each point represents the average of the activity measured in conditioned media from three independent transfections. APC resistance assays were performed as described in the experimental procedures. APC was added to the reactions at t = 0. (◊), Wild-type FV; (▵), FV R305Q; (□), FV R504Q; (○), FV R305Q/R504Q.
APC resistance of recombinant wild-type and mutant murine FV. Each point represents the average of the activity measured in conditioned media from three independent transfections. APC resistance assays were performed as described in the experimental procedures. APC was added to the reactions at t = 0. (◊), Wild-type FV; (▵), FV R305Q; (□), FV R504Q; (○), FV R305Q/R504Q.
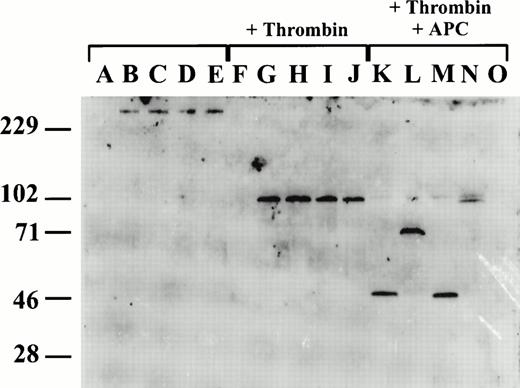
Because murine FV was not recognized by available anti-human FV antibody reagents (data not shown), we generated an affinity-purified rabbit polyclonal anti-murine FV heavy-chain antibody (see Materials and Methods). This antibody detects intact single-chain mouse FV, and FV heavy-chain in both conditioned media from transfected COS-1 cells and mouse plasma by Western blot analysis, but does not recognize the light chain (Fig 5). To assess the fragments resulting from APC cleavage of murine FV, conditioned media were reacted with human thrombin and APC (for 25 minutes) and the products were analyzed by Western blot (Fig 5). Intact single-chain FV (∼300 kD) is evident in unreacted conditioned media for wild-type and all the mutant FVs. After the addition of thrombin, identical ∼110-kD heavy-chain fragments are generated from the wild type and all three mutants. After treatment with APC, an ∼50-kD band is seen in both wild-type and the R504Q mutant, consistent with the expected N-terminal fragment resulting from cleavage at R305, and associated with complete loss of FV cofactor activity (Fig 4). The antibody appears to recognize epitopes restricted to the N-terminal segment (residues 1-305) of the FV heavy chain, explaining the failure to visualize the C-terminal cleavage fragments. The ∼70-kD fragment generated by APC cleavage of the R305Q mutant is consistent with cleavage at R504 and the complete loss of the second N-terminal cleavage as a result of the R305Q mutation. Cleavage by APC of the R305Q/R504Q mutant generates a large ∼105-kD fragment, consistent with the loss of both heavy-chain cleavages. The doublet of this large fragment is consistent with slow cleavage at the third APC cleavage site (within residues 657-709 comprising the acidic domain). This cleavage could be responsible for the observed ∼30% loss of FV cofactor activity (Fig 4).
APC cleavage of recombinant wild-type and mutant murine FV. Western blot analysis was performed using a rabbit anti-murine FV antibody which recognizes an epitope(s) on the N-terminal half of the FV heavy chain (residues 1-305). Lanes A through E are untreated conditioned media, lanes F through J are following activation with thrombin, and lanes K through O are following thrombin activation and subsequent treatment with APC. Lanes A, F, and O are conditioned media from mock-transfected cells; lanes B, G, and K, from cells transfected with wild-type FV; lanes C, H, and L, from cells transfected with R305Q mutant FV; lanes D, I, and M, from cells transfected with R504Q mutant FV; lanes E, J, and N, from cells transfected with R305Q/R504Q mutant FV.
APC cleavage of recombinant wild-type and mutant murine FV. Western blot analysis was performed using a rabbit anti-murine FV antibody which recognizes an epitope(s) on the N-terminal half of the FV heavy chain (residues 1-305). Lanes A through E are untreated conditioned media, lanes F through J are following activation with thrombin, and lanes K through O are following thrombin activation and subsequent treatment with APC. Lanes A, F, and O are conditioned media from mock-transfected cells; lanes B, G, and K, from cells transfected with wild-type FV; lanes C, H, and L, from cells transfected with R305Q mutant FV; lanes D, I, and M, from cells transfected with R504Q mutant FV; lanes E, J, and N, from cells transfected with R305Q/R504Q mutant FV.
APC resistance and FV evolution.
Interest in FV function has increased recently as a result of the identification of a common sequence variation in the human FV gene, which appears to be a major risk factor for thrombosis. Substitution of glutamine for arginine 506, resulting in a mutant FV that is partially resistant to APC inactivation, has been identified in as many as 7% of normal individuals in some populations (FV Leiden).30 This common mutation is associated with a marked increase in the risk of thrombosis.25,31,58 Despite this frequent variation in humans, our studies confirm the maintenance of very similar FV structure and function across species. Although previous studies have suggested fundamental differences in the interaction of human APC with protein S and FV from other species including rats,59 our data are consistent with conservation of the FV/APC interaction among mammalian species. Variation in carbohydrate structure, which has recently been shown to modulate FV inactivation by APC,60may also explain some previously observed differences in the interaction between FV and APC across species. The location of APC cleavage sites is conserved, and resistance to APC similar to that observed in human FV Leiden is obtained with a mutation at the homologous position in murine FV. In addition, the functional assays performed in human plasma indicate that FV procoagulant functions is also highly conserved.
Taking all of these observations together, it is perhaps surprising that mutation at the critical R506 APC cleavage site should be tolerated at the high population frequency observed for the human R506Q mutation, given the negative selection that would be anticipated. These observations suggest that a significant recent change in hemostatic balance may have decreased dependence on APC function in humans compared with other mammals. Alternatively, a recent, as yet unidentified positive selection in humans for the mutant R506Q allele may counteract the strong negative selection that would appear to have been present previously in evolution. Recent genetic evidence of a founder effect for this mutation further supports this latter hypothesis.61 The high degree of structural and functional conservation between human and murine FV in both procoagulant and APC anticoagulant function suggests that the mouse should be an appropriate model in which to study FV function in vivo. The mouse may also provide the necessary in vivo tools to identify the positive selection responsible for the high population frequency of the human FV R506Q mutation.
Supported by National Institutes of Health Grants No. 1P01HL57346-01A1 (D.G.), HL39639 (D.G.), HL52173 (R.J.K.), and HL53777 (R.J.K.).
Address reprint requests to David Ginsburg, MD, The University of Michigan Medical School, 4520 MSRB I, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0650.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Schematic of murine FV cDNA and protein structure. The top bar represents the murine FV protein with the domain boundaries numbered above. The two putative APC cleavage sites at R305 and R504 in murine FV are indicated by arrows. FV domains are labeled as A1-A3, B, and C1-C2. The FV coding sequence (lower bar) was cloned from a C57BL/6J BM cDNA library using the human FV cDNA as probe. The seven unique cDNAs identified are shown as single lines and labeled mFV1-7. The clone labeled “PCR” indicates the DNA fragment obtained through RT-PCR, and “exon 13” is the segment obtained from a genomic library. The major restriction enzyme sites used in the assembly are labeled on the top of the bar [Sac I (781),Apa I (1258), Pst I (1834), Nde I (2073),Kpn I (3976)]. Cla I and Sal I cut the vector sequences adjacent to the FV cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4593/4/m_blod41202001y.jpeg?Expires=1769096516&Signature=vcDQKpPfC3VCWxtT2xY5ylVI00Q8aaKjwE2aFU92rKPV9Ml4SG7C-afTno8cpw~vnzUGOHQXLBph4ITcTkkhNuuvQVwu~xKHKlV4-nIokylqgBw4ppPHeGXOyfVY0QILZmct-rto4ydJQI8fdElBwiff6KY8stAVttQ0usB3~~2~nvVILwvPv6p9ZT-XcWgqx2GZMrxXwFyVc4fwTkZPthuBBkvcKQRtZJ7FpGcupRa8OiG-YoRXMExA5BWexlMpA0Xl-EqQRzOTk-l-OBHoSeKW9xxF1W26pAqMVVPITaM4~iMjW-CZixZsgAq0UGnsSfaNN8jkrKt7yYGNj6QSag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal