Abstract
SHP-1 protein tyrosine phosphatase is a critical negative regulator of mitogenic signaling, as demonstrated by the heightened growth responses to hematopoietic growth factors in hematopoietic cells of motheaten mice, which lack functional SHP-1 expression due to mutations in the SHP-1 gene. The mitogenic signaling molecules dephosphorylated by SHP-1 have not been fully identified. We detected two proteins (p32/p30) that are hyperphosphorylated in a DA3/erythropoietin receptor (EpoR) cell line that expresses a mutant containing the SHP-1 C-terminus that suppresses the function of the endogenous phosphatase and induces hyperproliferative responses to interleukin-3 (IL-3) and Epo. Hyperphosphorylated p32/p30 are also detected in motheaten hematopoietic cells, demonstrating an association of p32/p30 hyperphosphorylation with SHP-1-deficiency and growth factor-hyperresponsiveness. The hyperphosphorylated p32/30 associate with SHP-1 via its C-terminus, because they coimmunoprecipitate with the phosphatase and the C-terminal mutant and they bind in vitro to a synthetic peptide of the mutant but not the GST fusion proteins of SHP-1 SH2 domains. Induction of p32/p30 phosphorylation by IL-3 or Epo occurs mainly at 2 to 18 hours poststimulation in the DA3/EpoR cell line, indicating p32/p30 as novel signaling molecules during cell cycle progression. These data demonstrate a function for the SHP-1 C-terminus in recruiting potential substrates p32/p30 and suggest that SHP-1 may regulates mitogenic signaling by dephosphorylating p32/p30.
HEMATOPOIETIC growth factors, such as interleukin-3 (IL-3) and erythropoietin (Epo), play critical roles in hematopoiesis. Signal transduction for both IL-3 and Epo depends on the induction of tyrosine phosphorylation to activate the Ras and the Jak2 signaling pathways.1 The receptors for IL-3 (IL-3R) and Epo (EpoR) have no intrinsic kinase activity. Ligand binding activates the receptor-coupled Jak2 protein tyrosine kinase.2,3 As shown by mutational analysis, the IL-3R and EpoR have distinct functional domains mediating the two signaling pathways.4,5 The membrane proximal part of the receptor cytoplasmic region is required for the activation of the Jak2 tyrosine kinase, STAT5 phosphorylation, and c-myc expression. The carboxyl terminal region of the receptor is responsible for activating the Ras pathway, leading to MAPK phosphorylation and c-fos expression. Whereas the activation of the Ras pathway is not required for mitogenic signaling, it is important for cell viability by preventing apoptosis6 and may also promote cell proliferation.7 On the other hand, activation of the Jak2 pathway and the induction of c-myc expression correlate with signaling of cell proliferation.5 However, the molecule(s) that mediates mitogenic signals downstream from the Jak/Ras pathways to apparatus controlling cell cycle progression has not been unequivocally identified. Although STAT5 activation enhances the cell proliferative response,8 it is not required for mitogenic signaling.5 Importantly, the signal transduction for IL-3 and Epo is also regulated by protein tyrosine phosphatases (PTPases), because the tyrosine phosphorylation induced by either of the growth factors is transient and returns to basal levels shortly after ligand stimulation.9 10
SHP-1 (previously called PTP1C/HCP/SHPTP1/SHP) is a cytoplasmic protein tyrosine phosphatase that contains two SH2 domains and is expressed predominantly in hematopoietic cells.11-15 Previous studies from our laboratory and others demonstrate that SHP-1 is a critical negative regulator of the mitogenic signaling of IL-3 and Epo. Hematopoietic cells from the motheaten mice, which lack functional SHP-1 due to mutations in the SHP-1 gene,16,17 are hyperproliferative in response to Epo,18 macrophage colony-stimulating factor (M-CSF),19 and granulocyte-macrophage colony-stimulating factor (GM-CSF).20 This hyperproliferation is likely one of the factors responsible for the elevated myelopoiesis of erythroid, monocytic, and granulocytic lineages in motheaten mice and for the early death of the mice from massive accumulation of myeloid cells in vital organs.21 The observation that overexpression of SHP-1 suppresses IL-3–induced cell proliferation in a murine IL-3–dependent cell line provides additional support for a negative role for the phosphatase in mitogenic signaling.22Furthermore, we and others have shown that SHP-1 associates with the receptors for hematopoietic growth factors, such as IL-3,22Epo,23,24 and stem cell factor,25 through its SH2n domain that bind to phosphotyrosine sites in the cytoplasmic regions of the receptors. In addition, SHP-1 also associates with and regulates signals from membrane molecules of other receptor complexes, such as FcγRIIB1,26 CD22,27 the nature killer cell inhibitory receptor (KIR),28 the B-cell antigen receptor,29 the receptor of interferon α/β,30 and the member of the signal-inhibition regulatory proteins (SRP).31 32
SHP-1 regulates mitogenic signaling by dephosphorylating key substrates essential for cell proliferation. The Jak family kinases have been implicated as SHP-1 substrates in recent studies. It was shown23 that an EpoR mutant, which has two of the tyrosines in the cytoplasmic domain substituted with phenylalanines and was not recognized by SHP-1 SH2 domains, caused marked and prolonged Jak2 hyperphosphorylation in response to Epo stimulation. This suggests that inhibition of SHP-1 binding to the receptor prevented it from dephosphorylating Jak2 and may have enhanced mitogenic signaling. However, the hypothesis is complicated by the observation that comparable Jak2 phosphorylation was detected from engagement of the wild-type EpoR or an EpoR null mutant with all of the tyrosines in the receptor cytoplasmic domain substituted by phenylalanines.8Moreover, it was shown recently that SHP-1 directly binds to and dephosphorylates Jak family kinase.33 Importantly, we found that GM-CSF stimulation of motheaten macrophages, which are hyperproliferative in response to the growth factor, induces only a rather modest and transient Jak2 hyperphosphorylation, whereas the cells contain several yet unidentified proteins that are markedly hyperphosphorylated.24 This suggests that SHP-1 may play a limited role in Jak2 dephosphorylation and that SHP-1 may regulate mitogenic signaling by dephosphorylating additional substrates.
To identify key substrates of SHP-1 and elucidate mitogenic signaling pathways, we sought to suppress the endogenous SHP-1 activity in a murine myeloid cell line (DA3EpoR) that depends on IL-3 or Epo for proliferation by introducing dominant negative SHP-1 mutants into the cells and to characterize hyperphosphorylated proteins induced by the mutant. Previous studies showed that deletion of the C-terminus of SHP-1 increased the SHP-1 PTPase catalytic activity,34-36suggesting that the C-terminus is inhibitory to the phosphatase. We examined Epo/IL-3 growth responses and phosphotyrosine proteins of DA3EpoR cells expressing an SHP-1 mutant containing the C-terminus of phosphatase. Two SHP-1–associated proteins, p32/p30, were detected whose hyperphosphorylation correlates with defective SHP-1 activity and cell hyperproliferation. Our data indicate that p32/p30 are novel SHP-1 substrates that function understream of Jak/Ras pathways and during cell cycle progression in mitogenic signaling of Epo/IL-3.
MATERIALS AND METHODS
Cells and cell culture.
PA317 cells37 were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS). The growth factor-dependent murine myeloid cell line DA3EpoR10 and DA3EpoR-H/Y343F5 were maintained in RPMI 1640 medium supplemented with 10% FCS and 10% WEHI-3 conditioned medium (WCM) as a source of IL-3 (∼30 U/mL). Spleen cells and bone marrow-derived macrophages from normal and viable motheaten mice were prepared as previously described.20,33 38
Cell proliferation responses were determined by an MTT assay, as described.20 For growth factor stimulation, cells were washed in 10% CSF RPMI 1640 and incubated in the medium without IL-3 for 16 hours at 37°C. Recombinant murine IL-3 (200 U/mL) or recombinant Epo (100 U/mL) was added to the cell cultures, which were then incubated at 37°C for various times, as indicated. Stimulation reactions were terminated by lysing the cells in cold lysis buffer (50 mmol/L Tris, pH 7.4, 50 mmol/L NaCl, 0.5% sodium deoxycholate, 0.2 mmol/L Na3VO4, 20 mmol/L NaF, 1% NP-40, 2 mmol/L phenylmethyl sulfonyl fluoride, 20 μg/mL of aprotinin, and 10% glycerol).
SHP-1-C mutant, transfection, and retroviral infection of DA3EpoR cells.
The SHP-1 C-terminus cDNA was generated by polymerase chain reaction39 from a murine SHP-1 cDNA clone15with synthetic oligonucleotide primers (5′-GGAATTCAGGATGAAGGCCTCGCGTACTTCC and 5′-GGTCGACCTTCCTCTTGAGAGA). The resulting cDNA fragment encodes SHP-1 C-terminus from amino acid 551 to 595, with a translation initiation codon incoporated into the 5′ end. The cDNA fragment was then ligated via its 3′ end to a double-strand DNA fragment encoding KT3 epitope (VDKPPTPPPEPET) of the SV40 T antigen40 to derive the cDNA clone of the KT3-tagged SHP-1 C mutant. The cDNA encoding this mutant was sequenced by the chain termination method,41 cloned into the pBabe/puro vector containing the puromycin resistant gene,42 and transfected24 into the retroviral packaging cell line PA317.37 DA3EpoR cells were infected by coculturing with the transfected PA317 cells for 48 hours, followed by selection in the presence of puromycin (2 μg/mL; Sigma, St Louis, MO) for 2 weeks in medium supplemented with 10% FCS and 10% WCM. DA3EpoR cells infected with the pBABE/puro vector alone were also generated under comparable conditions as a control.
RNA isolation and Northern hybridization.
Total cellular RNA was isolated from cells following the procedures previously described.20,33,38,39 43 The RNA samples (∼20 μg/well) were separated in 1.2% agarose formaldehyde gel by electrophoresis. The RNA samples were then blotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH), probed with a32P-labeled random-primed cDNA fragment encoding the SHP-1-C mutant, and detected by autoradiography.
Antibodies, immunoprecipitation, binding assays, and Western blotting.
Antibodies against SHP-1,15 Jak2,3EpoR,24 and IL-3R22 have been described previously. The antiserum against SHP-1 C-terminus was developed in rabbits with a synthetic peptide (KVKKQRSADKEKNKGSLKRK), whereas the anti–SHP-1-M antibody recognizes a peptide sequence from the middle section of the phosphatase (QKQEVKNLHQRLEGQRPENK). The hybridoma cell line of anti-KT3 monoclonal antibody was a gift from Dr G. Walter (San Diego, CA) and the antibody was purified from mouse ascites following established procedures.40 Antibodies against phosphotyrosine (anti-ptyr; UBI, Lake Placid, NY), STAT5 (Santa Cruz, Santa Cruz, CA), phospho-MAPK (Promega, Madison, WI), c-myc (UBI), and c-fos (Santa Cruz) were purchased from commercial sources.
Immunoprecipitation and Western blotting were performed as described previously.24 Immune complexes were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, blotted onto nitrocellulose membrane (Schleicher & Schuell), probed with specific antibodies, and detected using an enhanced chemiluminescence kit (ECL; Amersham, Arlington Heights, IL). For the detection of SHP-1-C protein, which has a calculated size of 6 kD and thus was difficult to quantitate by SDS-PAGE/Western blotting, nitrocellulose membrane in a dot blotting apparatus was coated with anti-KT3 antibody (20 mg/well) for 1 hour, washed three times, blocked with 5% milk solution, and incubated with various amounts of cell lysates for 2 hours. The membrane was probed with an anti–SHP-1-C antiserum (1:5,000 dilution) and detected by ECL as described above.
For binding assays,28 cell lysates were incubated with a GST-fusion protein of SHP-1 SH2 domains or with a synthetic peptide of SHP-1-C (SSKHKEEVYENVHSKSQKEEKVKKQRSADKEKNKGSLKRK; Quality Controlled Biochemicals, Inc) that was conjugated to Affi-gel 10 beads (BioRad Laboratories, Richmond, CA). Cellular proteins associated with the fusion proteins (2 μg/reaction) and peptides (1 μg/reaction) after washing in lysis buffer were analyzed by SDS-PAGE and Western blotting.
Phosphatase assays.
The preparation of GST fusion protein of SHP-1 has been described previously.28 The phosphatase (PTPase) activity of the GST-SHP-1 fusion protein was determined using pNPP (Sigma) as a substrate. The PTPase assay was performed in the absence or presence of synthetic peptide SHP-1-C (20 to 40 μmol/L) at 22°C for 30 minutes in 50 mL of reaction mixture (100 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 10 mmol/L pNPP, and 20 nmol/L GST-SHP-1). The reaction was terminated by adding 950 mL of 1 N NaOH. The reaction product p-nitrophenolate was quantified by measuring absorbance at 405 nm.
RESULTS
An SHP-1 mutant (SHP-1-C) containing the C-terminus of the phosphatase induces heightened growth response to IL-3 and Epo in DA3EpoR cells.
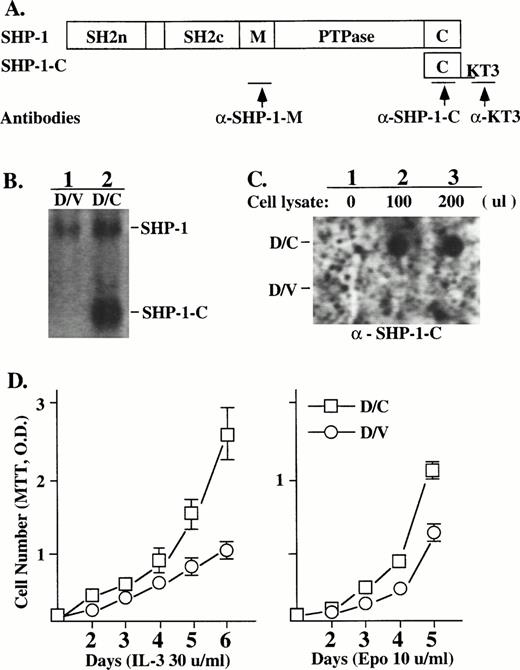
An SHP-1 mutant (SHP-1-C) containing the C-terminal 45 amino acids of the phosphatase was tagged with the KT3 epitope (Fig 1A) and introduced, by retroviral infection in pBabe vector carrying the puromycin-resistant gene, into the murine myeloid cell line DA3EpoR that depends on IL-3 or Epo for proliferation. Puromycin-resistant populations of DA3EpoR cells infected with the mutant (D/C) or the control vector (D/V) were further characterized. SHP-1-C transcript was detected by Northern hybridization in D/C but not D/V cells (Fig 1B, lanes 1 and 2). The SHP-1-C transcript in D/C cells was expressed at about twofold to threefold higher than that of the endogenous SHP-1. SHP-1-C protein expression was detected in D/C (Fig 1C, upper panel, lanes 2 and 3) but not in D/V cells (Fig 1C, lower panel, lanes 2 and 3), as expected.
The SHP-1-C mutant induced heightened growth responses to Epo/IL-3 in DA3EpoR cells. (A) The SHP-1-C mutant containing the C-terminus of the SHP-1 was tagged with the KT3 epitope and introduced into DA3EpoR cells by retroviral infection. The antigenic epitopes recognized by antibodies specific for SHP-1 (a-SHP-1-M) or SHP-1-C (a-KT3) or for both are indicated by the arrows. (B) The expression of SHP-1-C transcript in cells infected with the SHP-1-C construct (D/C) or the vector control (D/V) was determined by Northern hybridization with a SHP-1-C probe, which also detected the endogenous SHP-1. (C) The expression of SHP-1-C protein was determined by dot blotting. A membrane coated with the KT3 antibody was incubated without (lane 1) or with lysates from D/V or D/C cells (lanes 2 and 3) and probed with an anti–SHP-1-C antibody as indicated. (D) The growth responses of D/V and D/C cells to Epo or IL-3 were determined by cell proliferation assays using an MTT method and the values are the mean ± SD of three replicates.
The SHP-1-C mutant induced heightened growth responses to Epo/IL-3 in DA3EpoR cells. (A) The SHP-1-C mutant containing the C-terminus of the SHP-1 was tagged with the KT3 epitope and introduced into DA3EpoR cells by retroviral infection. The antigenic epitopes recognized by antibodies specific for SHP-1 (a-SHP-1-M) or SHP-1-C (a-KT3) or for both are indicated by the arrows. (B) The expression of SHP-1-C transcript in cells infected with the SHP-1-C construct (D/C) or the vector control (D/V) was determined by Northern hybridization with a SHP-1-C probe, which also detected the endogenous SHP-1. (C) The expression of SHP-1-C protein was determined by dot blotting. A membrane coated with the KT3 antibody was incubated without (lane 1) or with lysates from D/V or D/C cells (lanes 2 and 3) and probed with an anti–SHP-1-C antibody as indicated. (D) The growth responses of D/V and D/C cells to Epo or IL-3 were determined by cell proliferation assays using an MTT method and the values are the mean ± SD of three replicates.
Whereas both D/V and D/C populations displayed a significant growth response to IL-3 or Epo in cell proliferation assays, the D/C cells showed heightened growth response to IL-3 (Fig 1D, left) and Epo (Fig1D, right) in comparison to the D/V cells. D/C cells also showed an increased cell proliferation to different doses of Epo or IL-3 (data not shown). This increased cell growth of D/C cells was not due to changes in cell survival or caused by growth factor independent proliferation, because both populations underwent apoptosis in a similar manner after growth factor deprivation and could not be maintained in the absence of IL-3 or Epo (data not shown). A similarly increased cell growth was also detected in two other SHP-1C–expressing cell populations derived from independent transfection experiments (data not shown).
SHP-1-C induced hyperphosphorylation of p32/p30 but had no marked effect on the activation of Jak2 and Ras pathways.
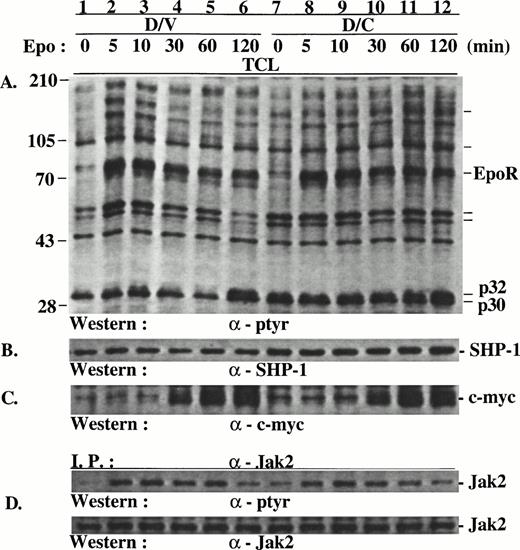
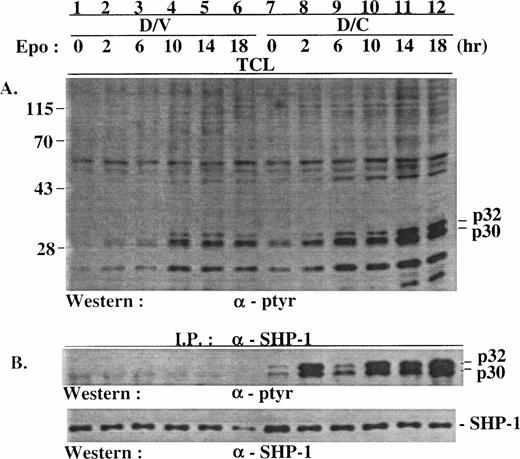
Because suppression of endogenous SHP-1 by the SHP-1-C mutant should cause hyperphosphorylation in the substrates of SHP-1, we examined Epo-induced protein tyrosine phosphorylation in D/V and D/C cells. In comparison with D/V cells, D/C cells contained several distinct proteins that were hyperphosphorylated. Among these, two proteins of approximately 32 and 30 kD (p32/p30) were prominent (Fig 2A), with p32 hyperphosphorylation more pronounced than p30. The phosphorylation of p32/p30 in D/V cells, as in the parental DA3EpoR cells (data not shown), was modestly and transiently induced by Epo at 10 minutes (Fig 2A, lane 3) and then again at 120 minutes after stimulation (Fig 2A, lane 6). p32/p30 phosphorylation in D/C cells was generally onefold to fourfold higher than that in D/V cells with or without Epo stimulation (Fig 2A, lanes 7 through 11; see also Figs 4 and 5) and were also further induced at 120 minutes after stimulation (Fig 2A, lane 12). In addition, increased phosphorylation was also detected in yet unidentified proteins of approximately 150, 104, 56, and 53 kD (Fig 2A, lanes 7 through 12). In contrast, Epo-induced phosphorylation of EpoR (Fig 2A), Jak2 (Fig 2D), and Stat5 (data not shown) were comparable in D/V and D/C cells, with a minor increase of Jak2 phosphorylation in growth factor-deprived D/C cells (compare Fig 3C and D, lanes 1 and 7). Consistent with the lack of effect of SHP-1-C mutant on Jak2/Stat5 phosphorylation, Epo induced similar expression levels of c-myc (Fig2C) and c-fos (data not shown) in both cell populations. Similar results were obtained when the cells were stimulated with IL-3 (data not shown).
SHP-1-C induced hyperphosphorylation of p32/p30 but had no marked effect on the phosphorylation of EpoR and Jak2 and c-myc expression. Total cell lysates (TCL) were prepared from D/V and D/C cells stimulated with Epo for various times. The lysates were analyzed directly by SDS-PAGE/Western blotting or used in immunoprecipitation with antibodies as indicated. The positions of hyperphosphorylated proteins of approximately 30, 32, 53, 56, 104, and 150 kD and protein size markers (in kilodaltons) are indicated.
SHP-1-C induced hyperphosphorylation of p32/p30 but had no marked effect on the phosphorylation of EpoR and Jak2 and c-myc expression. Total cell lysates (TCL) were prepared from D/V and D/C cells stimulated with Epo for various times. The lysates were analyzed directly by SDS-PAGE/Western blotting or used in immunoprecipitation with antibodies as indicated. The positions of hyperphosphorylated proteins of approximately 30, 32, 53, 56, 104, and 150 kD and protein size markers (in kilodaltons) are indicated.
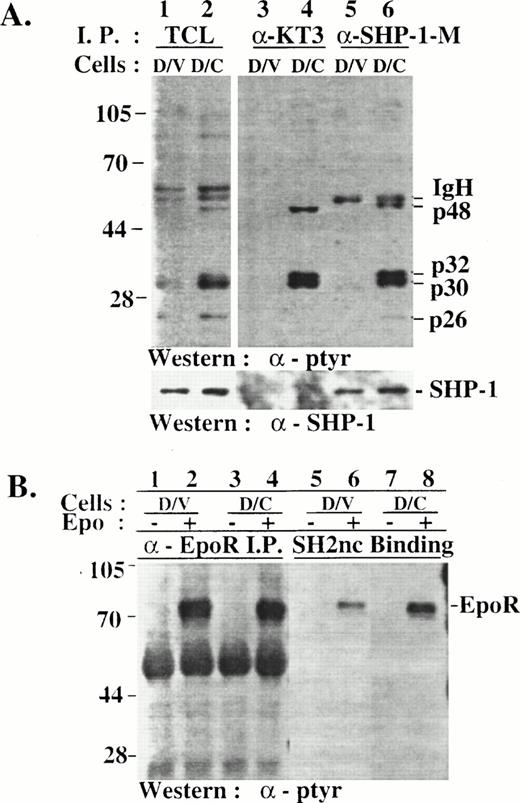
Hyperphosphorylated p32/p30 coimmunoprecipitated with SHP-1 and SHP-1-C from D/C cells but were not recognized by SHP-1 SH2 domains in vitro. (A) TCLs were prepared from D/V and D/C cells and were used for immunoprecipitation with antibodies specific for SHP-1 (lanes 5 and 6) or SHP-1-C (lanes 3 and 4). TCLs and the immunocomplexes were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (B) Cell lysates of D/V or D/C cells stimulated without (−) or with (+) Epo for 5 minutes were used in anti-EpoR immunoprecipitation (lanes 1 through 4) or in in vitro binding assays with a GST fusion protein of SHP-1 SH2 domains containing amino acids 1-198 of the murine SHP-1 (lanes 5 through 8). Phosphoproteins were analyzed by SDS-PAGE/Western blotting with an anti-ptyr antibody. The higher level of EpoR phosphotyrosine signaling from D/C cells (lane 8) was not reproducible and may have been caused by variations in the amount of fusion proteins. The positions of phosphoproteins and protein size markers are indicated.
Hyperphosphorylated p32/p30 coimmunoprecipitated with SHP-1 and SHP-1-C from D/C cells but were not recognized by SHP-1 SH2 domains in vitro. (A) TCLs were prepared from D/V and D/C cells and were used for immunoprecipitation with antibodies specific for SHP-1 (lanes 5 and 6) or SHP-1-C (lanes 3 and 4). TCLs and the immunocomplexes were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (B) Cell lysates of D/V or D/C cells stimulated without (−) or with (+) Epo for 5 minutes were used in anti-EpoR immunoprecipitation (lanes 1 through 4) or in in vitro binding assays with a GST fusion protein of SHP-1 SH2 domains containing amino acids 1-198 of the murine SHP-1 (lanes 5 through 8). Phosphoproteins were analyzed by SDS-PAGE/Western blotting with an anti-ptyr antibody. The higher level of EpoR phosphotyrosine signaling from D/C cells (lane 8) was not reproducible and may have been caused by variations in the amount of fusion proteins. The positions of phosphoproteins and protein size markers are indicated.
Synthetic peptide of SHP-1-C binds to p32/p30 from D/V and D/C cells but has no marked effect on SHP-1 PTPase activity in vitro. (A) Total cell lysates (TCL) from D/V and D/C cells were incubated in binding assays with a synthetic peptide of SHP-1C conjugated to Affi-gel 10. TCL (lanes 1 and 2) and cellular proteins associated with peptide (lanes 3 and 4) were analyzed by SDS-PAGE and Western blotting with antibodies as indicated. The positions of phosphotyrosine proteins and protein size markers are indicated. (B) The PTPase activity of a GST-SHP-1 fusion protein was determined in the absence or presence of the synthetic SHP-1-C peptide (20 to 40 μmol/L). The data represent the mean ± SD values of duplicated samples.
Synthetic peptide of SHP-1-C binds to p32/p30 from D/V and D/C cells but has no marked effect on SHP-1 PTPase activity in vitro. (A) Total cell lysates (TCL) from D/V and D/C cells were incubated in binding assays with a synthetic peptide of SHP-1C conjugated to Affi-gel 10. TCL (lanes 1 and 2) and cellular proteins associated with peptide (lanes 3 and 4) were analyzed by SDS-PAGE and Western blotting with antibodies as indicated. The positions of phosphotyrosine proteins and protein size markers are indicated. (B) The PTPase activity of a GST-SHP-1 fusion protein was determined in the absence or presence of the synthetic SHP-1-C peptide (20 to 40 μmol/L). The data represent the mean ± SD values of duplicated samples.
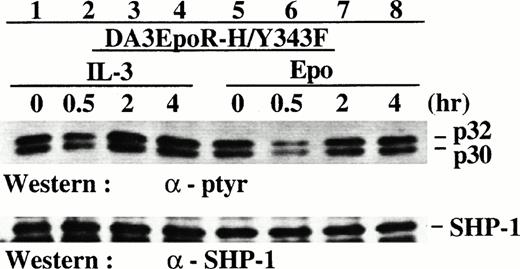
Reduced induction of p32/p30 phosphorylation from the EpoR mutant H/Y343F that was defective in Ras but not Jak2 activation. DA3 cells expressing the EpoR mutant H/Y343F were deprived of growth factors and then stimulated with IL-3 or Epo for various times. Cell lysates were prepared and analyzed by SDS-PAGE/Western blotting with antibodies as indicated.
Reduced induction of p32/p30 phosphorylation from the EpoR mutant H/Y343F that was defective in Ras but not Jak2 activation. DA3 cells expressing the EpoR mutant H/Y343F were deprived of growth factors and then stimulated with IL-3 or Epo for various times. Cell lysates were prepared and analyzed by SDS-PAGE/Western blotting with antibodies as indicated.
The data presented in Fig 2A were protein samples separated in a 7.5% SDS-PAGE gel that failed to distinctively resolute p32/p30 proteins. We used an 8.5% gel in the following experiments for p32/p30 analysis to achieve better results.
Reduced induction of p32/p30 phosphorylation from the EpoR mutant H/Y343F that was defective in Ras but not Jak2 activation.
The late induction of p32/p30 phosphorylation at 2 hours after Epo stimulation indicates that it is a signaling event downstream of the Jak/Ras signaling pathways that are activated transiently (1 to 60 minutes) after Epo/IL-3 stimulation. Using an IL-3–responsive cell line (DA3EpoR-H/Y343F) that expresses an EpoR mutant competent in activating the Jak2 but not the Ras pathway,5 we examined the relative roles of the Jak2 and Ras activations in the induction of p32/p30 phosphorylation.
As reported previously, stimulating DA3EpoR-H/Y343F cells with IL-3 or Epo induced rapid Jak2 phosphorylation (data not shown). We found that IL-3 induced p32/p30 phosphorylation in DA3EpoR-H/Y343F cells at 2 to 4 hours after stimulation (compare Fig 3, lanes 3 and 1), similar to the induction of p32/p30 phosphorylation via the wild-type EpoR in D/V and D/C cells (Fig 2A). However, p32/p30 phosphorylation in DA3EpoR-H/Y343F cells stimulated with Epo for 2 to 4 hours (Fig 3, lanes 7 and 8) was barely above the levels of growth factor-deprived cells (Fig 3, lane 5), indicating that the EpoR-H/Y343F mutant was less effective in inducing p32/p30 phosphorylation. Curiously, there were reduced p32/p30 phosphorylation signals in these cells at 30 minutes after stimulation by Epo (Fig 3, lane 7) or IL-3 (Fig 3, lane 2), which was also visible in D/V cells at 30 to 60 minutes (Fig 2A, lanes 4 and 5).
p32/p30 phosphoproteins associate with SHP-1-C and SHP-1 in D/C cells.
To detect phosphotyrosine proteins that interact with SHP-1-C or the endogenous SHP-1, we immunoprecipitated the mutant and SHP-1 from D/V and D/C cells using antibodies specific for the mutant (anti-KT3) or SHP-1 (anti-SHP-1-M; Fig 1A). p32 and p30 phosphoproteins were detected in the SHP-1-C and SHP-1 immunocomplexes from the D/C (Fig 4A, lanes 4 and 6), demonstrating that p32/p30 associated with SHP-1-C and SHP-1 in vivo in D/C cells. This association was specific, because the hyperphosphorylated p56 and p53 in D/C cells were not detected in the immunocomplexes under comparable conditions. The result also showed that SHP-1 and SHP-1-C associated with the phosphoproteins independently because SHP-1 was not detected in the SHP-1-C immunocomplexes (Fig 4A, lane 4). Because the GST fusion protein of SHP-1 SH2 domains bound to phosphorylated EpoR but not p32/p30 in vitro (Fig 4B, lane 8), it indicated that the association of p32/p30 with SHP-1 was mediated via the C-terminus of the phosphatase and that the SHP-1-C mutant competes against SHP-1 for p32/p30 association. In addition, phosphotyrosine proteins of approximately 48 and 26 kD (p48 and p26) were also detected in the immunocomplexes (Fig4A, lanes 4 and 6) with weaker and less consistent signals, suggesting that their association with SHP-1 and SHP-1-C was unstable or indirect. Interestingly, phosphorylated p32/p30 were not detected in SHP-1 immunocomplexes from D/V cells (Fig 4A, lane 3). Whether unphosphorylated forms of p32/p30 associate with SHP-1 in D/V cells remains to be determined.
Synthetic peptide of SHP-1-C bind to p32/p30 in D/V and D/C cells and is inactive against SHP-1 PTPase in vitro.
To further define the interactions of SHP-1 with p32/p30, we examined the binding of a synthetic peptide of the SHP-1-C to the phosphoproteins in vitro. The peptide bound to p32/p30 in D/V and D/C cells (Fig 5A, lanes 3 and 4 of the upper panel). This association of p32/p30 with the SHP-1-C peptide did not require the SHP-1 phosphatase (Fig 5A, lanes 3 and 4 of the lower panel), consistent with the independent association of p32/p30 with SHP-1-C and SHP-1 in vivo (Fig 4A). Interestingly, phosphorylated p32/p30 in association with the peptide from D/V were twofold to threefold higher than those from D/C cells. This indicated that pre-existing complexes of SHP-1-C mutant and p32/p30 in D/C cells (Fig4A, lane 6) reduced the amount of p32/p30 available for binding to the SHP-1-C peptide. It also demonstrated that p32/p30 in D/V cells had SHP-1-C binding activity. Thus, the failure of p32/p30 to coimmunoprecipitate with SHP-1 in D/V cells (Fig 4A, lane 3) may result from failure of the C-terminus of SHP-1 to interact with p32/p30.
To examine the effect of the SHP-1-C peptide on SHP-1 PTPase activity, we performed in vitro SHP-1 PTPase assays. We found that the peptide had no marked effect on the PTPase activity of a GST fusion protein of SHP-1 in the absence (Fig 5B) or presence of phosphotyrosine peptides that bind to the SH2 domains of SHP-1 (data not shown). Similarly, we failed to detect marked differences in the PTPase activities of the SHP-1 proteins immunoprecipitated from D/V and D/C cells (data not shown).
Induction of p32/p30 phosphorylation at 2 to 18 hours after Epo stimulation.
The induction of p32/p30 phosphorylation at 2 hours after stimulation (Fig 2A) prompted us to examine p32/p30 phosphorylation in D/V and D/C cells at later stages after stimulation. Consistent with data shown in Fig 2, p32/p30 phosphorylation was induced about twofold to threefold at 2 hours after Epo stimulation in both cell populations (Fig 6A, lanes 2 and 8). The phosphorylation of p32/p30 was further induced twofold to fourfold from 6 to 18 hours after stimulation, with those in the D/C cells threefold to sixfold higher than those in the D/V cells at each of the time points (lanes 3 through 6 and 9 through 12). Increased phosphorylation in proteins of 48 and 26 kD (p48 and p26) in D/C cells was also detected (Fig 6A). Phosphorylated p32/p30 coimmunoprecipitated with SHP-1 (Fig 6B, lanes 1 through 6) and the SHP-1-C mutant (data not shown) from D/C but not D/V cells (Fig 6B, lanes 1 through 6), despite their existence in D/V cell lysates (Fig 6A, lanes 1 through 6). Similar results were derived from D/V and D/C cells stimulated with IL-3 (data not shown).
p32/p30 phosphorylation in D/V and D/C cells was mainly induced at 2 to 18 hours after Epo stimulation. Growth factor-deprived D/V and D/C cells were stimulated with Epo for various times. Cell lysates were prepared and used for immunoprecipitation with an anti–SHP-1 antibody. The cell lysates and immunocomplexes were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. The positions of phosphoproteins and protein size markers are indicated.
p32/p30 phosphorylation in D/V and D/C cells was mainly induced at 2 to 18 hours after Epo stimulation. Growth factor-deprived D/V and D/C cells were stimulated with Epo for various times. Cell lysates were prepared and used for immunoprecipitation with an anti–SHP-1 antibody. The cell lysates and immunocomplexes were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. The positions of phosphoproteins and protein size markers are indicated.
Hyperphosphorylated p32/p30 are major novel proteins in SHP-1-C immunocomplexes.
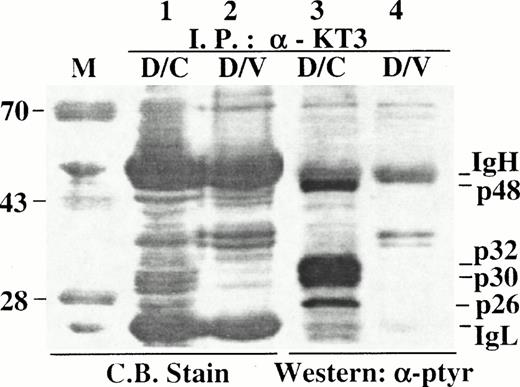
To determine the relative amounts of p32/p30 in SHP-1-C immunocomplexes, anti-KT3 immunocomplexes from D/V or D/C cells were separated in an SDS-PAGE gel in duplicates and analyzed by either direct Coomassie Blue staining or by Western blotting with an anti-ptyr antibody. As expected, hyperphosphorylated p32/p30 were detected by Western blotting in the immunocomplex from D/C cells (Fig 7, lane 3) but not from D/V cells (Fig7, lane 4). Two proteins that comigrate with the hyperphosphorylated p32/p30 were detected uniquely in the immunocomplex from D/C cells (Fig7, lane 1), indicating that these two proteins were likely to be the hyperphosphorylated p32/p30 and were among the major proteins in association with SHP-1-C. In addition, tyrosine-phosphorylated p48 and p26 were also detected in complexes with p32/p30 (Fig 7, lane 3). Using a similar approach, we found that proteins of 32 and 30 kD were also among the major cellular proteins associated with the SHP-1-C peptide in binding assays (data not shown). However, it was not clear whether SHP-1-C interacted with p32/p30 directly or indirectly, because additional cellular proteins were also presented in the immunoprecipitation and peptide binding complexes.
Hyperphosphorylated p32/p30 are major novel proteins in SHP-1-C immunocomplexes. Cell lysates were prepared from approximately 1 × 108 D/V or D/C cells and incubated with anti-KT3 antibody. Immunocomplexes were separated in a 10% SDS-PAGE gel in duplicates with 90% of the immunocomplexes loaded in lanes 1 and 2 and about 0.1% in lanes 3 and 4. Samples in lanes 1 and 2 were detected by direct staining with Coomassie Blue R-250 and those in lanes 3 and 4 were detected by Western blotting with an anti-ptyr antibody. The positions of p32/p30, p48, p26, IgH/IgL, and protein size markers (M) are indicated.
Hyperphosphorylated p32/p30 are major novel proteins in SHP-1-C immunocomplexes. Cell lysates were prepared from approximately 1 × 108 D/V or D/C cells and incubated with anti-KT3 antibody. Immunocomplexes were separated in a 10% SDS-PAGE gel in duplicates with 90% of the immunocomplexes loaded in lanes 1 and 2 and about 0.1% in lanes 3 and 4. Samples in lanes 1 and 2 were detected by direct staining with Coomassie Blue R-250 and those in lanes 3 and 4 were detected by Western blotting with an anti-ptyr antibody. The positions of p32/p30, p48, p26, IgH/IgL, and protein size markers (M) are indicated.
A number of cellular proteins have similar sizes as p32/p30 and are tyrosine phosphorylated or regulated during cell cycle progression. These include p34Cdc2, p33Cdk2, p38Cdk2, p33Cdk4, p40Cdk6, p36cyclinD1, p37/42CAK, p33cyclinD3, p36PCNA, and p27Kip1. Antibodies against these proteins failed to react with p32/p30 (data not shown).
Hyperphosphorylation and SHP-1 association of p32/p30 in motheaten hematopoietic cells.
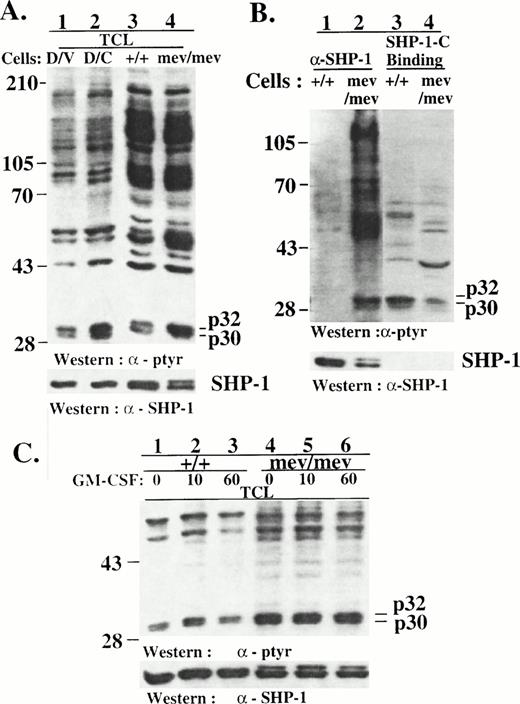
The hyperphosphorylation of p32/p30 in D/C cells indicated that p32/p30 are potential novel SHP-1 substrates. In support of this, we found that hyperphosphorylated proteins that comigrated with the p32/p30 of D/C cells in SDS-PAGE were also detectable in the spleen cells (Fig 8A) and bone marrow macrophages (Fig8C) of viable motheaten mouse, which express two forms of catalytically inactive SHP-1 proteins.16 Reminiscent of the results from D/V and D/C cells, we also found that the hyperphosphorylated p32/30 coimmunoprecipitated with the inactive SHP-1 proteins from viable motheaten spleen cells (Fig 8B, lane 2) and macrophages (data not shown), whereas the SHP-1-C peptide bound to p32/p30 from normal as well as motheaten cells. In consistence with previous studies, we detected a phosphoprotein of 120 to 130 kD20 and several yet-unidentified proteins in the SHP-1 immunocomplexes from the motheaten cells (Fig 8B, lane 2). The larger number of phosphoproteins in association with the catalytically inactive SHP-1 in motheaten cells than with the SHP-1 in D/C cells is consistent with the notion that SHP-1-C only block the C-terminal function of the phosphatase. As in D/V cells, GM-CSF induced an early and modest p32/p30 phosphorylation in normal mouse macrophages (Fig 8C, lane 2). But we failed to detect marked induction of p32/p30 phosphorylation in normal or motheaten macrophages after CSF-1 or GM-CSF stimulation at later stages (data not shown). It is known that the primary macrophages express lower levels of hematopoietic receptors and proliferate at a much slower rate than the established DA3 cell lines. Whether the lack of marked late induction of p32/p30 in these primary cells was due to these differences or whether it was a cell lineage-specific character has not been determined.
Hyperphosphorylation and SHP-1 association of p32/p30 in motheaten macrophages. (A) Total cell lysates were prepared from D/V or D/C cells and from the spleen cells of normal (+/+) or viable motheaten mice (mev/mev). The lysates were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (B) Cell lysates from the spleen cells of normal (+/+) or viable motheaten mice (mev/mev) were incubated with an anti–SHP-1 antibody for immunoprecipitation assays or with the synthetic SHP-1-C peptide in binding assays. SHP-1 immunocomplexes (lanes 1 and 2) and SHP-1-C peptide-binding proteins (lanes 3 and 4) were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (C) Bone marrow-derived macrophages from normal (+/+) or viable motheaten mice (mev/mev) were deprived of growth factors and then stimulated with GM-CSF for various times. Lysates from these cells were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. Positions of phosphotyrosine proteins and protein size markers are indicated.
Hyperphosphorylation and SHP-1 association of p32/p30 in motheaten macrophages. (A) Total cell lysates were prepared from D/V or D/C cells and from the spleen cells of normal (+/+) or viable motheaten mice (mev/mev). The lysates were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (B) Cell lysates from the spleen cells of normal (+/+) or viable motheaten mice (mev/mev) were incubated with an anti–SHP-1 antibody for immunoprecipitation assays or with the synthetic SHP-1-C peptide in binding assays. SHP-1 immunocomplexes (lanes 1 and 2) and SHP-1-C peptide-binding proteins (lanes 3 and 4) were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. (C) Bone marrow-derived macrophages from normal (+/+) or viable motheaten mice (mev/mev) were deprived of growth factors and then stimulated with GM-CSF for various times. Lysates from these cells were analyzed by SDS-PAGE/Western blotting with antibodies as indicated. Positions of phosphotyrosine proteins and protein size markers are indicated.
DISCUSSION
DA3EpoR cells expressing the SHP-1-C mutant (D/C cells) show heightened cell proliferation in response to Epo/IL-3 and contain hyperphosphorylated proteins similar to those detected in the motheaten cells that lack functional SHP-1. This indicates that the mutant blocks the function of the endogenous SHP-1 in D/C cells and causes hyperphosphorylation in SHP-1 substrates involved in mitogenic signaling of Epo/IL-3. Identification of these substrates is essential in understanding SHP-1 function and in elucidating mitogenic signaling pathways of the hematopoietic growth factors.
p32/p30 are likely major SHP-1 substrates, because they are among the prominently hyperphosphorylated proteins in D/C cells as well as in motheaten cells. The observation that p32/p30 coimmunoprecipitate with SHP-1 in D/C and the motheaten cells demonstrates their interaction with the phosphatase in vivo and supports a role for SHP-1 in directly dephosphorylating the two proteins. Indeed, we found that the phosphorylation of p32/p30 was sensitive to SHP-1 PTPase activity in vitro (unpublished data). Moreover, the marked and constitutive hyperphosphorylation of p32/p30 in these SHP-1–deficient cells demonstrates that their dephosphorylation is regulated mainly by SHP-1. This is in contrast to Jak2 dephosphorylation, in which SHP-1 appears to play a limited role as indicated by the transient and modest nature of Jak2 hyperphosphorylation after GM-CSF stimulation in motheaten macrophages20 and the lack of major effect of SHP-1-C mutant on Jak2 phosphorylation in D/C cells (Fig 2). It is expected that the Jak family kinases are regulated by additional phosphatase(s), because they are ubiquitously expressed, whereas SHP-1 expression is restricted predominantly in hematopoietic cells. Because of their late induction and small sizes, p32/30 escaped detection in previous studies focused on the relatively large Jak/Stat proteins and on signaling events induced immediately after Epo/IL-3 stimulation.
Several lines of evidence indicate that p32/p30 are likely to be involved in the mitogenic signaling of Epo and IL-3. Because they are hyperphosphorylated in D/C cells and motheaten cells, which are hyperproliferative in response to Epo/IL-3 and GM-CSF, respectively, a correlation exists between p32/p30 hyperphosphorylation and a heightened growth response to hematopoietic growth factors. Moreover, we found that SHP-1 deficiency in D/C cells (Fig 2) and motheaten cells20 has little or limited effect on the phosphorylation of Jak2/Stat5 and Epo/IL-3 receptors. On the other hand, the other hyperphosphorylated proteins in D/C cells (eg, p56/53 in Fig 2) were not detected in association with the phosphatase and thus may be not directly dephosphorylated by SHP-1. Thus, SHP-1 may downregulate mitogenic signaling of IL-3/Epo in large part by dephosphorylating p32/p30. SHP-1 regulates additional signaling pathways in hematopoietic cells, as indicated by its association with various membrane receptors and the multiple hematopoietic abnormalities in motheaten mice. The involvement of p32/p30 in these signaling pathways remain to be determined.
p32/30 is clearly involved in late signaling events, as indicated by the marked induction of p32/p30 phosphorylation at 2 to 18 hours. Their potential involvement in early signaling is suggested by the modest, but reproducible, induction immediately after ligand stimulation. They appear to function downstream of the early Jak2/Ras pathways, because Epo stimulation of the EpoR-H/Y343F mutant that activates the Jak2, but not the Ras, pathway results in reduced induction of p32/p30 phosphorylation. Because p32/p30 phosphorylation is still modulated modestly by the mutant that activates the Jak2 pathways, it is likely that both Ras and Jak2 pathways are required for optimal induction of p32/p30 phosphorylation. On the other hand, the marked induction of p32/p30 phosphorylation at 2 to 18 hours indicates them as signaling molecules in cell cycle progression. Consistent with this, we found that D/C cells showed a 16% increase in G2/M population and a corresponding decrease in G0/G1 population in comparison to D/V cells when maintained in the presence of IL-3 (unpublished data), indicating that D/C cells progress faster during cell cycle than D/V cells. Because p32/p30 phosphorylation may be cell cycle related, the differential cell cycle distributions of D/V and D/C cells may contribute to the differences of p32/p30 phosphorylation in these cells. Further studies to identify p32/p30 and characterize their function are clearly needed to define their role in mitogenic signaling and cell cycle progression. In this regard, the stable association of p32/p30 with SHP-1-C mutant in D/C cells allows us, from a unique position, to identify the proteins by micropeptide sequencing and to define their roles in mitogenic signaling in hematopoietic cells.
p32/p30 phosphorylation is regulated by SHP-1 via its C-terminus. We demonstrate that a synthetic peptide containing the SHP-1 C-terminal 40 amino acids has no marked effect on the PTPase activity of recombinant SHP-1 in vitro (Fig 5B). Moreover, the peptide forms stable complexes with p32/p30 but not SHP-1 in vitro in binding assays (Fig 5A) and in vivo when it was expressed as the SHP-1-C mutant (Fig 4A). p32/p30 binding activity is also detectable in intact SHP-1, as indicated by the association of p32/p30 with SHP-1 in D/C and motheaten cells. Because expression of the peptide and its association with p32/p30 in vivo correlates with an increase in p32/p30 phosphorylation, it indicates that the SHP-1-C mutant competes against the phosphatase for binding to p32/p30, resulting in p32/p30 hyperphosphorylation. We also found that p32/p30 in D/V and normal mouse cells are capable of binding to the SHP-1-C peptide in vitro, although they do not associate with the endogenous SHP-1 in vivo. This suggests that the p32/p30 binding activity in the endogenous SHP-1 is regulated and that SHP-1-C mutant may interfere with the regulation to cause SHP-1/p32/p30 interactions in D/C cells. In this regard, it is interesting to note that a tyrosine phosphorylation site (Y564) resides in the SHP-1-C mutant.44 The demonstrated binding of the synthetic peptide of SHP-1 C-terminus to p32/p30 suggests the possibility that phosphorylation of C-terminal tyrosines Y564 may affect SHP-1 configurations and its interactions with p32/p30. Alternatively, it may regulate SHP-1 phosphatase activity and SHP-1 dephosphorylation of p32/p30. Additional studies are clearly needed to define this regulation and the role of Y564 phosphorylation in p32/p30 association.
An intramolecular inhibitory function was proposed previously for the C-terminus of SHP-1, because truncation of the last 35 to 41 amino acids of SHP-1 activates the PTPase.34-36 However, direct interactions of C-terminus of SHP-1 with the SH2 domains or the PTPase catalytic domain have not been demonstrated.35 Furthermore, the enzyme with truncation to the C-terminal 60 amino acids35 or the C-terminal 20 amino acids (our unpublished data) behaves like the wild-type SHP-1. Thus, the activation of SHP-1 caused by C-terminal truncation of amino acid 35-41 is complicated. Although our data indicate that the SHP-1-C mutant affects the function of SHP-1 PTPase and causes p32/p30 hyperphosphorylation primarily by a competition mechanism, it remains possible that SHP-1-C may inhibit SHP-1 through unstable and/or transient interactions with the catalytic domain of SHP-1 in vivo and affect SHP-1 substrates. Nevertheless, the small size of the SHP-1-C and its potent effect in suppressing SHP-1 function make it an appealing target for further development of biochemical and pharmaceutical reagents to block SHP-1 activities and manipulate hematopoiesis and immunity. The approach described in this study could also be useful for analyzing the functions of other enzymes and for identification of their substrates.
Previous studies from our group and others showed that SHP-1 binds, via its SH2 domains in N-terminal region, to receptors and kinases in hematopoietic cells. The association leads to activation of SHP-1 PTPase and dephosphorylation of potential substrates, including the Jak kinases and ZAP-70 kinases. We demonstrate here that SHP-1 regulates p32/p30 phosphorylation through an alternative mechanism involving the C-terminus of the phosphatase and that p32/p30 may be key molecules through which SHP-1 regulates mitogenic signals. Despite marked advances in our understanding of mitogenic pathways, critical elements that link signals from the early Ras/Jak pathways and the later cell cycle progression remain to be fully characterized. p32/p30 phosphorylation is induced at early and, more significantly, late stages of Epo/IL-3 mitogenic signaling and thus may be involved in linking the early and late signaling events. The cloning of p32/p30 and characterization of their functions will lead to the elucidation of their role in mitogenic signaling pathways in hematopoietic cells.
ACKNOWLEDGMENT
The authors thank Dr G. Walter for the anti-KT3 hybridoma cell line, Dr J. Ihle for the DA3EpoR-H/Y343F cell line, and Drs Christine Campbell and Robert Silverman for critical reading of the manuscript.
Supported by grants from the American Cancer Society (DB-74554) and the American Heart Association (NEO-94—074-GIA ) to T.Y.
Address reprint requests to Taolin Yi, PhD, Department of Cancer Biology, The Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Ave, NN1-25, Cleveland, OH 44195; e-mail:yit@cesmtp.ccf.org.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal