Abstract
Protein tyrosine phosphorylation plays a crucial role in signaling from the receptor for erythropoietin (Epo), although the Epo receptor (EpoR) lacks the tyrosine kinase domain. We have previously shown that the Jak2 tyrosine kinase couples with the EpoR to transduce a growth signal. In the present study, we demonstrate that Lyn, a Src family tyrosine kinase, physically associates with the EpoR in Epo-dependent hematopoietic cell lines, 32D/EpoR-Wt and F36E. Coexpression experiments in COS7 cells further showed that Lyn induces tyrosine phosphorylation of the EpoR and that both LynA and LynB, alternatively spliced forms of Lyn, bind with the membrane-proximal 91-amino acid region of the EpoR cytoplasmic domain. In vitro binding studies using GST-Lyn fusion proteins further showed that the Src homology (SH)-2 domain of Lyn specifically binds with the tyrosine-phosphorylated EpoR in lysate from Epo-stimulated cells, whereas the tyrosine kinase domain of Lyn binds with the unphosphorylated EpoR. Far-Western blotting and synthetic phosphopeptide competition assays further indicated that the Lyn SH2 domain directly binds to the tyrosine-phosphorylated EpoR, most likely through its interaction with phosphorylated Y-464 or Y-479 in the carboxy-terminal region of the EpoR. In vitro binding studies also demonstrated that the Lyn SH2 domain directly binds to tyrosine-phosphorylated Jak2. In vitro reconstitution experiments in COS7 cells further showed that Lyn induces tyrosine phosphorylation of Stat5, mainly on Y-694, and activates the DNA-binding and transcription-activating abilities of Stat5. In agreement with this, Lyn enhanced the Stat5-dependent transcriptional activation when overexpressed in 32D/EpoR-Wt cells. In addition, Lyn was demonstrated to phosphorylate the EpoR and Stat5 on tyrosines in vitro. These results suggest that Lyn may play a role in activation of the Jak2/Stat5 and other signaling pathways by the EpoR.
ERYTHROPOIETIN (Epo) is a hematopoietic growth factor that regulates the growth and differentiation of erythroid progenitor cells through activation of its specific receptor expressed on the cell surface.1-3 The receptor for Epo (EpoR) belongs to the cytokine receptor family and lacks a tyrosine kinase domain. However, we previously showed that protein tyrosine phosphorylation plays a pivotal role in the EpoR-mediated growth signaling4,5 and further demonstrated that Jak2, a member of the JAK family of tyrosine kinases, physically associates with the EpoR membrane-proximal domain, which contains the Box1 and Box2 motifs conserved in the cytokine receptor family, and becomes activated upon Epo stimulation.6,7 Epo stimulation also induces tyrosine phosphorylation of the EpoR itself4 and various signaling molecules, such as Stat5, Shc, SHP-2, Vav, Cbl, CrkL, and SHIP, which are directly or indirectly recruited to the tyrosine-phosphorylated EpoR through the interaction between phosphotyrosines in the EpoR cytoplasmic domain and the Src homology (SH)-2 domain, a conserved modular domain that binds to phosphotyrosine-containing sequences, of these signaling molecules.8 Upon tyrosine phosphorylation, Stat5 dissociates rapidly from the EpoR to form a homodimer through the reciprocal SH2 domain-phosphotyrosine interaction and is then translocated to the nucleus, where it activates target genes by binding to specific regulatory sequences.9 Epo also induces the recruitment of other SH2-containing signaling molecules, including the p85 regulatory subunit of phosphatidyl inositol 3′-kinase (PI3K) and SHP-1, although their tyrosine phosphorylation state does not show any significant change after Epo stimulation.8 Previously, we showed that Jak2 plays a critical role in inducing tyrosine phosphorylation of the EpoR as well as the various cellular substrates, because, in cells expressing mutant EpoRs that failed to associate with and thus activate Jak2, Epo failed to induce tyrosine phosphorylation of the mutant receptors or any of these cellular substrates.5-7,10 11 However, it has remained to be known whether Jak2 is the only tyrosine kinase that is directly involved in Epo-induced tyrosine phosphorylation of the EpoR as well as the variety of cellular substrates.
Other than the JAK family of tyrosine kinases, Lyn, a member of the Src family tyrosine kinases, is abundantly expressed in the hematopoietic cells,12,13 including erythrocytes.14 The Src family kinases have a common domain organization. The N-terminal segment contains a myristylation site, which is required for membrane localization, and a unique domain of 5 to 70 residues that does not show any significant similarity among the family members. The SH3, SH2, and catalytic (SH1) domains follow in order. The 56-kD and 53-kD forms of Lyn, designated as LynA and LynB, respectively, are translated from alternatively spliced transcripts and expressed simultaneously in hematopoietic cells. LynB lacks the amino acid residues 24 through 44 in the unique domain of LynA.13 Recent studies onlyn−/− mice15,16 have demonstrated that Lyn plays crucial roles in signaling mediated through the B-cell antigen receptor and the high affinity receptor for Fcε. Lyn has also been implicated in signaling from the receptors for interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-5,17-22 which belong to the cytokine receptor superfamily and share a common signal-transducing subunit, βc,23 as well as from the granulocyte colony-stimulating factor (G-CSF) receptor.24 Although abnormalities of hematopoiesis have not been reported in lyn-deficient mice, the role of Lyn might have been complemented by the redundantly expressed other Src family members. Consistent with this idea, single knock-out experiments of any Src kinases have not been reported to result in abnormalities of murine hematopoiesis.25 Interestingly, the receptors sharing βc also activate Jak2 and induce tyrosine phosphorylation of a similar set of cellular proteins with the EpoR.8 In addition, a very recent study has suggested that Lyn may play an important role in Epo-induced differentiation.26 In the present study, we thus examined the physical interaction of Lyn with the EpoR and its involvement in the EpoR-mediated signaling.
MATERIALS AND METHODS
Cells and reagents.
IL-3–dependent 32D cells and a clone of 32D cells expressing the wild-type murine EpoR (32D/EpoR-Wt)5 were previously described and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10% WEHI-3 conditioned medium as a source of IL-3. An Epo-dependent human erythroleukemia cell line, F36E,27 was obtained through the Riken Gene Bank (Ibaraki, Japan) and cultured in RPMI 1640 medium supplemented with 10% FCS and 4 U/mL of Epo. COS7 cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% FCS. Recombinant human Epo was kindly provided by Chugai Pharmaceutical Co Ltd (Tokyo, Japan). Recombinant murine IL-3 was purchased from PeproTech Inc (Rocky Hill, NJ).
Expression plasmids for the murine wild-type and mutant EpoRs were described previously.4,28 A β-casein promoter luciferase construct, pZZ1,29 and cDNA clones coding for murine Jak2,30 murine Stat5A,31 murine LynA,13 murine LynB,13 wild-type ovine Stat5,32 and mutant ovine Stat5 with a substitution of Tyr694 with Phe29 33 have also been described previously. Control Renilla luciferase plasmids pRL-TK and pRL-SV40 were purchased from Promega (Madison, WI).
A rabbit antiserum raised against a glutathione S-transferase (GST) fusion protein containing amino acids 257 to 441 of the EpoR cytoplasmic domain was previously described.34 Rabbit antibodies against Lyn, Stat5A, SHP-2, and the carboxy-terminal region of EpoR (M-20) and a monoclonal antibody against GST were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies against phosphotyrosine (4G10) and the influenza virus hemagglutinin (HA) epitope were from Upstate Biotechnology, Inc (Lake Placid, NY) and from Boehringer Mannheim (Indianapolis, IN), respectively.
Immunoprecipitation and immunoblotting.
Cells were washed free of IL-3, cultured overnight, and left unstimulated as a negative control or stimulated with Epo or IL-3 at a saturating concentration, unless otherwise described. Cells were solubilized with a lysis buffer composed of 1% Triton X-100, 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, and 10 μg/mL leupeptin. Cell lysates were subjected to immunoprecipitation as described previously.10For double immunoprecipitation, immunoprecipitates were eluted with 1× Laemmli's sodium dodecyl sulfate (SDS) sample buffer, diluted 100-fold with lysis buffer supplemented with 0.1% bovine serum albumin, and subjected to reimmunoprecipitation with a second antibody or normal rabbit serum, as indicated. For immunoblot analysis of total cell lysates, an aliquot of the clarified supernatant was directly mixed with equal volumes of 2× Laemmli's sample buffer and heated at 100°C for 5 minutes. Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to Immobilon P membranes (Millipore, Bedford, MA). The membranes were probed with a relevant antibody followed by detection using enhanced chemiluminescence Western blotting detection system (Amersham, Buckinghamshire, UK). For reprobing of the membranes, they were treated with stripping buffer composed of 100 mmol/L 2-mercaptoethanol, 2% SDS, and 62.5 mmol/L Tris-HCl (pH 6.7) at 50°C for 30 minutes and subsequently probed with a different antibody.
In vitro binding studies using GST-Lyn fusion proteins.
To prepare GST-Lyn fusion proteins, the fragments of LynA cDNA coding for amino acid residues 1 through 230 (Lyn1-230) and 231 through 512 (Lyn231-512) were amplified by the polymerase chain reaction (PCR) method using Pwo DNA polymerase (Boehringer Mannheim, Mannheim, Germany). Using restriction enzyme recognition sites artificially added in the 5′- and 3′-primers, the PCR-generated fragments were subcloned into pGEX-4T-3 (Pharmacia, Uppsala, Sweden). In addition, the fragment coding for Lyn1-230 was digested with Bal I, and the two fragments coding for amino acid residues 1 through 118 (Lyn1-118) and 119 through 230 (Lyn119-230) were separately subcloned into pGEX-4T-3. The expression plasmids were transformed intoEscherichia coli DH5α, and the recombinant fusion proteins were purified by affinity chromatography on glutathione-Sepharose beads (Pharmacia).
In vitro binding of cellular proteins to GST-Lyn fusion proteins was examined essentially as described previously.10 In brief, parental 32D or 32D/EpoR-Wt cells were lysed in the lysis buffer described above, mixed with GST fusion proteins on glutathione-Sepharose beads, and incubated at 4°C for 2 hours. The beads were washed twice with the lysis buffer, and proteins bound to the beads were eluted by boiling in 1× SDS sample buffer and examined by immunoblotting with indicated antibodies. For competition assays with previously described EpoR-derived phosphotyrosine peptides,35 36 200 μmol/L or indicated concentrations of a synthetic peptide was added to cell lysate from Epo-stimulated cells before being subjected to binding analysis using GST-Lyn119-230. For Far-Western blotting, GST-Lyn fusion proteins were eluted from glutathione-Sepharose beads with a buffer composed of 50 mmol/L Tris-HCl (pH 8.0) and 5 mmol/L reduced glutathione. After immunoprecipitates were separated by SDS-PAGE and electrotransferred to Immobilon P membranes, the membrane was first incubated overnight at 4°C with an indicated GST-Lyn fusion protein followed by detection with anti-GST immunoblotting.
Affinity purification of DNA-binding proteins.
Cells were solubilized with a lysis buffer composed of 0.5% NP-40, 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.1 mmol/L EDTA, 10 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1 mmol/L dithiothreitol, 1 mmol/L PMSF, 10 μg/mL aprotinin, and 10 μg/mL leupeptin. The oligonucleotide sequence used was derived from the prolactin-inducible element (PIE) of the ovine β-casein gene (5′-AGATTTCTAGGAATTCAAT CC-3′).29 32 After preclarification, 500 μL of cell lysates was incubated at 4°C for 2 hours with 1 μg of double-stranded, 5′-biotinylated PIE-oligonucleotide and 10 μL of streptavidin-agarose (Pierce, Rockford, IL). After washing twice with the lysis buffer, bound proteins were eluted by heating at 100°C for 5 minutes in Laemmli's sample buffer and analyzed by immunoblotting with antiphosphotyrosine or anti-Stat5.
Transient expression in COS7 cells.
Transfection of expression plasmids into COS7 cells was performed using the Lipofectamin reagent (GIBCO-BRL, Grand Island, NY) according to the manufacturer's instructions. Two days after transfection, cells were stimulated with Epo (or left unstimulated), solubilized, and subjected to immunoprecipitation or affinity purification with the PIE-oligonucleotide, followed by immunoblotting with indicated antibodies as described above.
Luciferase reporter assays.
COS7 cells were cotransfected with the expression vector for Jak2 (pcDNA-Jak2) or Lyn (pXM-LynA and pXM-LynB) or with pcDNA3 as a control along with the Stat5-responsive luciferase reporter construct (pZZ1), a control Renilla luciferase plasmid (pRL-TK), and the expression plasmids for murine Stat5 (pRK-Stat5) and the EpoR (pXM-EpoR-Wt). Two days after transfection, transfected cells were incubated with or without Epo (5 U/mL) for 5 hours and harvested for the luciferase assay using Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. For transient overexpression experiments in 32D/EpoR-Wt, cells were electroporated at 960 μF and 300 V with the expression vector for Jak2 (pcDNA-Jak2) or Lyn (pXM-LynA and B) or pcDNA3 along with pZZ1 and a control Renilla luciferase plasmid, pRL-SV40. After a recovery period of 1 day, cells were starved overnight, incubated for 5 hours in medium with or without 4 U/mL of Epo, and harvested for the luciferase assay.
In vitro kinase assays.
The in vitro kinase assay of anti-Lyn immunoprecipitates was performed as described previously.7 13 In brief, anti-Lyn immunoprecipitates were subjected to the in vitro kinase reaction in kinase buffer (10 mmol/L HEPES, pH7.5, 50 mmol/L NaCl, 5 mmol/L MgCl2, 5 mmol/L MnCl2, 100 μmol/L sodium orthovanadate) containing [γ-32P]-ATP, with or without rabbit enolase added as an exogenous substrate, were resolved by SDS-PAGE, and were analyzed by autoradiography.
In vitro tyrosine phosphorylation of the EpoR and Stat5 by Lyn was examined by using GST-EpoR and GST-Stat5 fusion proteins. A GST-EpoR fusion protein containing the cytoplasmic domain of the EpoR (amino acid residues 257 through 483) has been described previously.36 To prepare a GST-Stat5 fusion protein, the portion of the murine Stat5A cDNA coding for amino acid residues 515 through 793 was amplified by the PCR and subcloned into the pGEX-4T-3 plasmid, as described above. The GST-EpoR and GST-Stat5 fusion proteins, eluted from glutathione-Sepharose beads, were incubated at room temperature for 30 minutes with anti-Lyn immunoprecipitates from unstimulated or Epo-stimulated 32D/EpoR-Wt cells in the kinase buffer supplemented with or without 1 mmol/L cold ATP. After the kinase reaction, the reaction products were mixed with equal volumes of 2× Laemmli's sample buffer, heated at 100°C for 5 minutes, and subjected to antiphosphotyrosine immunoblotting followed by reprobing with anti-EpoR or anti-Stat5.
RESULTS
Lyn physically associates with the EpoR in hematopoietic cells.
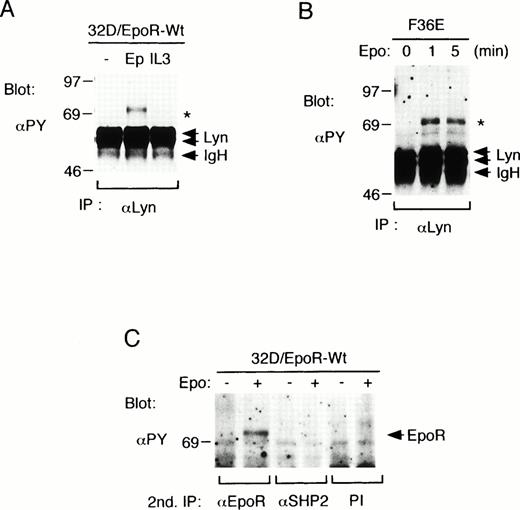
To investigate whether Lyn is involved in signaling through the EpoR, we stimulated 32D/EpoR-Wt cells, a clone of IL-3–dependent 32D cell line that expresses the EpoR, with Epo or IL-3 and examined anti-Lyn immunoprecipitates with antiphosphotyrosine blotting. As shown in Fig 1A, neither Epo nor IL-3 induced any significant effect on the tyrosine phosphorylation status of two alternatively spliced forms of Lyn, 56-kD LynA and 53-kD LynB. The kinase activity of Lyn, as determined by the immune complex in vitro kinase assays, was also not significantly changed after Epo or IL-3 stimulation in repeated experiments (negative data not shown). However, as indicated with an asterisk in Fig 1A, a tyrosine-phosphorylated 72-kD protein was found to be coimmunoprecipitated with Lyn only after Epo stimulation. The association of the 72-kD phosphotyrosyl protein with Lyn was also observed in an Epo-dependent human erythroleukemia cell line, F36E, after stimulation with Epo for 1 or 5 minutes (Fig 1B). Because both the EpoR and SHP-2 are inducibly tyrosine phosphorylated after Epo stimulation and comigrate on SDS-PAGE as 72-kD proteins, the anti-Lyn immunoprecipitates from 32D/EpoR-Wt cell lysates were subjected to a second immunoprecipitation with anti-EpoR, anti-SHP-2, or preimmune rabbit serum and then examined by antiphosphotyrosine blotting. As shown in Fig 1C, the 72-kD phosphotyrosyl protein was reimmunoprecipitated with anti-EpoR but not with anti-SHP-2. These results indicate that Lyn is associated directly or indirectly with the tyrosine-phosphorylated EpoR in Epo-stimulated hematopoietic cells.
Lyn associates with the tyrosine-phosphorylated EpoR in hematopoietic cell lines. (A) 32D/EpoR-Wt cells, a clone of IL-3–dependent 32D cell line expressing the transfected wild-type murine EpoR cDNA, were starved overnight and left unstimulated (−) or stimulated with 100 U/mL of Epo (Ep) or 25 ng/mL of IL-3 (IL3) for 5 minutes at 37°C before solubilization. Cell lysates were immunoprecipitated with anti-Lyn. Immunoprecipitates were resolved by SDS-PAGE and subjected to immunoblotting with an antiphosphotyrosine monoclonal antibody, 4G10 (αPY). (B) A human erythroleukemic cell line, F36E, was starved overnight and stimulated with 100 U/mL Epo for the indicated times. Cells were then lysed and analyzed as described above. (C) 32D/EpoR-Wt cells were left unstimulated or stimulated with Epo, as indicated. Cells were then lysed and immunoprecipitated with anti-Lyn. Anti-Lyn immunoprecipitates were then subjected to a second immunoprecipitation with anti-EpoR, anti-SHP-2, or preimmune serum (PI), as indicated, and analyzed by antiphosphotyrosine (αPY) immunoblotting. A 72-kD phosphotyrosyl protein that coimmunoprecipitates with Lyn from Epo-stimulated cell lysates is indicated with an asterisk. The positions of EpoR, Lyn, and the Ig heavy chain (IgH) are indicated. The molecular weight markers are indicated and given in kilodaltons.
Lyn associates with the tyrosine-phosphorylated EpoR in hematopoietic cell lines. (A) 32D/EpoR-Wt cells, a clone of IL-3–dependent 32D cell line expressing the transfected wild-type murine EpoR cDNA, were starved overnight and left unstimulated (−) or stimulated with 100 U/mL of Epo (Ep) or 25 ng/mL of IL-3 (IL3) for 5 minutes at 37°C before solubilization. Cell lysates were immunoprecipitated with anti-Lyn. Immunoprecipitates were resolved by SDS-PAGE and subjected to immunoblotting with an antiphosphotyrosine monoclonal antibody, 4G10 (αPY). (B) A human erythroleukemic cell line, F36E, was starved overnight and stimulated with 100 U/mL Epo for the indicated times. Cells were then lysed and analyzed as described above. (C) 32D/EpoR-Wt cells were left unstimulated or stimulated with Epo, as indicated. Cells were then lysed and immunoprecipitated with anti-Lyn. Anti-Lyn immunoprecipitates were then subjected to a second immunoprecipitation with anti-EpoR, anti-SHP-2, or preimmune serum (PI), as indicated, and analyzed by antiphosphotyrosine (αPY) immunoblotting. A 72-kD phosphotyrosyl protein that coimmunoprecipitates with Lyn from Epo-stimulated cell lysates is indicated with an asterisk. The positions of EpoR, Lyn, and the Ig heavy chain (IgH) are indicated. The molecular weight markers are indicated and given in kilodaltons.
Lyn induces tyrosine phosphorylation of the EpoR and binds to the membrane-proximal cytoplasmic region of the EpoR in COS7 cells.
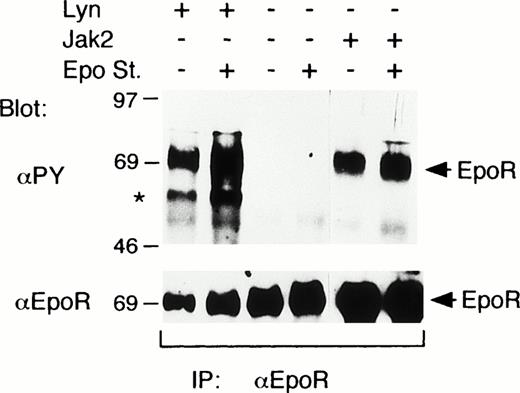
To explore the possibility that Lyn may induce the tyrosine phosphorylation of the EpoR, the EpoR was coexpressed with Lyn or Jak2 in COS7 cells. When the EpoR was expressed alone, Epo did not induce any detectable tyrosine phosphorylation of the EpoR (Fig 2). On the other hand, the coexpression of Lyn or Jak2 induced a prominent tyrosine phosphorylation of the EpoR even without Epo stimulation (Fig 2). In accordance with this, Jak2 was tyrosine phosphorylated and thus activated without Epo stimulation when overexpressed with the EpoR in COS cells (data not shown). In repeated experiments, Lyn and Jak2 induced comparable levels of tyrosine phosphorylation of the EpoR, which were only marginally enhanced by Epo stimulation. In addition, a phosphotyrosyl protein corresponding in size to Lyn was coimmunoprecipitated with the EpoR from cells coexpressing the EpoR and Lyn, as indicated by an asterisk in Fig 2.
Lyn induces tyrosine phosphorylation of the EpoR in COS7 cells. In COS7 cells, the EpoR was transiently coexpressed with Lyn or Jak2, as indicated. Transfected cells were either stimulated with 100 U/mL Epo for 5 minutes or left unstimulated, as indicated (Epo st. + or −, respectively). Cells were lysed and immunoprecipitated with anti-EpoR. Immunoprecipitates were analyzed by antiphosphotyrosine (αPY) immunoblotting followed by reprobing with anti-EpoR, as indicated. A coimmunoprecipitated phosphotyrosyl protein that corresponds in size to Lyn is indicated with an asterisk.
Lyn induces tyrosine phosphorylation of the EpoR in COS7 cells. In COS7 cells, the EpoR was transiently coexpressed with Lyn or Jak2, as indicated. Transfected cells were either stimulated with 100 U/mL Epo for 5 minutes or left unstimulated, as indicated (Epo st. + or −, respectively). Cells were lysed and immunoprecipitated with anti-EpoR. Immunoprecipitates were analyzed by antiphosphotyrosine (αPY) immunoblotting followed by reprobing with anti-EpoR, as indicated. A coimmunoprecipitated phosphotyrosyl protein that corresponds in size to Lyn is indicated with an asterisk.
We next examined the binding of Lyn with the previously characterized EpoR mutants,4 as shown in Fig3A, in COS7 cells. The H- and S-mutant EpoRs lack 72- and 142-amino acid residues, respectively, by carboxy-terminal truncation, whereas the PB-mutant EpoR lacks 20-amino acid residues by an internal deletion within the membrane proximal cytoplasmic domain required for the activation of Jak2.6,7 As demonstrated in Fig 3B, anti-EpoR blotting of anti-Lyn immunoprecipitates from transfected COS7 cells indicated that all the three mutant EpoRs as well as the wild-type EpoR physically associated with Lyn when coexpressed. Anti-Lyn immunoblotting of anti-EpoR immunoprecipitates, shown in Fig 3C, confirmed the Lyn association with the wild-type and mutant EpoRs and further indicated that both LynA and LynB associated with the EpoR. To confirm this, LynA and LynB were individually coexpressed with the wild-type EpoR in COS7 cells. Anti-Lyn blotting of anti-Lyn immunoprecipitates demonstrated that LynA and LynB were expressed as 56- and 53-kD proteins, respectively, although slower-migrating minor species were observed for both LynA and LynB (Fig 3D, lower panel). Anti-EpoR blotting of anti-Lyn immunoprecipitates (Fig 3D, upper panel) as well as anti-Lyn blotting of anti-EpoR immunoprecipitates (Fig 3E, upper panel) confirmed the physical association of both forms of Lyn with the EpoR in transfected COS7 cells. In repeated experiments, the binding of Lyn with the EpoR in COS7 cells was observed independently of Epo stimulation (data not shown). These data demonstrate that both LynA and LynB bind with the membrane-proximal 91-amino acid region of the EpoR cytoplasmic domain. However, amino acids 281 to 300 within this region were dispensable for the association of Lyn, which is in contrast to the binding of Jak2.6 7 In addition, Lyn was found to induce tyrosine phosphorylation of the EpoR in COS7 cells.
Binding of LynA and LynB to various mutant EpoRs in COS7 cells. (A) Schematic representation of EpoR mutants used in binding studies in COS7 cells. The transmembrane domain (TM) is represented with a hatched box, whereas the conserved sequence motifs, Box1 and Box2, are represented with solid boxes. The positions of eight tyrosine residues in the cytoplasmic region are indicated with vertical lines. Numbers in parentheses indicate the amino acid number at the carboxy terminus of the EpoR or at the sites of truncation. EC, extracellular region; IC, intracellular region. (B and C) The wild-type (W) or mutant EpoRs (H, S, or PB) were coexpressed with both LynA and LynB in COS7 cells, as indicated. Cells were lysed and immunoprecipitated with anti-Lyn or anti-EpoR, as indicated. Immunoprecipitates were subjected to immunoblotting with indicated antibodies. (D and E) LynA (A) or LynB (B) was coexpressed with the wild-type EpoR in COS7 cells, as indicated, and examined as described above.
Binding of LynA and LynB to various mutant EpoRs in COS7 cells. (A) Schematic representation of EpoR mutants used in binding studies in COS7 cells. The transmembrane domain (TM) is represented with a hatched box, whereas the conserved sequence motifs, Box1 and Box2, are represented with solid boxes. The positions of eight tyrosine residues in the cytoplasmic region are indicated with vertical lines. Numbers in parentheses indicate the amino acid number at the carboxy terminus of the EpoR or at the sites of truncation. EC, extracellular region; IC, intracellular region. (B and C) The wild-type (W) or mutant EpoRs (H, S, or PB) were coexpressed with both LynA and LynB in COS7 cells, as indicated. Cells were lysed and immunoprecipitated with anti-Lyn or anti-EpoR, as indicated. Immunoprecipitates were subjected to immunoblotting with indicated antibodies. (D and E) LynA (A) or LynB (B) was coexpressed with the wild-type EpoR in COS7 cells, as indicated, and examined as described above.
In vitro binding of GST-Lyn fusion proteins to the EpoR.
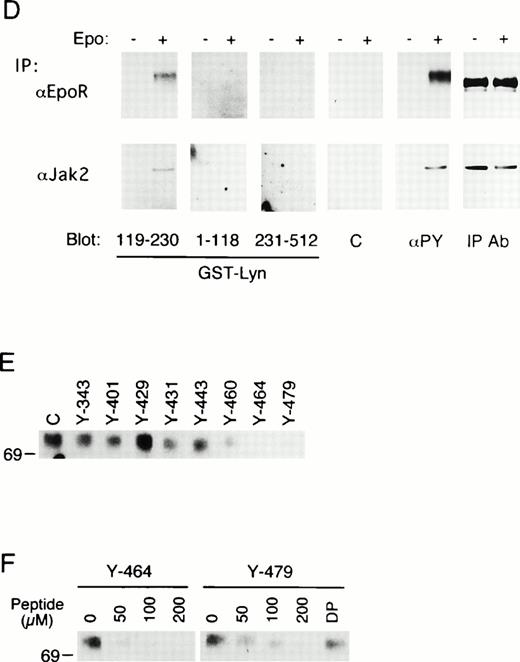
To explore the mechanisms of Lyn binding with the EpoR, in vitrobinding of GST-Lyn fusion proteins, shown in Fig 4A, with the EpoR was examined. First, cell lysates from parental 32D cells or 32D/EpoR-Wt cells were incubated with the GST-Lyn fusion proteins, and cellular proteins bound to these fusion proteins were examined by anti-EpoR blotting. As shown in Fig 4B, GST-Lyn231-512, which encompasses the Lyn tyrosine kinase domain, bound with the 62- to 66-kD forms of the EpoR in lysates from 32D/EpoR-Wt cells. In contrast, GST-Lyn119-230, which contains the Lyn SH2 domain, bound specifically with the 72-kD form of the EpoR from Epo-stimulated cells, which represents a small fraction of the receptor that was transported to the cell surface and tyrosine phosphorylated after Epo stimulation.4 37 In accordance with this, antiphosphotyrosine blotting of the proteins bound to GST-Lyn119-230 confirmed that the 72-kD EpoR is the predominant phosphotyrosyl protein that binds the Lyn SH2 domain (Fig 4C, upper and middle panels). On the other hand, GST-Lyn1-119, which comprises the unique and SH3 domains of Lyn, did not show any binding with the EpoR (Fig 4B). Antiphosphotyrosine blotting of proteins bound to the GST-Lyn fusion proteins also showed that a phosphotyrosyl 130-kD protein in Epo-stimulated cell lysate specifically bound with GST-Lyn119-230 (Fig4C, upper panel). This phosphotyrosyl protein was identified as Jak2 by reprobing with anti-Jak2 (Fig 4C, lower panel).
In vitro binding studies with GST-Lyn fusion proteins. (A) Schematic representation of LynA and recombinant Lyn proteins used in in vitro binding studies. The SH3 and SH2 domains are represented by hatched and solid boxes, respectively. Amino acid numbers are shown under Lyn. (B) Cell lysate from parental 32D (EpoR −) or 32D/EpoR-Wt (EpoR +) cells, which were unstimulated or stimulated with Epo as indicated (Epo st. − or +, respectively), was incubated with GST-Lyn fusion proteins indicated below the panel. Affinity-purified proteins were resolved by SDS-PAGE and subjected to anti-EpoR immunoblotting. The tyrosine-phosphorylated 72-kD EpoR as well as the 62- to 66-kD forms of the EpoR is indicated. (C)Cell lysate from Epo-stimulated or unstimulated 32D/EpoR-Wt was incubated with GST (C) or GST-Lyn fusion proteins indicated below the panel. Affinity-purified proteins were subjected to antiphosphotyrosine immunoblotting followed by reprobing with anti-EpoR and anti-Jak2, as indicated. (D) 32D/EpoR-Wt cells were starved overnight and stimulated with 100 U/mL of Epo for 5 minutes or left unstimulated, as indicated, before solubilization. Cell lysates were immunoprecipitated with anti-EpoR or anti-Jak2 (as indicated), resolved by SDS-PAGE, and electrotransferred onto a PVDF membrane. The membrane was probed with GST-Lyn119-230 followed by detection with anti-GST immunoblotting. The membrane was then reprobed with GST-Lyn1-118, GST-Lyn231-512, GST (C), antiphosphotyrosine (αPY), and the antibody used for immunoprecipitation, as indicated. (E) Cell lysate from Epo-stimulated 32D/EpoR-Wt was mixed with synthetic phosphopeptides (200 μmol/L) corresponding to potential tyrosine phosphorylation sites, as indicated, and incubated with GST-Lyn119-230. Proteins bound to GST-Lyn119-230 were then subjected to immunoblotting with anti-EpoR. (F) Cell lysate from Epo-stimulated 32D/EpoR-Wt was mixed with indicated concentrations of the Y-464 and Y479 phosphopeptides, as indicated, or the unphosphorylated equivalent of Y-479 polypeptide (479DP) and analyzed as described above.
In vitro binding studies with GST-Lyn fusion proteins. (A) Schematic representation of LynA and recombinant Lyn proteins used in in vitro binding studies. The SH3 and SH2 domains are represented by hatched and solid boxes, respectively. Amino acid numbers are shown under Lyn. (B) Cell lysate from parental 32D (EpoR −) or 32D/EpoR-Wt (EpoR +) cells, which were unstimulated or stimulated with Epo as indicated (Epo st. − or +, respectively), was incubated with GST-Lyn fusion proteins indicated below the panel. Affinity-purified proteins were resolved by SDS-PAGE and subjected to anti-EpoR immunoblotting. The tyrosine-phosphorylated 72-kD EpoR as well as the 62- to 66-kD forms of the EpoR is indicated. (C)Cell lysate from Epo-stimulated or unstimulated 32D/EpoR-Wt was incubated with GST (C) or GST-Lyn fusion proteins indicated below the panel. Affinity-purified proteins were subjected to antiphosphotyrosine immunoblotting followed by reprobing with anti-EpoR and anti-Jak2, as indicated. (D) 32D/EpoR-Wt cells were starved overnight and stimulated with 100 U/mL of Epo for 5 minutes or left unstimulated, as indicated, before solubilization. Cell lysates were immunoprecipitated with anti-EpoR or anti-Jak2 (as indicated), resolved by SDS-PAGE, and electrotransferred onto a PVDF membrane. The membrane was probed with GST-Lyn119-230 followed by detection with anti-GST immunoblotting. The membrane was then reprobed with GST-Lyn1-118, GST-Lyn231-512, GST (C), antiphosphotyrosine (αPY), and the antibody used for immunoprecipitation, as indicated. (E) Cell lysate from Epo-stimulated 32D/EpoR-Wt was mixed with synthetic phosphopeptides (200 μmol/L) corresponding to potential tyrosine phosphorylation sites, as indicated, and incubated with GST-Lyn119-230. Proteins bound to GST-Lyn119-230 were then subjected to immunoblotting with anti-EpoR. (F) Cell lysate from Epo-stimulated 32D/EpoR-Wt was mixed with indicated concentrations of the Y-464 and Y479 phosphopeptides, as indicated, or the unphosphorylated equivalent of Y-479 polypeptide (479DP) and analyzed as described above.
To examine whether the Lyn SH2 domain binds directly to the EpoR and Jak2, the binding was next examined by Far-Western blotting. As shown in Fig 4D, GST-Lyn119-230 specifically bound to the tyrosine-phosphorylated 72-kD form of EpoR from Epo-stimulated 32D/EpoR-Wt cells, whereas GST-Lyn1-118 and GST-Lyn231-512 failed to show any binding to the EpoR. Similarly, tyrosine-phosphorylated Jak2 bound specifically with GST-Lyn119-230 in Far-Western blotting (Fig 4D, lower panels). These data indicate that the Lyn SH2 domain directly binds to the tyrosine-phosphorylated forms of EpoR and Jak2.
Next, to determine which tyrosine residues in the EpoR are involved in binding with the Lyn SH2 domain, in vitro binding competition assays were performed using synthetic phosphopeptides corresponding to the potential tyrosine phosphorylation sites in the EpoR cytoplasmic domain. When added at 200 μmol/L to cell lysate from Epo-stimulated 32D/EpoR-Wt, the phosphopeptide containing Y-464 almost completely inhibited the binding of GST-Lyn119-230 with the EpoR, as shown in Fig4E. The Y-479 containing peptide also showed a prominent inhibitory effect on the Lyn SH2 binding to the EpoR, whereas the other phosphopeptides had less significant or no effects. Various concentrations of the Y-464 and Y-479 peptides were then added to cell lysate to determine the inhibitory efficiency, as shown in Fig 4F. Densitometric scanning of the immunoblot showed that less than 50 μmol/L of the Y-464 peptide was required to inhibit half of the Lyn SH2 binding to the EpoR. On the other hand, the half-inhibitory concentration of the Y-479 peptide was between 50 and 100 μmol/L and thus was higher than that of the Y-464. Nevertheless, the inhibition was shown to be dependent on phosphorylation of Y-479, because the unphosphorylated peptide with the identical sequence did not show any inhibition at 200 μmol/L. These results thus indicate that Y-464 and Y-479 in the carboxy-terminal region of the EpoR may be involved in binding with the Lyn SH2 domain.
Lyn induces phosphorylation of Stat5 on Y-694 and activates its DNA-binding activity in COS7 cells.
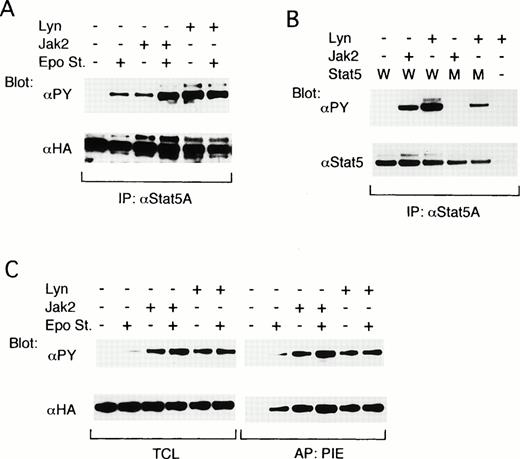
To explore the possibility that Lyn may be involved in activation of the down-stream signaling pathways from the EpoR, we next examined the possible effect of Lyn on the Stat5 activation. The effect of Lyn on the tyrosine phosphorylation of Stat5 was first examined by reconstituting the EpoR signaling pathways in COS7 cells. As shown in Fig 5A, when ovine Stat5, tagged with the HA epitope alone was coexpressed with the EpoR, a moderate tyrosine phosphorylation of Stat5 was observed only after Epo stimulation, as demonstrated by antiphosphotyrosine blotting of anti-Stat5A immunoprecipitates. When Jak2 was additionally coexpressed, the tyrosine phosphorylation of Stat5 was observed constitutively but was significantly augmented by Epo stimulation. On the other hand, the coexpression of Lyn induced a strong constitutive tyrosine phosphorylation of Stat5, which was comparable with that attained in the Epo-stimulated Jak2-coexpressing cells and was not increased by Epo stimulation (Fig 5A, upper panel). Anti-HA blotting of the anti-Stat5A immunoprecipitates confirmed that ovine Stat5 was comparably expressed under each condition (Fig 5A, lower panel).
Lyn induces tyrosine phosphorylation of Stat5 and activates its DNA-binding ability in COS7 cells. (A) The EpoR and ovine Stat5 tagged with an epitope recognized with anti-HA were transiently coexpressed with Jak2 or with both LynA and LynB, as indicated, in COS7 cells. Cells were left unstimulated (−) or stimulated (+) with Epo for 5 minutes before solubilization, as indicated. Cell lysates were subjected to immunoprecipitation with anti-Stat5A followed by immunoblotting with the indicated antibodies. (B) The EpoR and wild-type ovine Stat5 (W) or its mutant with a substitution of Tyr694 with Phe (M) were coexpressed in COS7 cells with Jak2 or Lyn, as indicated. Cell lysates were immunoprecipitated with anti-Stat5A and subjected to immunoblotting with indicated antibodies. (C) The EpoR and ovine Stat5 were coexpressed with Jak2 or Lyn in COS7 cells, as indicated. Cells were left unstimulated (−) or stimulated (+) with Epo for 15 minutes before solubilization, as indicated. Cell lysates were subjected to affinity purification with a PIE oligonucleotide. Total cell lysates (TCL) or affinity-purified proteins (PIE) were analyzed by immunoblotting with indicated antibodies.
Lyn induces tyrosine phosphorylation of Stat5 and activates its DNA-binding ability in COS7 cells. (A) The EpoR and ovine Stat5 tagged with an epitope recognized with anti-HA were transiently coexpressed with Jak2 or with both LynA and LynB, as indicated, in COS7 cells. Cells were left unstimulated (−) or stimulated (+) with Epo for 5 minutes before solubilization, as indicated. Cell lysates were subjected to immunoprecipitation with anti-Stat5A followed by immunoblotting with the indicated antibodies. (B) The EpoR and wild-type ovine Stat5 (W) or its mutant with a substitution of Tyr694 with Phe (M) were coexpressed in COS7 cells with Jak2 or Lyn, as indicated. Cell lysates were immunoprecipitated with anti-Stat5A and subjected to immunoblotting with indicated antibodies. (C) The EpoR and ovine Stat5 were coexpressed with Jak2 or Lyn in COS7 cells, as indicated. Cells were left unstimulated (−) or stimulated (+) with Epo for 15 minutes before solubilization, as indicated. Cell lysates were subjected to affinity purification with a PIE oligonucleotide. Total cell lysates (TCL) or affinity-purified proteins (PIE) were analyzed by immunoblotting with indicated antibodies.
To determine whether Lyn induces the phosphorylation of Stat5 on Y-694, which is required for homodimerization and activation of Stat5, we next examined the effect of Lyn on tyrosine phosphorylation of an ovine Stat5 mutant in which Y-694 is replaced with a Phe residue.29 33 As shown in Fig 5B, the tyrosine phosphorylation of mutant Stat5 was induced by the coexpression of Lyn but not by that of Jak2 in COS7 cells coexpressing the EpoR. Densitometric analysis showed that the expression and tyrosine phosphorylation levels of wild-type Stat5 coexpressed with Lyn were 1.8 and 3.7 times higher, respectively, than those of mutant Stat5. Accordingly, the intensity of tyrosine phosphorylation of wild-type Stat5 induced by Lyn was about two times higher than that of mutant Stat5, thus suggesting that Lyn phosphorylates Y-694 as well as other tyrosine residues.
The ability of Lyn to activate the DNA-binding activity of Stat5 was then examined. For this purpose, proteins that bound to the PIE oligonucleotide, which contains the Stat5-binding sequence, were affinity purified from COS7 cells in which the EpoR signaling pathway was reconstituted by coexpressing the EpoR, HA-tagged ovine Stat5, and Jak2 or Lyn. In accordance with the previous results shown in Fig 5A, antiphosphotyrosine blotting of total cell lysates showed that Stat5 was constitutively and prominently tyrosine phosphorylated in COS7 cells coexpressing Jak2 or Lyn, whereas Epo stimulation was required to induce any detectable tyrosine phosphorylation of Stat5 when these kinases were not coexpressed (Fig 5C, left upper panel). Antiphosphotyrosine and anti-HA blotting of proteins bound to the PIE oligonucleotide showed that the DNA-binding activity of Stat5 closely correlated with its tyrosine phosphorylation state in these transfected cells (Fig 5C). It was thus demonstrated that Lyn and Jak2 comparably activated the DNA-binding activity of Stat5, which agrees with the fact that Lyn induces phosphorylation of Stat5 mainly on Y-694.
Lyn enhances Stat5-mediated transcriptional activation of the β-casein promoter in COS7 and 32D/EpoR-Wt cells.
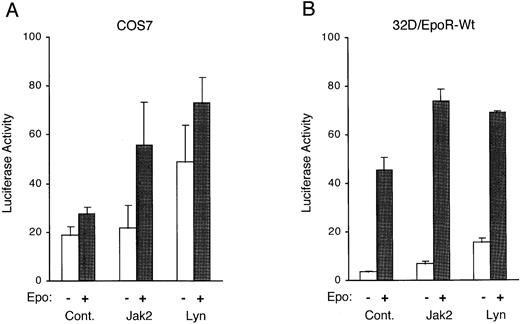
To investigate whether Lyn activates Stat5 to induce transcription from responsive genes, we coexpressed Lyn or Jak2 in COS7 cells along with the EpoR, Stat5, and a Stat5-responsive luciferase reporter plasmid, pZZ1, in which the expression of the luciferase gene is controlled by the β-casein promoter containing two Stat5-binding sites. Figure 6A shows that the coexpression of Lyn significantly increased the transcriptional activation of the β-casein promoter with or without Epo stimulation. To further confirm that Lyn activates the transactivation potential of Stat5, Lyn was next transiently overexpressed in 32D/EpoR-Wt cells along with the Stat5-responsive reporter plasmid. As shown in Fig 6B, the overexpression of Lyn or Jak2 increased the basal transcription level of the β-casein promoter as well as the level obtained after Epo stimulation. These results are in agreement with the idea that Lyn enhances Epo-induced gene transcription through the action of Stat5.
Lyn enhances expression of a Stat5-responsive reporter plasmid. (A) COS7 cells were cotransfected with either pcDNA3 (Cont.), pcDNA-Jak2 (Jak2), or pXM-LynA and pXM-LynB (Lyn), as indicated, along with the Stat5-responsive luciferase reporter pZZ1 plasmid, the control Renilla luciferase pRL-TK plasmid, pRK5-Stat5, and pXM-EpoR-Wt. Two days after transfection, cells were either left unstimulated or stimulated with 5 U/mL for 5 hours, as indicated, and harvested for the dual-luciferase assay. The luciferase activity was normalized by the Renilla luciferase activity and expressed in arbitrary units. The data represent averages ± standard deviations of three independent experiments. (B) 32D/EpoR-Wt cells were transiently transfected with either pcDNA3 (Cont.), pcDNA-Jak2 (Jak2), or pXM-LynA and pXM-LynB (Lyn), as indicated, along with pZZ1 and pRL-SV40. One day after transfection, cells were starved overnight, incubated for 5 hours in medium with or without 4 U/mL of Epo (as indicated), and harvested for the dual-luciferase assay.
Lyn enhances expression of a Stat5-responsive reporter plasmid. (A) COS7 cells were cotransfected with either pcDNA3 (Cont.), pcDNA-Jak2 (Jak2), or pXM-LynA and pXM-LynB (Lyn), as indicated, along with the Stat5-responsive luciferase reporter pZZ1 plasmid, the control Renilla luciferase pRL-TK plasmid, pRK5-Stat5, and pXM-EpoR-Wt. Two days after transfection, cells were either left unstimulated or stimulated with 5 U/mL for 5 hours, as indicated, and harvested for the dual-luciferase assay. The luciferase activity was normalized by the Renilla luciferase activity and expressed in arbitrary units. The data represent averages ± standard deviations of three independent experiments. (B) 32D/EpoR-Wt cells were transiently transfected with either pcDNA3 (Cont.), pcDNA-Jak2 (Jak2), or pXM-LynA and pXM-LynB (Lyn), as indicated, along with pZZ1 and pRL-SV40. One day after transfection, cells were starved overnight, incubated for 5 hours in medium with or without 4 U/mL of Epo (as indicated), and harvested for the dual-luciferase assay.
Lyn phosphorylates the EpoR and Stat5 on tyrosines in vitro.
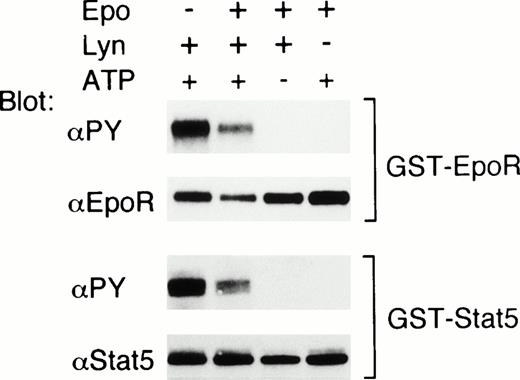
To confirm that the EpoR and Stat5 are substrates for Lyn, we next examined whether Lyn phosphorylates the EpoR and Stat5 in vitro. For this purpose, a GST-EpoR fusion protein containing the EpoR intracellular domain or a GST-Stat5 protein containing a carboxy-terminal portion of Stat5 including Y-694 was incubated, in the presence or absence of ATP, with Lyn immunoprecipitated from 32D/EpoR-Wt cells. As shown in Fig 7, antiphosphotyrosine blotting showed that the tyrosine phosphorylation of these fusion proteins was induced only in the presence of both Lyn and ATP. On the other hand, Lyn did not induce any detectable tyrosine phosphorylation of the GST protein in vitro (negative data not shown). These data indicate that the EpoR and Stat5 are substrates for tyrosine phosphorylation by Lyn in vitro. In the experiment shown in Fig 7, the phosphorylation of the EpoR and Stat5 induced by Lyn immunoprecipitated from Epo-stimulated cells was less prominent than that induced by Lyn from nonstimulated cells. However, in a repeated experiment, Epo stimulation of 32D/EpoR-Wt cells before solubilization did not have any significant effect on the ability of Lyn to phosphorylate these fusion proteins.
Lyn phosphorylates the EpoR and Stat5 on tyrosine in vitro. 32D/EpoR-Wt cells were left unstimulated or stimulated with Epo, as indicated. Cells were then lysed and immunoprecipitated with anti-Lyn (Lyn +) or with normal rabbit serum (Lyn −). GST-EpoR (upper panels) and GST-Stat5 (lower panels) proteins were then added to immunoprecipitates as substrates and incubated at room temperature for 30 minutes in in vitro kinase buffer with or without 1 mmol/L ATP, as indicated. Reaction products were then subjected to immunoblotting with antiphosphotyrosine followed by reprobing with anti-EpoR or anti-Stat5, as indicated.
Lyn phosphorylates the EpoR and Stat5 on tyrosine in vitro. 32D/EpoR-Wt cells were left unstimulated or stimulated with Epo, as indicated. Cells were then lysed and immunoprecipitated with anti-Lyn (Lyn +) or with normal rabbit serum (Lyn −). GST-EpoR (upper panels) and GST-Stat5 (lower panels) proteins were then added to immunoprecipitates as substrates and incubated at room temperature for 30 minutes in in vitro kinase buffer with or without 1 mmol/L ATP, as indicated. Reaction products were then subjected to immunoblotting with antiphosphotyrosine followed by reprobing with anti-EpoR or anti-Stat5, as indicated.
DISCUSSION
The present study has demonstrated that Lyn physically associates with the EpoR in Epo-responsive hematopoietic cells. Transient expression experiments in COS7 cells further showed that both LynA and LynB binds to the membrane-proximal 91-amino acid residues of the EpoR cytoplasmic domain. However, in contrast to the binding of Jak2 to the EpoR, the membrane-proximal amino acid residues 281 through 300 of the EpoR were dispensable for the binding of Lyn. In vitro binding studies using GST-Lyn fusion proteins showed that the tyrosine kinase domain of Lyn binds to the EpoR and further indicated that the binding of Lyn to the EpoR may be additionally mediated through interaction between the Lyn SH2 domain and phosphorylated Y-464 and Y-479 residues in the carboxy-terminal region of the EpoR. In vitro binding studies also raised a possibility that the Lyn SH2 may bind directly to tyrosine-phosphorylated Jak2. When coexpressed in COS7 cells, Lyn induced tyrosine phosphorylation of the EpoR. Furthermore, in vitroreconstitution experiments in COS7 cells as well as overexpression experiments in 32D/EpoR-Wt cells indicated that Lyn has the ability to induce tyrosine phosphorylation of Stat5 to activate its DNA-binding and transcription-activating activities. Lyn was also shown to phosphorylate the EpoR and Stat5 on tyrosines in vitro. Together, these results strongly suggest that Lyn may play a role in activation of the EpoR-mediated signaling pathways including the Jak2/Stat5 pathway.
The coexpression experiments in COS7 cells demonstrated that Lyn binds to the membrane-proximal 91-amino acid region of the EpoR cytoplasmic domain, which is required for growth signaling from the EpoR.4,5,7,36 However, the 20-amino acid region between the Box1 and Box2 motifs, required for the binding of Jak2, was dispensable for that of Lyn, thus suggesting that the two tyrosine kinases bind to the EpoR membrane-proximal region in different manners. Because the membrane-proximal 91-amino acid region does not contain any tyrosine residues, it is expected that the binding to this region is mediated through the Lyn tyrosine kinase domain, which was shown to bind to the EpoR irrespective of its tyrosine phosphorylation status in the in vitro binding study. The physical association between the Src family tyrosine kinase domain and the cytokine receptor cytoplasmic domain has previously been reported in the case of binding of Lck to the IL-2 receptor β subunit (IL-2Rβ).38 Intriguingly, when expressed in cells not expressing Lck, IL-2Rβ has been shown to couple with Lyn instead of Lck,39 40 which implies that the Lyn tyrosine kinase domain may also have the ability to bind IL-2Rβ.
The in vitro binding studies using GST-Lyn fusion proteins further showed that the Lyn SH2 domain specifically bound to the tyrosine-phosphorylated 72-kD form of the EpoR in cell lysate from Epo-stimulated cells. In fact, anti-EpoR immunoblotting showed that the amount of the EpoR that was bound to the SH2 domain of Lyn was much higher than that bound to the tyrosine kinase domain (Fig 4B). It is thus suggested that the SH2 domain of Lyn binds to the EpoR with an affinity that is substantially higher than that of the tyrosine kinase domain. The difference in binding affinities may be quite remarkable, because the amount of the tyrosine-phosphorylated EpoR in cells is much smaller than that of the unphosphorylated EpoR; only a small portion of the EpoR synthesized in cells as the 62- to 66-kD forms is expressed on the cell surface and becomes tyrosine phosphorylated as the 72-kD form upon Epo binding.4,37 41 Consistent with this idea, we could detect the EpoR binding of the Lyn SH2 domain but not that of the tyrosine kinase domain by Far Western blotting. It is thus possible that, unlike when overexpressed in COS7 cells, the Lyn binding to the EpoR in hematopoietic cells may be mediated mainly through the interaction between the Lyn SH2 domain and phosphotyrosines in the activated EpoR and thus induced mainly after Epo stimulation. However, this possibility remains to be proved, because the small quantity of the surface expressed EpoR prohibited the direct detection of the EpoR that is associated with Lyn in hematopoietic cells by anti-EpoR blotting of Lyn immunoprecipitates (data not shown).
The Lyn SH2 domain was shown to bind directly with the EpoR by Far-Western blotting (Fig 4D). Competition binding assays using synthetic phosphopeptides further indicated that the binding may involve phosphorylated Y-464 and Y-479 residues at the EpoR carboxy-terminal region (Fig 4E and F). Notably, the amino acids following Y-464 (Y-464 ENS) are similar to the optimal binding sequences for the SH2 domains of the Src family kinases with the general motif pY-hydrophilic-hydrophilic-I/P,42 although those following Y-479 (Y-479 VAC) are quite different. Previously, we showed that the p85 regulatory subunit of PI3K binds through its SH2 domain to the tyrosine-phosphorylated EpoR carboxy-terminal region, which is, however, not required for growth signaling.10Recently, Damen et al43 have shown that Y-479 of the EpoR, one of the Lyn SH2 binding sites identified in the present study, is involved in binding to the p85 SH2 domain. Intriguingly, a very recent study by Klingmüller et al44 has further shown that Y-479 may be involved in transducing signals for proliferation and differentiation of the erythroid progenitor cells. It is thus possible that Lyn may play a physiologically significant role by binding to this potentially important tyrosine residue or to Y-464 in the vicinity of Y-479. In this regard, it should be noted that Lyn has been shown to bind directly or indirectly with p85 and to activate the PI3K activity.20 45-48 It is also conceivable that Lyn may compete with p85 for binding to Y-479 and thus inhibit the EpoR-mediated activation of PI3K. The effect of Lyn on the EpoR-mediated activation of the PI3K pathway is currently under investigation in our laboratory using cells stably overexpressing Lyn.
As the present study was being completed, Tilbrook et al26reported that Lyn physically associates with the EpoR and is essential for Epo-induced differentiation of the J2E cell line, which was generated by transforming immature erythroid cells with theraf/myc-containing J2 retrovirus. Using the yeast two-hybrid analysis, Tilbrook et al26 assigned the region of Lyn involved in binding to the EpoR to the N-terminal 162-amino acid residues, which contains the unique and SH3 binding domains and only a portion of the SH2 domain. This is in contrast to the present study, which demonstrated that the SH2 and catalytic domains of Lyn are involved in in vitro binding studies. However, because Tilbrook et al26 examined only this N-terminal region of Lyn in the yeast two-hybrid analysis, it has remained to be determined whether the other domains of Lyn may also mediate binding with the EpoR in the yeast cells. Furthermore, it should be noted that the binding that requires the tyrosine phosphorylation of the EpoR may not be examined by the method used by Tilbrook et al.26 Further studies are thus required to determined which domain of Lyn mainly mediates binding to the EpoR in hematopoietic cells.
Previous studies have implicated Lyn in signaling from the receptors for IL-3, GM-CSF, and IL-5,17-22 which share the common signal-transducing subunit βc, because Lyn physically associates with βc and its kinase activity shows a moderate increase after activation of these receptors in several cell lines, including 32D cells.19 In contrast, we have not observed any persistent and significant increase in the autokinase or exokinase activity of Lyn by Epo stimulation of 32D/EpoR-Wt cells in the present study (negative data not shown). This may be due to the high constitutive activity of Lyn in cells we used, because a moderate increase in activity of a fraction of Lyn that is associated with the EpoR could not be then detectable by the in vitro kinase assay of Lyn immunoprecipitated from the whole cell. Alternatively, because we have not observed any significant increase in Lyn activity even after IL-3 stimulation of 32D/EpoR-Wt cells (data not shown), the differences in reagents, cells, or method we used as compared with those used in previous studies might have caused our inability to demonstrate an increase in Lyn activity after cytokine stimulation. In this regard, it should be noted that Tilbrook et al26 have shown that Epo induces a marginal increase in the Lyn kinase activity in the J2E cells. Another possibility is that the binding of Lyn to the EpoR may not modulate its kinase activity but may allow the kinase access to substrates for phosphorylation, which include the EpoR itself and signaling molecules recruited to the tyrosine-phosphorylated EpoR.
Previously, v-Src has been shown to activate Stat3 in NIH3T3 and rat embryo fibroblast cells.49,50 Furthermore, it has been proposed that c-Src, which physically associates with growth factor receptors with intrinsic tyrosine kinase activity,51mediates the activation of Stat3 by these receptors.50 When expressed in 32D cells, v-Src was shown to activate Stat1, Stat3, and Stat5 and to abrogate IL-3 dependence of this cell line.52These observations led us to speculate that Lyn, the most abundantly expressed Src family kinase in 32D cells, may play a role in the EpoR-mediated activation of Stat5. In accordance with this speculation, the present study demonstrated that Stat5 as well as the EpoR is a substrate for Lyn, because Lyn induced the tyrosine phosphorylation of both the EpoR and Stat5 in COS7 cells (Figs 2 and 5A) and phosphorylated these proteins on tyrosines in vitro (Fig 7). Although the Lyn-induced tyrosine phosphorylation of the EpoR and Stat5 was independent of Epo stimulation in COS7 cells, this could be due to the high expression levels of Lyn and these substrates in these cells, because Jak2 also induced the constitutive tyrosine phosphorylation of the EpoR and Stat5 when coexpressed in COS7 cells (Figs 2 and 5A). By using a Stat5 mutant, it was further demonstrated that Lyn induced the phosphorylation of Stat5 on Y-694, which is required for its activation (Fig 5B).29 In accordance with this finding, Lyn stimulated the DNA-binding and transactivation abilities of Stat5 in COS7 cells (Figs 5C and 6A). The ability of Lyn to increase the Stat5-mediated transcriptional activation was confirmed in 32D/EpoR-Wt cells by transient overexpression of Lyn (Fig 6B). These data strongly support a role for Lyn in enhancing the EpoR-mediated Stat5 activation.
Importantly, the Lyn SH2 domain was shown to bind to tyrosine-phosphorylated Jak2 in vitro (Fig 4C and D), although the in vivo binding could not be demonstrated by coimmunoprecipitation (data not shown). Similarly, Uddin et al53 have very recently reported that Fyn, another member of the Src family, associates via its SH2 domain with Tyk2 or Jak2 in cells stimulated with interferon (IFN) α or IFNγ, respectively. In addition, the Src family kinases have been shown to associate through their SH2 domains with several receptor-type tyrosine kinases, resulting in the mutual stimulation of catalytic activity and enhanced phosphorylation of downstream targets of each of the tyrosine kinases.51 It is thus tempting to speculate that the Lyn-Jak2 interaction may similarly activate or stabilize the catalytic activity of both tyrosine kinases. In vitro and in vivo binding studies have previously shown that Lyn also binds to Cbl, Shc, PI3K, phospholipase Cγ, MAPK, and GAP,47 all of which have also been implicated in signaling mediated through the EpoR. Therefore, Lyn may also directly phosphorylate these signaling molecules involved in EpoR-mediated signaling or indirectly potentiate their tyrosine phosphorylation. Further studies are currently in progress to address these possibilities and to investigate the effect of Lyn on anti-apoptotic, growth-promoting, and differentiation-inducing abilities of the EpoR.
In summary, this study suggests that Lyn constitutively binds to the 91-amino acid membrane-proximal EpoR region and, upon the activation and tyrosine phosphorylation of the EpoR, further interacts through its SH2 domain with the phosphorylated tyrosine residues, Y-464 and Y-479, in the EpoR carboxy-terminal region. It is speculated that the binding of these phosphotyrosines to the Lyn SH2 domain may increase the kinase activity of Lyn by displacing the inhibitory phosphotyrosine at the carboxy-terminal region of Lyn. Alternatively, Lyn may be newly recruited to the activated EpoR through the phosphotyrosine-SH2 interaction, thus gaining access to substrates for phosphorylation, which include the EpoR itself and signaling molecules recruited to the tyrosine-phosphorylated EpoR. It is thus hypothesized that Lyn plays a role in the EpoR-mediated signaling, which was supported by the finding that Lyn enhanced the Epo-induced activation of Stat5.
ACKNOWLEDGMENT
The authors are grateful to Drs James N. Ihle and Taolin Yi for invaluable discussions and for the generous gifts of the murine Lyn cDNAs and the EpoR-derived phosphotyrosine peptides. We thank Drs Yoji Ikawa, Atsushi Miyajima, and Koh Yamamoto for helpful discussions and Kaori Okada for excellent technical assistance.
Supported by grants from the Ministry of Education, Science and Culture of Japan.
Address reprints requests to Osamu Miura, MD, First Department of Internal Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyoku, Tokyo 113, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal