Abstract
An unexpectedly high incidence of blast transformation after splenectomy has been reported in patients with myelofibrosis with myeloid metaplasia. However, whether this was associated with spleen removal after adjustment for risk factors was not determined. We conducted a multicenter historical cohort study of patients with myelofibrosis with myeloid metaplasia diagnosed from January 1970 through January 1994. A total of 549 patients (325 men and 224 women from 22 to 92 years of age; median age, 63 years) were included in the final data set. The Cox's proportional-hazards model was used to identify factors associated with blast transformation and death. To further adjust for factors related to spleen removal assignment, a propensity score for splenectomy was estimated using recursive-partitioning analysis. Blast transformation developed in 78 patients (14.2%). Patients who underwent splenectomy developed more blast transformations than those who were not splenectomized (23 of 87 [26.4%] v 55 of 462 [11.9%]; P < .001). The cumulative incidence of blast transformation 12 years after diagnosis was 27.0% in nonsplenectomized patients and 55.0% in splenectomized ones (P = .01). The risk factors independently predictive of blast transformation included prior splenectomy (relative risk = 2.61), platelet count less than 100 × 109/L at diagnosis (relative risk = 2.45), and the presence of blasts in peripheral blood at diagnosis (relative risk = 2.31). The relative risk of blast transformation in splenectomized patients increased from 2.2 at 48 months from diagnosis to 14.3 at 12 years. Patients with the same propensity score for splenectomy showed a higher risk for blast transformation on the basis of having undergone splenectomy (P= .02). In conclusion, the risk of blast transformation is significantly increased in subjects who underwent splenectomy and appears to be independent of factors related to spleen removal assignment.
BLAST TRANSFORMATION, ie, an accumulation of blasts in bone marrow and peripheral blood associated with clinical deterioration, is reported to occur in 5% to 30% of patients with myelofibrosis with myeloid metaplasia.1-12 Erythroid failure, severe anemia, a high number of circulating immature myeloid cells or white blood cells at diagnosis, and chromosomal aberrations have all been recognized as markers of leukemic evolution.12-14
Two previous studies aimed at evaluating the outcome of splenectomy in myelofibrosis with myeloid metaplasia reported an unexpectedly high incidence of blast transformation in splenectomized patients. In one study of 71 patients, blast transformation accounted for 42.8% of deaths;15 in another study of 39 cases, blast transformation occurred in 46.6% of surviving patients observed for more than 2 years.16 However, the design of those studies did not eliminate the possible bias related to whether the splenectomized patients were inclined to proceed toward leukemic evolution or whether it became clinically manifest with splenectomy.
We conducted a follow-up study of patients with myelofibrosis with myeloid metaplasia that combined the results from Italian centers in an attempt to reassess the risk factors for the development of blast transformation and the effect of splenectomy on the outcome. The main reason that brought us to address this problem with a large study was that splenectomy in myelofibrosis with myeloid metaplasia is performed with a critical balance between risks and benefits. Thus postsplenectomy complications that could shorten the length and worsen the quality of life should be perceived as particularly grave. Moreover, the hypothesis of this study, with the reports on the effect of splenectomy in increasing the risk of acute leukemia in Hodgkin's disease17-23 and aplastic anemia,24 could support a general role for splenectomy in the disruption of the tumor-host relationship.
MATERIALS AND METHODS
A collaborative study group was formed comprising 13 large hospitals in Italy. The centers were asked to include in this study all patients who were diagnosed with myelofibrosis with myeloid metaplasia from January 1970 through January 1994. Confirmation of diagnosis required a bone marrow biopsy demonstrating moderate to severe bone marrow fibrosis and two of the following criteria: typical peripheral blood morphology showing immature erythroid and myeloid cells with teardrop erythrocytes in the absence of absolute monocytosis; signs of myeloproliferation such as thrombocytosis or leukocytosis; and myeloid metaplasia documented by splenomegaly, ferrokinetic evaluation, or extramedullary tissue biopsy.25 Patients with postpolycythemia vera or postessential thrombocythemia myelofibrosis were excluded.
The rate of disease progression was evaluated by three indices: anemia, thrombocytopenia, and splenomegaly progression. They were defined as the ratio of the change in one progression parameter to the time interval from diagnosis to the change point. Change points were established at the minimum value for hemoglobin concentration and platelet count and at the maximum value for spleen size. Only the period before splenectomy was considered in splenectomized patients. Patients with fewer than three measurements for each parameter from diagnosis to change points were excluded from this assessment. Confirmation of a diagnosis of blast transformation required a percentage of peripheral blood blasts greater than 20% of the white blood cell count and/or a percentage of blasts in the bone marrow greater than 40%.
Five hundred and sixty-four patients were recruited. Anyone with clinical evidence of acute leukemia at diagnosis (n = 8) was excluded from further analysis. Three patients were excluded because they had undergone splenectomy from 5 to 20 years before diagnosis for reasons other than a myeloproliferative disorder; 4 others were excluded for insufficient data. Patients were censored from the analysis of blast transformation risk factors if they died within 1 month after splenectomy for causes related to surgery (n = 7). A total of 549 patients were included in the final data set.
Data from patient groups were compared using the Student'st-test, Mann-Whitney U test, or χ2 test as appropriate. Actuarial blast transformation-free curves were calculated using the method of Kaplan and Meier,26 with censoring at death or at the last follow-up. The log-rank test was used to assess differences between curves. We used the Cox's proportional-hazards model27 to analyze the association between splenectomy and blast transformation. The following baseline variables were included in the univariate model: sex, age at diagnosis, white blood cell count corrected for circulating erythroblasts, hemoglobin concentration, platelet count, percentage of immature myeloid cell in peripheral blood (excluding blasts), percentage of circulating erythroblasts, presence of circulating blasts, and splenomegaly grade. Given the wide variation in white blood cell counts and platelet counts at diagnosis, natural logs were applied to these covariates before their evaluation in the Cox's model. For the sake of homogeneity among different types of measurements, the degree of splenomegaly was assessed semiquantitatively on a scale of 0 to 3+ according to whether the spleen was of normal size or detected over, at, or below the umbilical line. Follow-up variables were the use of cytostatics, cumulative dose of cytostatics (converted to an ordinal variable), the progression indices, and splenectomy. Predictors with two-tailed P values less than .05 were entered into the multivariate models, and a series of models was constructed. To account for different times of splenectomy, the operation was entered as a time-dependent covariate. The assumption of a constant risk ratio over time for the proportional-hazards analysis was tested by the Schoenfeld residuals method28 and found to be valid for all the variables modeled except splenectomy. To accommodate risk time-dependency, a separate approach was used by modelling a time-by-covariate interaction as the product term in the Cox regression equation, and an exponential function of time was modeled.
To further separate the influence of splenectomy assignment on the risk for blast transformation, we used a propensity score adjustment.29 Recursive-partitioning analysis30was used to discriminate between patients who were assigned to splenectomy and those who were not. In addition to the variables applied in the Cox's model for the risk of blast transformation, the candidate predictor variables included the study center. Mantel-Haenszel statistics31 were used to compare the average incidence of blast transformation in splenectomized and nonsplenectomized patients in each stratum. Two-sided P values and 95% confidence intervals (CI) were used throughout. Statistical analysis was performed with the STATISTICA package (StataSoft, Tulsa, OK), except for the classification tree, for which implementation in S-PLUS (Statistical Science Inc, Seattle, WA) was used.
RESULTS
The number of evaluable patients and data on clinical and hematologic characteristics at diagnosis and during disease progression are presented in Table 1. The median follow-up period was 42.0 months (range, 1 to 297 months). Median follow-up duration was not statistically different (P = .3) between splenectomized (48 months; range, 2 to 297 months) and nonsplenectomized patients (40 months; range, 1 to 236 months) in the total patient population. This lack of difference was maintained when the centers were analyzed separately. Information about medical therapy was available in 516 patients (93.9%); a total of 305 (59.1%) received some kind of chemotherapy: 54.1% received hydroxyurea (mean total dose, 353 g; range, 1.50 to 1,500 g). Other patients received, alone or in association, busulphan, melphalan, 6-mercaptopurine, low-dose cytosine arabinoside, and pipobroman. Six patients underwent splenic irradiation, one of them before splenectomy.
Clinical Characteristics of the Patients With Myelofibrosis With Myeloid Metaplasia in the Study Cohort
| . | All Patients . | Patients Who Underwent Splenectomy . | Patients Who Did Not Undergo Splenectomy . | P . |
|---|---|---|---|---|
| (N = 549) | (N = 87) | (N = 462) | ||
| Patient age at diagnosis (yr), range (median) | 22-92 (63) | 29-72 (55) | 22-92 (64) | <.001 |
| M/F (no.) | 325/224 | 56/31 | 269/193 | NS |
| Hematologic data at diagnosis, range (median) | ||||
| Hemoglobin (g/dL) | 3.4-20.2 (10.6) | 4.6-14.8 (10.5) | 3.4-20.2 (10.6) | NS |
| White blood cell count (×109/L)-150 | 0.5-145 (9.1) | 2.0-73.0 (6.7) | 0.5-145 (9.8) | .01 |
| Platelet count (×109/L) | 14-1,768 (280) | 30-1,500 (214) | 14-1,768 (291) | .02 |
| (N = 371) | (N = 59) | (N = 312) | ||
| Immature myeloid cells in peripheral blood, range (median)-151 | 0-58 (4) | 0-42 (3) | 0-58 (4) | NS |
| Patients with detectable blasts cell in peripheral blood at diagnosis, no. (percentage of total) | 102 (27.4) | 16 (27.1) | 86 (27.6) | NS |
| Blast cells at diagnosis in patients with detectable blasts, range (median)-151 | 1-9 (2) | 1-8 (2) | 1-9 (3) | NS |
| Erythroblasts in peripheral blood, 109/L, range (median) | 0-38 (2) | 0-13 (1) | 0-38 (2) | NS |
| (N = 513) | (N = 84) | (N = 429) | ||
| Spleen size at diagnosis, no. of patients (percentage of total) | ||||
| Grade 0 | 44 (8.6) | 3 (3.5) | 41 (9.5) | NS |
| Grade I | 328 (63.9) | 49 (58.3) | 279 (65.0) | NS |
| Grade II | 121 (23.6) | 22 (26.2) | 99 (23.1) | NS |
| Grade III | 20 (3.9) | 10 (11.9) | 10 (2.3) | .02 |
| (N = 498) | (N = 67) | (N = 431) | ||
| Anemia progression index-152 (g/dL hemoglobin/mo), range (median) | 0.92 to −4.65 (−0.02) | 0.70 to −1.05 (−0.05) | 0.92 to −4.65 (−0.02) | NS |
| Thrombocytopenia progression index-152(109/L platelets/mo), range (median) | 38.8 to −286 (−3.5) | 38.1 to −38 (−2.86) | 38.8 to −286 (−3.73) | .002 |
| Splenomegaly progression index-152 (grade/mo), range (median) | −0.33 to 0.27 (0.01) | −0.25 to 0.25 (0.02) | −0.33 to 0.27 (0.01) | NS |
| . | All Patients . | Patients Who Underwent Splenectomy . | Patients Who Did Not Undergo Splenectomy . | P . |
|---|---|---|---|---|
| (N = 549) | (N = 87) | (N = 462) | ||
| Patient age at diagnosis (yr), range (median) | 22-92 (63) | 29-72 (55) | 22-92 (64) | <.001 |
| M/F (no.) | 325/224 | 56/31 | 269/193 | NS |
| Hematologic data at diagnosis, range (median) | ||||
| Hemoglobin (g/dL) | 3.4-20.2 (10.6) | 4.6-14.8 (10.5) | 3.4-20.2 (10.6) | NS |
| White blood cell count (×109/L)-150 | 0.5-145 (9.1) | 2.0-73.0 (6.7) | 0.5-145 (9.8) | .01 |
| Platelet count (×109/L) | 14-1,768 (280) | 30-1,500 (214) | 14-1,768 (291) | .02 |
| (N = 371) | (N = 59) | (N = 312) | ||
| Immature myeloid cells in peripheral blood, range (median)-151 | 0-58 (4) | 0-42 (3) | 0-58 (4) | NS |
| Patients with detectable blasts cell in peripheral blood at diagnosis, no. (percentage of total) | 102 (27.4) | 16 (27.1) | 86 (27.6) | NS |
| Blast cells at diagnosis in patients with detectable blasts, range (median)-151 | 1-9 (2) | 1-8 (2) | 1-9 (3) | NS |
| Erythroblasts in peripheral blood, 109/L, range (median) | 0-38 (2) | 0-13 (1) | 0-38 (2) | NS |
| (N = 513) | (N = 84) | (N = 429) | ||
| Spleen size at diagnosis, no. of patients (percentage of total) | ||||
| Grade 0 | 44 (8.6) | 3 (3.5) | 41 (9.5) | NS |
| Grade I | 328 (63.9) | 49 (58.3) | 279 (65.0) | NS |
| Grade II | 121 (23.6) | 22 (26.2) | 99 (23.1) | NS |
| Grade III | 20 (3.9) | 10 (11.9) | 10 (2.3) | .02 |
| (N = 498) | (N = 67) | (N = 431) | ||
| Anemia progression index-152 (g/dL hemoglobin/mo), range (median) | 0.92 to −4.65 (−0.02) | 0.70 to −1.05 (−0.05) | 0.92 to −4.65 (−0.02) | NS |
| Thrombocytopenia progression index-152(109/L platelets/mo), range (median) | 38.8 to −286 (−3.5) | 38.1 to −38 (−2.86) | 38.8 to −286 (−3.73) | .002 |
| Splenomegaly progression index-152 (grade/mo), range (median) | −0.33 to 0.27 (0.01) | −0.25 to 0.25 (0.02) | −0.33 to 0.27 (0.01) | NS |
Abbreviation: NS, not significant.
White blood cell counts were corrected for the presence of circulating erythroblasts.
Percentage of white blood cells.
Progression indices represent the rate of change of the parameter during the course of the disease before splenectomy. A positive value indicates an increase and a negative value indicates a decrease in the value of the parameter.
Eighty-seven patients (15.8%) were splenectomized from 1 to 190 months after diagnosis (median, 19 months; first and third quartiles, 5 and 45 months). The main reasons for splenectomy were symptomatic splenomegaly in 52 patients (59.7%) and transfusion-dependent anemia in 32 others (36.8%). Three patients were splenectomized for isolated thrombocytopenia in the absence of anemia and symptomatic splenomegaly. The percentage of splenectomized patients appears to differ across the study centers, ranging from 0% to 35.7% of the enrolled cases. The reasons for performing splenectomy were different from center to center. Transfusion-dependent anemia appeared to be the cause of spleen removal in 0% to 77.8% of the patients from the 7 centers that performed splenectomy in almost 10% of the cases. The cohort that subsequently underwent splenectomy were diagnosed at a younger age, had a lower white blood cell and platelet count, and a higher proportion of patients with the highest degree of splenomegaly (Table 1). More patients who were splenectomized received chemotherapy (62 of 86 [72.1%]) during the course of the disease and before the possible occurrence of blast transformation than patients who did not undergo splenectomy (240 of 430 [55.8%]; P < .005).
During the follow-up period, blast transformation occurred in 78 (43 men and 35 women) of 549 patients (14.2%) at 2 to 171 months after diagnosis (median, 36 months; first and third quartiles, 19 and 63 months). The cumulative proportion of patients estimated to undergo blast transformation 12 years after diagnosis was 36.7% (95% CI, 27.3% to 46.1%). Blast transformation was diagnosed in 47% of patients on the basis of findings at peripheral blood examination only and, in the remaining ones, on the results of bone marrow histologic examination as well. Studies aimed at characterizing blasts cells were available in 60 patients (76.9%). Forty-five blast transformations were classified as myeloid type, 10 as monocyte type, and 5 as megakaryocyte type.
At the time of diagnosis of blast transformation, the median percentage of blasts in peripheral blood was 42%, ranging from 20% to 92%. In 82% of the patients, the appearance of blast transformation caused death in 1 to 30 months (median, 2.7 months). The remaining 18% of the patients were alive at follow-up 0 to 26 months (median, 4.0 months) from the diagnosis of blast transformation.
In an unadjusted comparison, patients who underwent splenectomy developed more blast transformations than those who were not splenectomized (23 of 87 [26.4%] v 55 of 462 [11.9%];P < .001). Higher crude blast transformation incidences in splenectomized patients also resulted when patients were stratified by centers according to the proponent of the study (Pavia v all others), to the total number of cases enrolled (more or less than 50 patients per center), and according to the frequency with which splenectomy was performed (more or less than 10% of patients splenectomized). The Kaplan-Meier estimate of developing blast transformation after diagnosis was significantly higher among patients who had been splenectomized. By 12 years, the estimates were 27.0% (95% CI, 16.3% to 37.3%) among patients who had not been splenectomized and 55.0% (95% CI, 37.8% to 72.2%) among those who had (P = .01). In patients splenectomized within 5 years from diagnosis, ie, homogeneous with respect to risk time-dependence (73 cases; 83.9% of splenectomies), the estimated incidence of blast transformation by 12 years was 72.0% (95% CI, 53.6% to 92.2%;P = .001; Fig 1). In the splenectomized group, the estimated cumulative hazard of developing blast transformation after surgery showed a constant steep increase that began 3 months after the operation and increased to 43.4% (95% CI, 27.6% to 59.2%) at 6 years and 63.0% (95% CI, 43.0% to 83.2%) at 10 years (Fig 1, left upper corner).
Cumulative incidence of blast transformation in 73 patients who had been splenectomized within 5 years from diagnosis and in the 462 nonsplenectomized patients. The vertical lines represent 95% CIs. Actuarial data beyond 144 months were not reported because the number of patients remaining in each group was too small. The incidence of blast transformation from the time of splenectomy is also represented in the upper left corner.
Cumulative incidence of blast transformation in 73 patients who had been splenectomized within 5 years from diagnosis and in the 462 nonsplenectomized patients. The vertical lines represent 95% CIs. Actuarial data beyond 144 months were not reported because the number of patients remaining in each group was too small. The incidence of blast transformation from the time of splenectomy is also represented in the upper left corner.
In univariate analysis, the factors associated with blast transformation included prior splenectomy, the presence of blast cells in peripheral blood at diagnosis, a platelet count less than 100 × 109/L at diagnosis, and a hemoglobin concentration less than 9 g/dL at diagnosis. Prior therapy with hydroxyurea, considering any dose, was not significantly correlated to blast transformation, but considering only patients who had received doses greater than 300 g, was marginally correlated (P = .07). At multivariate analysis (Table 2), prior splenectomy, low platelet count, and the presence of blasts at diagnosis remained independently correlated with blast transformation. At subgroup analysis, patients splenectomized for symptomatic splenomegaly had a higher risk of blast transformation than the ones splenectomized for transfusion-dependent anemia, but the risk ratio was not significant. Also, the combination of splenectomy and hydroxyurea therapy significantly was not correlated with blast transformation. The time-dependent evolution of the relative risks of blast transformation according to prior splenectomy is represented in Fig 2. The relative risk was 2.2 at 4 years, 5.2 at 8 years, and 14.3 at 12 years from diagnosis.
Results of Cox Proportional Hazards Regression, Adjusted for Potential Confounders
| Variable . | Relative Risk* . | 95% CI . | P Value . |
|---|---|---|---|
| All patients (N = 356) | |||
| Splenectomy | 2.61 | 1.38-4.95 | .003 |
| Platelet count (<100 × 109/L) | 2.45 | 1.26-4.75 | .007 |
| Blast cells at diagnosis (>0) | 2.31 | 1.30-4.08 | .004 |
| Hemoglobin (<9 g/dL) | 1.66 | 0.92-2.98 | .09 |
| Hydroxyurea (dose >300 g) | 1.02 | 0.38-2.67 | .96 |
| Patients splenectomized (N = 85) | |||
| For symptomatic splenomegaly v for transfusion-dependent anemia | 1.59 | 0.61-4.11 | .33 |
| Receiving hydroxyurea v not receiving hydroxyurea | 1.23 | 0.52-2.91 | .62 |
| Variable . | Relative Risk* . | 95% CI . | P Value . |
|---|---|---|---|
| All patients (N = 356) | |||
| Splenectomy | 2.61 | 1.38-4.95 | .003 |
| Platelet count (<100 × 109/L) | 2.45 | 1.26-4.75 | .007 |
| Blast cells at diagnosis (>0) | 2.31 | 1.30-4.08 | .004 |
| Hemoglobin (<9 g/dL) | 1.66 | 0.92-2.98 | .09 |
| Hydroxyurea (dose >300 g) | 1.02 | 0.38-2.67 | .96 |
| Patients splenectomized (N = 85) | |||
| For symptomatic splenomegaly v for transfusion-dependent anemia | 1.59 | 0.61-4.11 | .33 |
| Receiving hydroxyurea v not receiving hydroxyurea | 1.23 | 0.52-2.91 | .62 |
*Data from a Cox proportional-hazards regression model giving the risk ratio and 95% CIs for blast transformation are shown. Risk ratios greater than 1.0 imply a higher risk of blast transformation. For all dichotomous variables, the patients with the characteristic were compared with those without it.
Relative risk of blast transformation according to whether splenectomy had been performed. Observed (•) and calculated risks (—) using a time-dependent function in the Cox's model are represented.
Relative risk of blast transformation according to whether splenectomy had been performed. Observed (•) and calculated risks (—) using a time-dependent function in the Cox's model are represented.
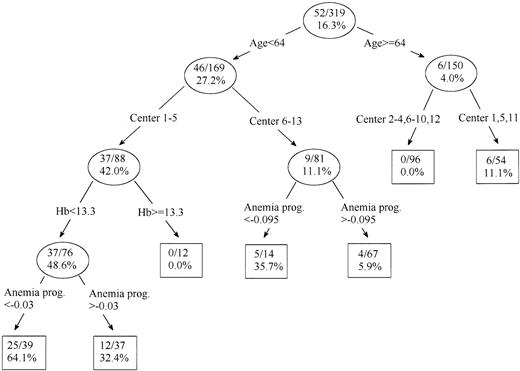
Three hundred nineteen candidates with a complete set of covariates were assigned by the recursive partitioning algorithm to one of seven groups according to propensity to be splenectomized, from null to high (64.1%; Fig 3). The model indicated that the age was the primary determinant of assignment to splenectomy. Among the patients in both groups, the next division was according to the clinical Center, and additional factors that were important were the hemoglobin concentration at diagnosis and the anemia progression index. The seven groups were aggregated in six strata with the same propensity score, and the stratum-specific incidence of blast transformation was plotted by splenectomized and nonsplenectomized groups (Fig 4). Patients who had been splenectomized showed a higher incidence of blast transformation than the nonsplenectomized patients in the same propensity score stratum (P = .008).
Derivation of the seven propensity groups for splenectomy on the basis of data available at the time of diagnosis and the progression indices. The recursive partitioning model shows the variables used to discriminate between subgroups according to the likelihood of the patients' undergoing splenectomy. Hb, hemoglobin at diagnosis in grams per deciliter; Anemia progr., anemia progression index hemoglobin per month. The size of the subgroup relative to the total population and the percentage of patients in that subgroup in whom splenectomy was performed are represented in each circle.
Derivation of the seven propensity groups for splenectomy on the basis of data available at the time of diagnosis and the progression indices. The recursive partitioning model shows the variables used to discriminate between subgroups according to the likelihood of the patients' undergoing splenectomy. Hb, hemoglobin at diagnosis in grams per deciliter; Anemia progr., anemia progression index hemoglobin per month. The size of the subgroup relative to the total population and the percentage of patients in that subgroup in whom splenectomy was performed are represented in each circle.
The incidence of blast transformation plotted by propensity score stratum for splenectomized and nonsplenectomized patients. The six propensity score strata were indexed in order of increasing probability of splenectomy.
The incidence of blast transformation plotted by propensity score stratum for splenectomized and nonsplenectomized patients. The six propensity score strata were indexed in order of increasing probability of splenectomy.
Data on current status (living or dead) was obtained for 409 patients (74.5% of the entire cohort). A total of 210 subjects (38.2%) died during follow-up. The median length of survival after diagnosis was 90 months (95% CI, 78 to 110 months). At Cox's multivariate analysis, mortality corrected for age, hemoglobin concentration, and presence of blasts at diagnosis was associated with both blast transformation (risk ratio = 2.94; 95% CI, 1.99 to 4.34; P < .001) and splenectomy (risk ratio = 1.59; 95% CI, 1.02 to 2.48; P = .03). Patients who developed blast transformation had a median survival of 51.9 months (95% CI, 35.5 to 64.0 months) as compared with 113.5 months (95% CI, 93.2 to 189.5 months) for patients who did not develop blast transformation (P < .001).
DISCUSSION
In this cohort of patients, the incidence of blast transformation progressively increased from diagnosis and the cumulative incidence at 12 years was 36.7%. Disease presentation with detectable blasts in peripheral blood or with thrombocytopenia was predictive of the transformation. Others found that erythroid failure, a high percentage of myeloid precursors, or a high white blood cell count and abnormal karyotype were associated with the conversion.12-14 Extreme heterogeneity of features and outcomes in myelofibrosis with myeloid metaplasia and the low number of patients in individual series may justify these differences in results. However, these risk factors, taken together, lead to the conclusion that high proliferation of the myeloid lineage with defective maturation associated with the failure of other hemopoietic series is the prime characteristic at presentation that predisposes to blast transformation.
The present study provided evidence that splenectomy is an adjunctive strong independent risk factor for blast transformation. The crude blast transformation rate in splenectomized patients was 26.4%, whereas it was 11.9% in nonsplenectomized patients, and the cumulative actuarial transformation rate at 12 years after diagnosis was 55.0% in splenectomized and 27.0% in nonsplenectomized patients. We found that the cumulative incidence of blast transformation after splenectomy began to increase from 3 months after surgery and at the 12 years of follow-up it had not reached a plateau. The overall relative risk of blast transformation was 2.61 times higher among patients who had been submitted to splenectomy, even when all contributing factors were corrected, and being splenectomized carried the highest risk of blast transformation during the late phase of follow-up, where it reached 14.3 times that of nonsplenectomized patients.
We were especially careful to separate the influence of splenectomy assignment in the analysis of risk for blast transformation. In addition to multivariate Cox regression analysis, we used the propensity score approach31 on the grounds that the two methodologies are conceptually different.32 Through the use of recursive partitioning analysis, the relatively young age retained major importance in selecting patients for splenectomy. The clinical center, anemia at diagnosis, and worsening of anemia during disease progression were the additional factors that played an independent role in choosing patients for spleen removal. When these factors were considered in a propensity score and when the occurrence of blast transformation was adjusted for it, splenectomized patients retained on average an increased risk of blast transformation. In conclusion, both methodological approaches used in this study served to exclude that splenectomy and blast transformation shared the same causes and contrasted the hypothesis of a selection bias in previous studies15 16 that showed a high incidence of blast transformation after splenectomy.
Our study is limited by its reliance on data drawn from clinical records, but there is no reason to believe that there was a systematic bias in the identification of MMM patients or in their follow-up. In particular, the slightly but significantly shorter survival of patients who had been submitted to splenectomy reduced the risk of a duration bias, ie, that splenectomy afforded additional survival time for a blast crisis to occur.
An additional important problem concerns the possibility of misdiagnosis of blast transformation. We used the definition of blast conversion reported in studies on chronic myeloproliferative disorders,12 33 but our most important reason for confidence in its accuracy derives from the fact that in our cohort of patients this diagnosis was associated with hematologic and clinical deterioration that substantiated an acute and terminal event of the disease.
The most compelling link between splenectomy and malignant diseases derives from clinical studies. Although not universally demonstrated,34-39 studies have reported that, in patients with Hodgkin's disease, the risk of secondary leukemia or solid tumors is higher in splenectomized patients.17-23 Moreover, splenectomy was found to correlate with increased risk for both acute leukemia and myelodysplastic syndromes in patients treated for aplastic anemia.24 There is no evident reason to justify an association between splenectomy and tumors; nevertheless, certain features may help us to understand the role of the spleen in the leukemia-host relationship in myelofibrosis with myeloid metaplasia. After splenectomy, a progressive increase in circulating mature and immature myeloid cells with massive liver enlargement due to myeloid metaplasia has been reported.15 These features may be associated with a progressive increase in blast cells in peripheral blood (data not evaluated in this study) preceding the evolution toward blast transformation.15 These observations, along with the finding that splenectomies in both healthy population40,41and animal models42 43 did not increase the risk of acute leukemia, indicate a direct effect of splenectomy in accelerating preexisting myeloid proliferation.
The results of the present investigation have little chance of being confirmed by additional prospective randomized studies due to the rarity of the disease and lack of a consensus about the indications for splenectomy. Given the observational nature of the present study, we are aware of the possibility of bias in patient selection and of confounding due to splenectomy indication or duration of postsplenectomy disease. However, in this study, two different methodologies of data analysis yielded the same result, providing sufficient evidence that splenectomy adds to the chances of blast transformation. The knowledge that, using splenectomy, we provide the patient with an additional chance for blast transformation and survival reduction, when considered together with his or her specific clinical profile, may influence the clinician's judgment about whether that person will benefit from splenectomy.
Address reprint requests to Giovanni Barosi, MD, Laboratorio di Informatica Medica, IRCCS Policlinico S. Matteo, 27100 Pavia, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal