Abstract
Previous studies have shown that daily multiple administration of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) markedly stimulates thrombopoiesis and effectively ameliorates thrombocytopenia, and in most cases anemia and neutropenia, in myelosuppressed animals. In this study, we evaluated the effects of a single intravenous injection of PEG-rHuMGDF on hematopoietic recovery after sublethal total-body irradiation in mice. A single injection of PEG-rHuMGDF (1 to 640 μg/kg) 1 hour after irradiation accelerated platelet, red blood cell (RBC), and white blood cell (WBC) recovery in a dose-dependent fashion. In the bone marrow of vehicle-treated mice, megakaryocytic, erythroid, and myeloid progenitors, as well as day 12 colony-forming unit–spleen (CFU-S), were dramatically decreased much earlier than the nadirs of peripheral blood cells, whereas megakaryocytes were modestly decreased. Treatment with PEG-rHuMGDF (80 μg/kg, an optimal dose) 1 hour after irradiation resulted in more rapid recovery of these four hematopoietic progenitors and also significantly facilitated megakaryocyte recovery. In addition, the same PEG-rHuMGDF administration schedule expanded bone marrow cells capable of rescuing lethally irradiated recipient mice. As the interval between irradiation and PEG-rHuMGDF treatment was longer, its effects on hematopoietic recovery were attenuated. In contrast to the effects of PEG-rHuMGDF, a single injection of recombinant human granulocyte colony-stimulating factor (rhG-CSF) 1 hour after irradiation exclusively accelerated WBC recovery, but only to a similar extent as PEG-rHuMGDF (80 μg/kg) treatment even when rhG-CSF doses were escalated to 1,000 μg/kg. This appeared related to different pharmacokinetics of these two factors after a single injection in irradiated mice. The concentrations of PEG-rHuMGDF after injection persisted in the plasma for a longer time compared with rhG-CSF. These results indicate that a single injection of PEG-rHuMGDF at an early time after irradiation is able to effectively improve thrombocytopenia, anemia, and leukopenia with concomitant accelerated recovery of both primitive and committed hematopoietic progenitors in irradiated mice. Our data also show that compared with the rhG-CSF shown to exert multilineage effects on hematopoiesis, PEG-rHuMGDF has more wide-ranging effects on peripheral blood cell recovery.
THROMBOPOIETIN (TPO), also termed the c-Mpl ligand, is the recently isolated hematopoietic factor that primarily regulates megakaryocytopoiesis and platelet production.1-6 Results from in vitro studies have shown that TPO stimulates the growth of committed megakaryocyte progenitors (colony-forming unit–megakaryocyte [CFU-MK]), progressive maturation of megakaryocytes, and proplatelet formation.3,7-14 In addition to acting on megakaryocytopoiesis, TPO enhances the growth of committed erythroid progenitors15,16 and primitive hematopoietic progenitors.17-20 In vivo administration of either glycosylated TPO or pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF), a pegylated, truncated molecule related to TPO, dramatically increases circulating platelets with little or no influence on red blood cells (RBCs) and white blood cells (WBCs) and markedly expands CFU-MK and megakaryocytes in the bone marrow of normal animals.3 21-24
In chemotherapy- and/or irradiation-induced myelosuppression in animals, treatment with TPO or PEG-rHuMGDF has a profound effect on thrombocytopenia, effectively reducing the platelet nadir and accelerating platelet recovery.25-31 Moreover, TPO or PEG-rHuMGDF significantly improves neutropenia and anemia associated with myelosuppression.25-33 In bone marrow transplantation models, PEG-rHuMGDF administration34,35 or transplantation of bone marrow cells from TPO-pretreated donor mice36facilitates platelet recovery.
It has been reported that a single injection of pegylated murine MGDF into normal mice causes dose-dependent and significant increases in megakaryocyte number, size, and ploidy in the bone marrow.24 One study using PEG-rHuMGDF has shown that a single injection of PEG-rHuMGDF is sufficient to increase circulating platelet counts.25 On the other hand, multiple-injection protocols have been used in all of the experiments to investigate the effects of TPO or PEG-rHuMGDF on hematopoietic recovery in myelosuppressed animals. Our previous study has shown that in chemotherapy-induced myelosuppressed mice, treatment with PEG-rHuMGDF once per week (on days 1 and 8) after chemotherapy on day 0 is almost as effective as daily multiple injections of PEG-rHuMGDF from day 1 in improving thrombocytopenia.31 This suggests that a single injection of PEG-rHuMGDF may have a significant effect on thrombocytopenia in myelosuppressed mice.
In this study, we therefore explored whether a single administration of PEG-rHuMGDF is able to improve impaired hematopoiesis in irradiated mice. Our data show that a single injection of PEG-rHuMGDF into irradiated mice at an early time after irradiation greatly accelerates the recovery of circulating platelets and significantly enhances the recovery of RBCs and WBCs, accompanied by accelerated recovery of both primitive and committed hematopoietic progenitors in the bone marrow.
MATERIALS AND METHODS
Animals.
Male BALB/c mice aged 8 weeks were purchased from Charles River Japan Inc (Atsugi, Japan). Mice were housed in autoclaved cages, and were maintained in an air-conditioned, specific pathogen–free animal room regulated at a temperature of 21° to 23°C and a relative humidity of 50% to 60%. The 12-hour lighting cycle began with lights on at 8am. Mice were given sterilized commercial rodent chow and water ad libitum. All experiments in this study were approved by the Institutional Animal Care and Use Committee of our laboratory.
Cytokines.
PEG-rHuMGDF was expressed in Escherichia coli using a plasmid that encodes a truncated mpl ligand related to TPO including the Mpl-binding amino-terminal domain, and then was purified to homogeneity. The molecule was further derivatized with polyethylene glycol. Filgrastim (rhG-CSF) expressed in E coli was prepared at Kirin Brewery Co (Tokyo, Japan).
Study design.
Before treatment with hematopoietic factors or vehicle, mice were exposed to total-body irradiation at a dose of 3.5 Gy with an x-ray apparatus (MBR-1520R; Hitachi, Tokyo, Japan). At various times after irradiation, mice were given a single intravenous injection of various doses of PEG-rHuMGDF, rhG-CSF, or vehicle (acetate-buffered solution) in a volume of 0.1 mL. Each experimental group consisted of five or six mice. Peripheral blood samples were obtained from the retro-orbital plexus using 75-mm heparinized capillary tubes (Funakoshi Pharmaceutical Co, Tokyo, Japan). Complete blood cell counts were performed with the Sysmex automatic microcell counter F-800 (Toa Medical Electronics, Kobe, Japan). Reticulocyte counts were determined as previously described.23
In vitro progenitor cell assays.
Bone marrow cells were harvested from mice by flushing the femoral contents with modified CATCH medium.37 CFU-MK cultures were performed according to the method previously described37 with minor modifications. Briefly, an appropriate number of marrow cells were cultured in the presence of 30 ng recombinant mouse interleukin-3 ([rmIL-3] Kirin Brewery Co)23 and 20 ng PEG-rHuMGDF in 1 mL Iscove's modified Dulbecco's medium (Sigma, St Louis, MO) containing 0.3% Noble agar (Difco, Detroit, MI), 10% fetal calf serum ([FCS] GIBCO, Grand Island, NY), 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, and 50 μmol/L 2-mercaptoethanol (Merck, Darmstadt, Germany) in a 35-mm tissue culture dish (Nunc, Naperville, IL) at 37°C in a humidified atmosphere of 5% CO2. After 7 days of culture, agar disks were detached from the culture dishes, placed onto glass slides, and stained with acetylcholinesterase (AchE) according to the method of Jackson.38 CFU-MK–derived colonies were defined as colonies with at least three megakaryocytes. Cultures of CFU–granulocyte-macrophage (CFU-GM) and burst-forming unit–erythroid (BFU-E) were performed with a methylcellulose method. Briefly, an appropriate number of marrow cells were cultured with 16 IU recombinant human erythropoietin (Kirin Brewery Co)23 and 5 ng rmIL-3 in 1 mL α-medium (Flow Laboratories, McLean, VA) containing 0.88% methylcellulose (Shinetsu Kagaku Kogyo, Tokyo, Japan), 30% FCS, 1% bovine serum albumin (Sigma), and 50 μmol/L 2-mercaptoethanol in a 35-mm tissue culture dish. After 7 days of culture, GM colonies (≥50 cells) or erythroid colonies (≥200 cells) were counted as CFU-GM– or BFU-E–derived colonies, respectively, using an inverted light microscope. The total number of each hematopoietic progenitor cell per femur was calculated as follows: the number of colonies generated per dish was multiplied by the total number of cells obtained from one femur divided by the number of cells seeded per dish.
Day 12 CFU–spleen assay.
Day 12 CFU-spleen (CFU-S) was assayed according to the method of Till and McCulloch.39 Briefly, bone marrow cells derived from mice treated postirradiation with PEG-rHuMGDF or vehicle were washed and suspended in α-medium, and aliquots of the cells were injected into recipients that had been irradiated at a lethal dose of 8.5 Gy. Twelve days after inoculation, the recipients were killed, and their spleens were removed and fixed with Bouin's solution. Colonies on the surface of the spleen were then counted as day 12 CFU-S. The total number of day 12 CFU-S per femur was calculated as follows: the number of day 12 CFU-S generated per spleen was multiplied by the total number of cells obtained from one femur divided by the number of cells injected per mouse.
Measurement of the number of marrow megakaryocytes.
Bone marrow cells were plated at 105 cells per well in 96-well immunoplates (Nunc) and fixed in 100 μL 0.1-mol/L phosphate buffer (PB), pH 6.0, containing 2.5% glutaraldehyde (Wako Pure Chemicals, Osaka, Japan) for 10 minutes at room temperature. After fixation, the plates were centrifuged at 120g for 5 minutes and the supernatant of each well was removed by aspiration, taking care not to disturb the cell pellet. A volume of 100 μL 0.1-mol/L PB was added to each well, and the plates were centrifuged as before. This washing procedure was repeated twice. Subsequently, the cell pellets were stained with AchE. The number of AchE-positive cells was counted as for megakaryocytes using an inverted light microscope. The total number of megakaryocytes per femur was calculated as follows: the number of AchE-positive megakaryocytes per well was multiplied by the total number of cells obtained from one-femur divided by the number of cells plated per well.
Bone marrow transplantation experiments.
Mice were intravenously given one dose of PEG-rHuMGDF (80 μg/kg) or vehicle 1 hour after total-body irradiation at a dose of 3.5 Gy. Two days later, the femoral bone marrow cells were collected, washed, and resuspended in α-medium. Aliquots of cells (106 cells per mouse) were inoculated intravenously into the lateral tail vein of syngeneic recipient mice that had been irradiated at a lethal dose of 8.5 Gy (six mice per experimental group), and then the survival of the transplanted mice was monitored for 90 days.
Enzyme-linked immunosorbent assays.
Plasma levels of PEG-rHuMGDF and rhG-CSF after a single injection in irradiated mice were measured by sandwich enzyme-linked immunosorbent assays (ELISAs) previously established for endogenous human TPO40 and endogenous human G-CSF,41respectively. One hour after irradiation, mice received a single intravenous injection of PEG-rHuMGDF (80 μg/kg) or rhG-CSF (1,000 μg/kg), and blood was then collected at different times within 72 hours after injection to prepare plasma samples. Cytokine standards (PEG-rHuMGDF and rhG-CSF) and test samples were assayed by the above-mentioned sandwich ELISAs, and all samples were analyzed in duplicate. Cytokine concentrations in test plasma were calculated by regression analysis from standard curves obtained with serially diluted cytokine standards. The limit of detection for each cytokine in mouse serum was 100 to 200 pg/mL.
Statistical analysis.
All data represent the mean ± SEM. The statistical significance of differences in the number of platelets, RBCs, WBCs, and hematopoietic progenitor cells between vehicle- and PEG-rHuMGDF–treated groups was assessed by Dunnett's multiple comparison test. The statistical significance of differences in megakaryocyte number between vehicle- and PEG-rHuMGDF–treated groups was assessed by Student'st-test.
RESULTS
A single injection of PEG-rHuMGDF significantly improves pancytopenia in irradiated mice.
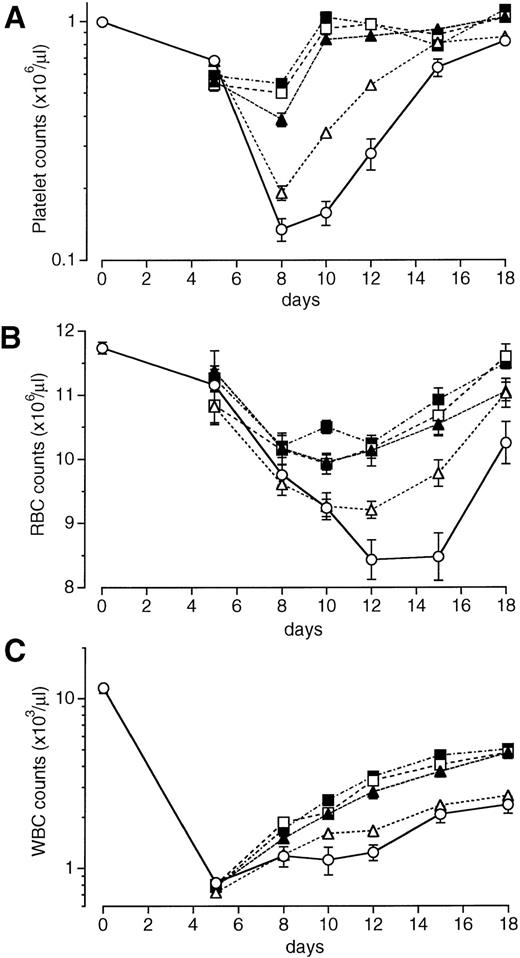
We examined the effects of a single intravenous injection of PEG-rHuMGDF at doses of 1 to 640 μg/kg on the recovery of peripheral blood cell counts in mice exposed to sublethal total-body irradiation. The data shown in Fig 1 were obtained with PEG-rHuMGDF administration 1 hour after irradiation. In vehicle-treated controls, the platelet nadir occurred on day 8 at approximately 11% of normal levels, and platelet numbers then began to increase but did not return to normal levels by day 18 (Fig 1A). A single injection of PEG-rHuMGDF had a dose-dependent, marked effect on thrombocytopenia in irradiated mice. The platelet nadir on day 8 was significantly reduced by treatment with at least 8 μg/kg PEG-rHuMGDF compared with the vehicle (P < .01; Fig 1A). Treatment with PEG-rHuMGDF even at the lowest dose (1 μg/kg) resulted in faster recovery of platelet counts after day 8 compared with the time-matched vehicle treatment (P < .01). When higher doses of PEG-rHuMGDF (80 or 640 μg/kg) were administered, platelet counts returned to basal levels as early as day 10 (Fig 1A). Based on these results, we chose 80 μg/kg as a single optimal dose.
Effects of a single injection of PEG-rHuMGDF on peripheral blood cell recovery in irradiated myelosuppressed mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 1 (▵), 8 (▴), 80 (□), or 640 (▪) μg/kg PEG-rHuMGDF, or vehicle (○). The data represent 1 of 2 separate experiments.
Effects of a single injection of PEG-rHuMGDF on peripheral blood cell recovery in irradiated myelosuppressed mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 1 (▵), 8 (▴), 80 (□), or 640 (▪) μg/kg PEG-rHuMGDF, or vehicle (○). The data represent 1 of 2 separate experiments.
Irradiation treatment produced moderate anemia with a nadir on days 12 through 15 (Fig 1B). A single injection of PEG-rHuMGDF caused a dose-dependent recovery of RBCs in irradiated mice. Treatment with even the lowest dose of PEG-rHuMGDF (1 μg/kg) resulted in a significant reduction in the RBC nadir (P < .05; Fig 1B). A single injection of PEG-rHuMGDF also accelerated reticulocyte recovery in a dose-dependent fashion, indicating enhanced RBC production by PEG-rHuMGDF treatment (data not shown).
Irradiated mice exhibited severe leukopenia, as well (Fig 1C). Although PEG-rHuMGDF treatment had no effect on the WBC nadir, a single injection of at least 80 μg/kg PEG-rHuMGDF modestly but significantly accelerated WBC recovery compared with the time-matched vehicle treatment (P < .01; Fig 1C).
To determine the effective administration schedules for PEG-rHuMGDF, mice were given a single injection of an optimal dose of PEG-rHuMGDF (80 μg/kg) at different times (within 60 hours) after irradiation (Fig 2). The earlier PEG-rHuMGDF was administered, the better the improvement of thrombocytopenia was achieved (Fig 2A). Thus, PEG-rHuMGDF treatment 1 hour after irradiation was most effective in improving thrombocytopenia. PEG-rHuMGDF treatment at an earlier time after irradiation was required for a significant reduction in the platelet nadir on day 8, compared with a significant platelet recovery after day 8. Platelet nadir counts were significantly reduced with PEG-rHuMGDF treatment within the first 24 hours after irradiation (P < .01; Fig 2A). On the other hand, mice receiving a single dose of PEG-rHuMGDF 48 hours after irradiation had significantly higher platelet counts on day 12 (P < .05) and day 15 (P < .01) compared with vehicle-treated, time-matched control mice, although PEG-rHuMGDF treatment 60 hours after irradiation no longer had a significant effect on platelet counts (Fig 2A). Similar to the effects on platelet counts, PEG-rHuMGDF treatment 1 hour after irradiation was most effective for recovery of both RBCs and WBCs (Fig 2B and C).
Effects of the interval between irradiation and a single injection of PEG-rHuMGDF on peripheral blood cell recovery in irradiated myelosuppressed mice. Mice were irradiated at a dose of 3.5 Gy and intravenously given 80 μg/kg PEG-rHuMGDF 1 (•), 12 (▵), 24 (▴), 36 (□), 48 (▪), or 60 (▿) hours after irradiation, or vehicle (○) 1 hour after irradiation. The data represent 1 of 2 separate experiments.
Effects of the interval between irradiation and a single injection of PEG-rHuMGDF on peripheral blood cell recovery in irradiated myelosuppressed mice. Mice were irradiated at a dose of 3.5 Gy and intravenously given 80 μg/kg PEG-rHuMGDF 1 (•), 12 (▵), 24 (▴), 36 (□), 48 (▪), or 60 (▿) hours after irradiation, or vehicle (○) 1 hour after irradiation. The data represent 1 of 2 separate experiments.
PEG-rHuMGDF treatment accelerates the recovery of hematopoietic progenitors and megakaryocytes in the bone marrow.
We next examined the effects of a single injection of PEG-rHuMGDF on the recovery of megakaryocytes and various hematopoietic progenitor cells in the bone marrow of irradiated mice. Total-body irradiation had a mild effect on megakaryocytes in the femur, as compared with its influence on hematopoietic progenitor cells (Fig 3). In vehicle-treated controls, megakaryocytes gradually decreased after irradiation and reached approximately 60% of normal levels by day 5. A single injection of PEG-rHuMGDF 1 hour after irradiation significantly increased the number of megakaryocytes on day 5 (P < .01; Fig 3).
Effects of a single injection of PEG-rHuMGDF on megakaryocyte recovery after irradiation in mice. Mice were irradiated at a dose of 3.5 Gy and intravenously given 80 μg/kg PEG-rHuMGDF (•) or vehicle (○) 1 hour after irradiation. The number of megakaryocytes in the femur of the mice before total-body irradiation and at indicated times after irradiation was examined.
Effects of a single injection of PEG-rHuMGDF on megakaryocyte recovery after irradiation in mice. Mice were irradiated at a dose of 3.5 Gy and intravenously given 80 μg/kg PEG-rHuMGDF (•) or vehicle (○) 1 hour after irradiation. The number of megakaryocytes in the femur of the mice before total-body irradiation and at indicated times after irradiation was examined.
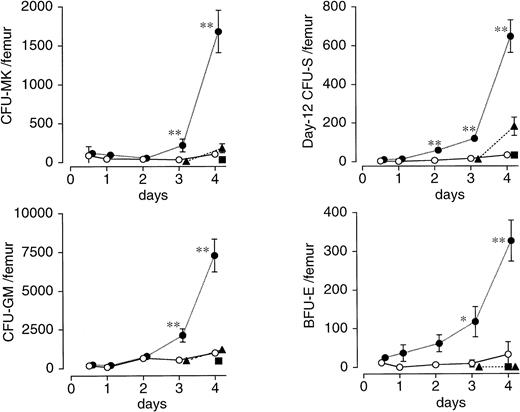
In the femur of vehicle-treated mice, drastic decreases in the number of CFU-MK, BFU-E, CFU-GM, and day 12 CFU-S were noted by 12 hours, much earlier than the nadirs for peripheral blood cells, and continued on day 3 (Fig 4). A single injection of PEG-rHuMGDF 80 μg/kg 1 hour after irradiation resulted in significantly accelerated recovery of CFU-MK, BFU-E, and CFU-GM by day 3, and of day 12 CFU-S by day 2 (Fig4). However, an injection of PEG-rHuMGDF 24 and 48 hours after irradiation had little or no effect on any of four hematopoietic progenitors tested (Fig 4).
Effects of a single injection of PEG-rHuMGDF on the recovery of various hematopoietic progenitor cells after irradiation in mice. Mice were intravenously given 80 μg/kg PEG-rHuMGDF 1 (•), 24 (▴), or 48 (▪) hours after irradiation, or vehicle (○) 1 hour after irradiation. The number of CFU-MK, BFU-E, CFU-GM, and day 12 CFU-S in femoral bone marrow before irradiation and at indicated times after irradiation was examined using the respective adequate progenitor assays. The number of CFU-MK, BFU-E, CFU-GM, and day 12 CFU-S per femur in untreated normal mice was 2,340 ± 314, 1,030 ± 474, 9,900 ± 1,772, and 2,436 ± 407, respectively. *P < .05, **P< .01: significantly greater than vehicle-treated, time-matched controls.
Effects of a single injection of PEG-rHuMGDF on the recovery of various hematopoietic progenitor cells after irradiation in mice. Mice were intravenously given 80 μg/kg PEG-rHuMGDF 1 (•), 24 (▴), or 48 (▪) hours after irradiation, or vehicle (○) 1 hour after irradiation. The number of CFU-MK, BFU-E, CFU-GM, and day 12 CFU-S in femoral bone marrow before irradiation and at indicated times after irradiation was examined using the respective adequate progenitor assays. The number of CFU-MK, BFU-E, CFU-GM, and day 12 CFU-S per femur in untreated normal mice was 2,340 ± 314, 1,030 ± 474, 9,900 ± 1,772, and 2,436 ± 407, respectively. *P < .05, **P< .01: significantly greater than vehicle-treated, time-matched controls.
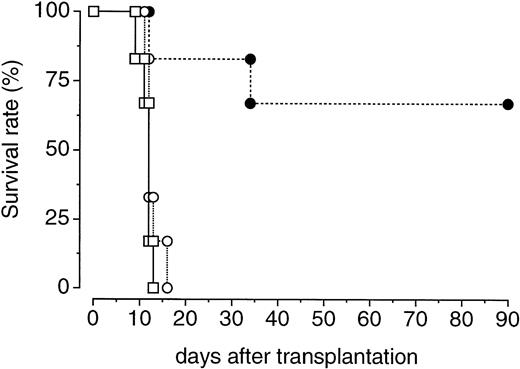
We further examined whether PEG-rHuMGDF treatment enhances the recovery of bone marrow hematopoietic progenitor cells with in vivo repopulating ability. One hour after irradiation, donor mice were treated with a single injection of PEG-rHuMGDF (80 μg/kg) or vehicle. Two days later, their femoral bone marrow cells were prepared and inoculated into lethally irradiated recipient mice. Transplantation of bone marrow cells derived from PEG-rHuMGDF–treated donor mice resulted in a 70% survival of recipient mice at 90 days. In contrast, all recipient mice that received bone marrow cells derived from vehicle-treated donor mice or no transplantation died by 14 or 17 days after transplantation, respectively (Fig 5).
Effects of a single injection of PEG-rHuMGDF on the expansion of bone marrow cells capable of surviving in lethally irradiated mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 80 μg/kg PEG-rHuMGDF (•) or vehicle (○). Two days later, the mice were killed and their bone marrow cells (106 cells per mouse) were intravenously injected into syngeneic recipient mice that had been lethally irradiated at a dose of 8.5 Gy. One group of lethally irradiated mice received no transplantation of bone marrow cells (□).
Effects of a single injection of PEG-rHuMGDF on the expansion of bone marrow cells capable of surviving in lethally irradiated mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 80 μg/kg PEG-rHuMGDF (•) or vehicle (○). Two days later, the mice were killed and their bone marrow cells (106 cells per mouse) were intravenously injected into syngeneic recipient mice that had been lethally irradiated at a dose of 8.5 Gy. One group of lethally irradiated mice received no transplantation of bone marrow cells (□).
Comparison of the effects of a single injection of PEG-rHuMGDF and rhG-CSF in irradiated mice.
The above-mentioned results indicate multilineage effects of PEG-rHuMGDF. It has already been shown that in addition to the effects on committed granulocyte precursors, G-CSF acts on primitive hematopoietic progenitors,42 and that G-CSF treatment accelerates the recovery of not only WBCs (or neutrophils) but also RBCs and platelets in myelosuppressed mice,33 43 indicating multilineage effects of G-CSF. Therefore, we compared the effects of a single injection of PEG-rHuMGDF 80 μg/kg and different doses of rhG-CSF 1 hour after irradiation on the recovery of peripheral blood cells in irradiated mice. A single injection of rhG-CSF at any dose up to 1,000 μg/kg could not reduce the WBC nadir, but higher doses of rhG-CSF significantly accelerated the WBC recovery as compared with the time-matched, vehicle treatment (P < .01 at 250 μg/kg on days 8 and 10 and P < .01 at 1,000 μg/kg on days 8 through 18; Fig 6C). However, even if rhG-CSF 1,000 μg/kg was used, its effects on WBC recovery were modest, being only comparable to the effects of PEG-rHuMGDF (Fig 6C). Moreover, a single injection of rhG-CSF had only minimal effects on the recovery of both platelets and RBCs in irradiated mice (Fig 6A and B).
Comparison of the effects of a single injection of rhG-CSF and PEG-rHuMGDF on the recovery of peripheral blood cells in irradiated mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 80 μg/kg PEG-rHuMGDF (•) or 10 (▵), 50 (▴), 250 (□), or 1,000 (▪) μg/kg rhG-CSF, or vehicle (○).
Comparison of the effects of a single injection of rhG-CSF and PEG-rHuMGDF on the recovery of peripheral blood cells in irradiated mice. One hour after irradiation at a dose of 3.5 Gy, mice were intravenously given 80 μg/kg PEG-rHuMGDF (•) or 10 (▵), 50 (▴), 250 (□), or 1,000 (▪) μg/kg rhG-CSF, or vehicle (○).
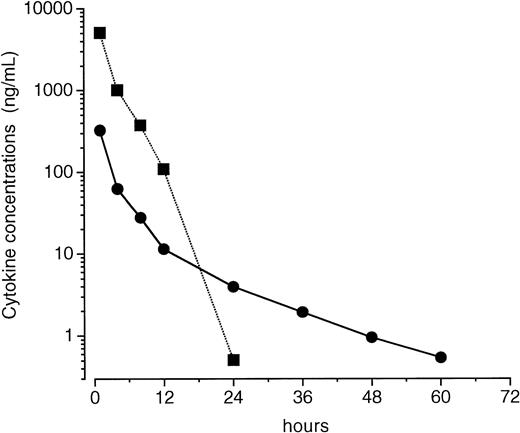
One possibility was thought to be that the different effects of PEG-rHuMGDF and rhG-CSF on pancytopenia are due to different pharmacokinetics of these two cytokines in irradiated mice. Therefore, we examined the time course of PEG-rHuMGDF and rhG-CSF concentrations in the plasma of mice that received a single intravenous injection of PEG-rHuMGDF 80 μg/kg or rhG-CSF 1,000 μg/kg 1 hour after irradiation. PEG-rHuMGDF concentrations decreased more slowly compared with rhG-CSF, and still remained at detectable levels (∼540 pg/mL) 60 hours after injection (Fig 7). However, although a much higher dose of rhG-CSF was administered to irradiated mice, rhG-CSF concentrations decreased to approximately 510 pg/mL 24 hours after injection, and afterward to undetectable levels (Fig 7). These data indicate a more prolonged presence of injected PEG-rHuMGDF in the peripheral blood of irradiated mice.
Plasma concentration profiles of PEG-rHuMGDF and rhG-CSF after a single injection in irradiated mice. One hour after irradiation, mice received a single intravenous injection of 80 μg/kg PEG-rHuMGDF (•) or 1,000 μg/kg rhG-CSF (▪). Plasma samples were prepared from blood drawn from mice at the indicated times after injection, and were assayed by sandwich ELISA for each cytokine. Each point represents the mean value from 4 mice.
Plasma concentration profiles of PEG-rHuMGDF and rhG-CSF after a single injection in irradiated mice. One hour after irradiation, mice received a single intravenous injection of 80 μg/kg PEG-rHuMGDF (•) or 1,000 μg/kg rhG-CSF (▪). Plasma samples were prepared from blood drawn from mice at the indicated times after injection, and were assayed by sandwich ELISA for each cytokine. Each point represents the mean value from 4 mice.
DISCUSSION
Previous studies have demonstrated that daily multiple injections of human or murine TPO or PEG-rHuMGDF starting the day after myelosuppressive treatment cause a dose-dependent improvement of thrombocytopenia, anemia, and leukopenia (or neutropenia) in myelosuppressed animals.25-31 Our previous study has shown that a daily multiple-injection schedule of PEG-rHuMGDF is not necessarily required for accelerated platelet recovery after myelosuppressive chemotherapy in mice.31 In this study, we therefore examined whether a single injection of PEG-rHuMGDF enhances hematopoietic recovery after irradiation in mice. Treatment with a single dose of PEG-rHuMGDF 1 hour after irradiation reduced the severity of thrombocytopenia and accelerated platelet recovery in a dose-dependent fashion, thereby shortening the duration of thrombocytopenia, in irradiated mice. Such effects of a single injection of PEG-rHuMGDF seem comparable to those of daily multiple injections of PEG-rHuMGDF or TPO as previously reported.25-31
A single dose of PEG-rHuMGDF at an early time after irradiation significantly accelerated RBC and WBC recovery, as well. The effects of a single injection of PEG-rHuMGDF on peripheral blood cells other than platelets are similar to results from previous studies using multiple injections of murine or human TPO or PEG-rHuMGDF in myelosuppressed animals.26-33 As already reported for multiple administrations of murine TPO30,32,33 or PEG-rHuMGDF,27,31 a single injection of PEG-rHuMGDF significantly enhanced erythroid and myeloid progenitor recovery. Previous in vitro studies have shown that although TPO alone has no influence on terminal differentiation in myelopoiesis and erythropoiesis, it can synergize with other cytokines to enhance the development of early progenitor cells other than CFU-MK. For example, TPO enhances the proliferation of erythroid progenitors in the presence of erythropoietin15,16,32 and proliferation of primitive multipotent progenitors in synergy with early-acting cytokines such as IL-3 or stem cell factor.17-20 Considering these in vitro results, administered PEG-rHuMGDF may interact with other endogenous cytokines to stimulate expansion of erythroid and myeloid progenitors, resulting in the accelerated recovery of RBCs and WBCs we observed.
The interval between PEG-rHuMGDF and irradiation is critical to the effects of PEG-rHuMGDF on pancytopenia in irradiated mice. By delaying the start of PEG-rHuMGDF treatment after irradiation, its therapeutic effects were lessened. A single injection 60 hours after irradiation had no effects on either the platelet nadir or platelet recovery. Although the explanation for the necessity of earlier administration of PEG-rHuMGDF is unclear at present, it is possible that at an earlier time after irradiation, more residual hematopoietic progenitors in the bone marrow respond to PEG-rHuMGDF, leading to accelerated peripheral blood cell recovery. Our results on the timing of a single injection of PEG-rHuMGDF are consistent with a previous report showing that an injection of adenovirus vector encoding TPO on the same day of chemotherapy and irradiation effectively accelerates platelet recovery in mice, but has little effect on thrombocytopenia when the vector administration is delayed by 3 days.44 Similarly, it has been reported that, like PEG-rHuMGDF treatment in this study, administration of G-CSF,43,45 IL-6,46 or IL-1147 to myelosuppressed mice immediately after myelosuppressive treatment is most effective for improving impaired hematopoiesis.
Hematologic analyses of mice engineered to lack either the TPO receptor (c-Mpl)48,49 or TPO49 have revealed substantial effects of TPO on the development of both primitive and various committed hematopoietic progenitors. Indeed, results from in vitro studies have shown that TPO stimulates proliferation of not only CFU-MK3,7-13 but also primitive hematopoietic progenitors17-20 and committed erythroid progenitors.15,16 Similar to the in vitro effects, in vivo administration of TPO or PEG-rHuMGDF expands the number of multipotent progenitors and various committed progenitors, including CFU-MK, in the bone marrow of normal mice23 and monkeys.21 The multilineage effects of TPO or PEG-rHuMGDF have been shown in myelosuppressed animals. Multiple injections of TPO or PEG-rHuMGDF enhance the recovery of both multipotent27,31,33 and committed27,30-33 progenitors. Our data show that a single injection of PEG-rHuMGDF markedly enhances the recovery of CFU-MK, BFU-E, CFU-GM, day 12 CFU-S, and primitive hematopoietic progenitors with in vivo repopulating ability in the femur of irradiated mice. Thus, a single injection of PEG-rHuMGDF is sufficient to accelerate multilineage progenitor recovery. Recent studies have shown that TPO prevents apoptosis of murine primitive hematopoietic progenitors50 and a human megakaryocytic cell line.51 Therefore, it is possible that a single administration of PEG-rHuMGDF suppresses apoptosis of both primitive multipotent and lineage-restricted progenitors in the bone marrow of irradiated mice, leading to an expansion of a wide range of hematopoietic progenitors. It is also possible that PEG-rHuMGDF exogenously administered acts in concert with elevated levels of other cytokines endogenously produced in response to myelosuppression to enhance hematopoietic progenitor recovery. It remains unknown whether the enhanced recovery of multipotent primitive progenitors contributes to the enhanced recovery of peripheral blood cells.
In this study, the number of megakaryocytes in the femur was gradually and modestly decreased after irradiation, clearly different from the drastic decreases in hematopoietic progenitors examined. These data are in agreement with a previous report showing that megakaryocytes are relatively radioresistant.52 A single injection of PEG-rHuMGDF into irradiated mice resulted in a significant enhancement of megakaryocyte numbers on day 5 after irradiation as compared with vehicle treatment. However, the size of megakaryocytes in PEG-rHuMGDF–treated mice on day 5 was smaller than in vehicle-treated controls (data not shown), suggesting generation of immature megakaryocytes from CFU-MK by PEG-rHuMGDF administration.
It has been shown that G-CSF stimulates primitive hematopoietic progenitors other than committed granulocyte progenitors in vitro.42 Treatment with G-CSF has a significant effect on the recovery of WBCs (or neutrophils), RBCs, and platelets after myelosuppressive treatment in mice.33,43,46 In this study, we therefore compared the therapeutic effects of a single injection of PEG-rHuMGDF and rhG-CSF 1 hour after irradiation in mice. Our data show that, unlike PEG-rHuMGDF treatment, a single injection of rhG-CSF to irradiated mice exerts a dominant effect on WBC recovery but has minimal effects on platelet and RBC recovery. It is conceivable that these different effects of PEG-rHuMGDF and rhG-CSF on peripheral blood cell recovery are due to different intrinsic properties. Further, our data suggest that compared with rhG-CSF, more prolonged circulating plasma levels of PEG-rHuMGDF after a single injection may be related to more widespread, pronounced effects of PEG-rHuMGDF on peripheral blood cell recovery. Previous studies have shown that PEG-rHuMGDF has much more potent platelet-promoting activity in normal mice, as compared with nonpegylated rHuMGDF.23 26 These findings together with our data suggest that pegylation results in a longer half-life of rHuMGDF in the circulation, thereby increasing its in vivo biologic activity.
In conclusion, our study has shown that a single injection of PEG-rHuMGDF in irradiated mice at an early time after irradiation markedly accelerates the recovery of both primitive and committed hematopoietic progenitors and effectively improves pancytopenia, including thrombocytopenia. Our data suggest that a single injection of PEG-rHuMGDF may be a clinically effective administration schedule.
ACKNOWLEDGMENT
We thank Miyuki Kato, Yuko Nitta, and Masumi Ida for excellent technical assistance.
Address reprint requests to Hiroshi Miyazaki, PhD, Pharmaceutical Research Laboratory, Kirin Brewery Co, Ltd, 3 Miyahara-cho, Takasaki, Gunma 370-12, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal