Abstract
Thrombopoietin (TPO) has been established as the major regulator of megakaryocyte and platelet production. In vitro and in vivo studies have demonstrated that TPO affects both megakaryocyte proliferation and maturation. In vitro, TPO has been reported to be essential for full development of megakaryocytes and platelets. These studies are in contrast to results observed in vivo in mice deficient in the TPO or c-mpl gene (TPO-/- and c-mpl-/-). Both TPO-/- and c-mpl-/- mice exhibit a 90% reduction in megakaryocyte and platelet levels. But even with this small number of circulating platelets, these mice do not have any excessive bleeding. Ultrastructural analysis indicates that platelets and megakaryocytes present in the knockout mice are morphologically normal. Characterization of platelet function shows that platelets from knockout mice are functionally identical to the wild-type platelets as measured by upregulation of 125I-fibrinogen binding to platelets in response to adenosine diphosphate (ADP) stimulation and by platelet attachment to the immobilized extracellular matrix proteins, collagen and von Willebrand factor (vWF). These results demonstrate that in vivo, TPO is required for the control of megakaryocyte and platelet number but not for their maturation. Other factors with megakaryocytopoietic activity may be able to compensate for the maturational role of TPO and lead to the formation of normal megakaryocytes and platelets in TPO-/- and c-mpl-/- mice.
NORMAL HEMOSTASIS requires the maintenance of an adequate number of circulating platelets in the peripheral blood to prevent bleeding. Many cytokines, including interleukin-1 (IL-1), IL-3, IL-6, IL-11, granulocyte-macrophage colony-stimulating factor, and erythropoietin, are capable of stimulating megakaryocytopoiesis both in vitro and in vivo.1,2 However, the ligand for the c-mpl proto-oncogene, thrombopoietin (TPO), has been shown to be the primary regulator of platelet production.3-6 In vitro and in vivo experiments with recombinant TPO (rTPO) indicate that it stimulates both megakaryocyte colony formation and megakaryocyte maturation.3,4,7,8 TPO supports the formation of colony-forming units–megakaryocyte both alone and in combination with early-acting factors6,9 and stimulates the production of megakaryocytes and functional platelets from enriched murine or human stem cell populations.10,11 Injection of rTPO in mice increases platelet counts fourfold to sixfold and causes up to a 20-fold increase in bone marrow megakaryocytes.5,6 Although rTPO dramatically stimulates platelet production, it has only a modest effect on platelet function. In vitro studies show that rTPO does not directly induce platelet aggregation, but does enhance agonist aggregation induced by another agonist.12 13 TPO appears to sensitize platelets, making them moderately more responsive to aggregation agonists. This raises the possibility that TPO may have prothrombotic effects in vivo. However, an increase in thrombotic episodes in animals treated with TPO has never been observed, even when platelet levels were 4- to 10-fold above normal.
The availability of TPO has for the first time made it possible to grow megakaryocytes in culture to study the mechanisms of regulation of thrombopoiesis.7,10,11,14,15 Megakaryocytes and platelets generated in vitro are functionally and morphologically identical to bone marrow megakaryocytes and blood-derived platelets. In vitro, it appears impossible to generate recognizable megakaryocytes in the absence of TPO,14 but we show in this study that TPO is not required for the formation of normal megakaryocytes and platelets in vivo. The importance of TPO in megakaryocytopoiesis and thrombopoiesis has been confirmed by generation of mice deficient in the TPO gene16 or its receptor c-mpl.17 18 These mice exhibit an 85% reduction in peripheral platelet and marrow and spleen megakaryocytes. But even with these reduced platelet counts, the mice do not bleed spontaneously. We show here that both megakaryocytes and platelets from the knockout mice appear normal by ultrastructural analysis. Their platelets are functionally normal as measured by the upregulation of fibrinogen binding sites in response to agonist stimulation and the ability to attach to extracellular matrix proteins. These results demonstrate that in vivo, TPO is not required for the formation of normal megakaryocytes and platelets but is necessary for the production of an adequate amount.
MATERIALS AND METHODS
Animals.Male 129/C57BL/6 mice aged 8 to 12 weeks crossbred to be deficient in either the c-mpl receptor17 (c-mpl-/-), TPO16 (TPO-/-), or both and their homozygous normal littermates were maintained in specific pathogen-free conditions.
Bleeding time.Bleeding time was determined as described by Dejana et al.19 Briefly, the most distal 2 mm of the tail was transected using a sharp razor blade, and the tail was immersed in 0.9% saline at 37°C. The time to cessation of erythrocyte streaming was recorded as the bleeding time.
Preparation of washed murine platelets.Whole blood was collected into 3.8% sodium citrate (9:1 vol/vol) in microfuge tubes and centrifuged for 3 seconds at 14,000 rpm to obtain platelet-rich plasma (PRP). The PRP was collected, and the residual whole blood was recombined and recentrifuged to collect a second PRP harvest added to the initial collection. PRP volumes were generally 1 to 1.5 mL from five mice. PGI2 was added to a final concentration of 300 ng/mL, and after 10 minutes, apyrase was added at 5 U/mL final concentration. After another 10 minutes, the PRP was centrifuged at 1,600g for 5 minutes. The platelets were resuspended in 5 mL Tyrode's buffer (0.14 mol/L NaCl, 2.7 mmol/L KCl, 12 mmol/L NaHCO3 , and 0.4 mmol/L Na2HPO4 , pH 7.4, with 5.5 mmol/L glucose, 10 mmol/L HEPES, and 2% bovine serum albumin [BSA], ie, Tyrode's-BSA) containing 300 ng/mL PGI2 and 1 U/mL apyrase. After centrifugation, the platelets were resuspended in 5 mL Tyrode's-BSA with 0.2 U/mL apyrase, centrifuged, and finally resuspended in Tyrode's-BSA. Platelets were counted on a Baker System 9000 Diff Model cell counter (Serono-Baker, Allentown, PA). To allow recovery from the PGI2 treatment, the washed platelets were allowed to stand in the dark for a minimum of 2 hours at room temperature (RT) before use.
125I-fibrinogen binding to washed platelets.Platelets (2.5 × 107/mL) were incubated in the presence of 125I-fibrinogen (20 nmol/L), CaCl2 (2 mmol/L, and TPO (2 μg/mL) for 10 minutes at RT. Various concentrations of adenosine diphosphate (ADP) were then added, and the binding proceeded for 60 minutes at RT. The binding solution was then layered on a 500-μL step of Tyrode's-BSA-20% sucrose in a microfuge tube and centrifuged at 14,000 rpm for 4 minutes. The liquid was aspirated, and the platelet pellet was then counted.
Murine PRP.Whole blood was collected into heparin (1.0 U/mL) in microfuge tubes and centrifuged at 14,000 rpm for 3 seconds to prepare PRP. Platelet-poor plasma was prepared from the residual cell/PRP left by centrifugation at 14,000 rpm for 10 minutes. PRP platelets were used at a final concentration of 6.5 × 107/mL in the same conditions already described.
Platelet adhesion assay.Two extracellular matrix proteins, collagen III and von Willebrand factor (vWF), were coated at 3 mg/mL in phosphate-buffered saline (PBS), 1 mmol/L CaCl2 , and 1 mmol/L MgCl2 in microtiter plates overnight at 4°C. The plates were washed three times with PBS and then blocked for 60 minutes with Tyrode's-BSA. Sixty microliters of washed murine platelets (150,000/μL) were added to each well in the presence of 1 mmol/L CaCl2 and 1 mmol/L MgCl2 in the presence or absence of 20 mmol/L ADP, and the plates were gently centrifuged for 5 minutes at 60g and incubated for 60 minutes at RT. The plates were then washed with PBS to remove unattached platelets. Attached cells were stained with 70 μL PNAG (p-nitrophenol-N-acetyl-B-D-glucosaminide; Sigma, St Louis, MO), a substrate for the lysosomal enzyme hexoseaminidase,20 in 50 mmol/L citrate, pH 5.0, and 0.25% Triton X-100. The plates were incubated 120 to 180 minutes at 37°C. The reaction was stopped by addition of 104 μL 50-mmol/L glycine and 5-mmol/L EDTA, pH 10.4, and absorbance was read at 405 nm.
Electron microscopy.Blood was collected by tail bleed directly into Karnovsky fixative (1% paraformaldehyde and 1.25% glutaraldehyde in 0.1 mol sodium cacodylate buffer, pH 7.2) and fixed for 2 hours at 4°C. Washed platelets were then prepared as already described. The sternum and spleen from normal, c-mpl-/-, and TPO-/- mice were fixed in Karnovsky fixative. The platelet, spleen, and sternum samples were then postfixed in 1% osmium in the same buffer, washed in distilled water, dehydrated through graded ethanols and propylene oxide, and embedded in Eponate 12 (Ted Pella Inc, Redding, CA), and ultrathin sections were cut on a Reichert Ultracut (Reichert-Jung, Vienna, Austria). Sections were counterstained with ethanolic uranyl acetate and lead citrate, examined at 80 kV, and photographed on a Philips CM12 transmission electron microscope (Philips, Eindhoven, The Netherlands).
Since only a small number of megakaryocytes could be identified in the bone marrow of both c-mpl-/- and TPO-/- mice compared with normal animals, semithick survey sections were cut to determine the presence of megakaryocytes. Ultrathin sections were cut only from areas containing a megakaryocyte. In all, 24 megakaryocytes were photographed at low (×3,500) and high (×12,000) magnification.
RESULTS
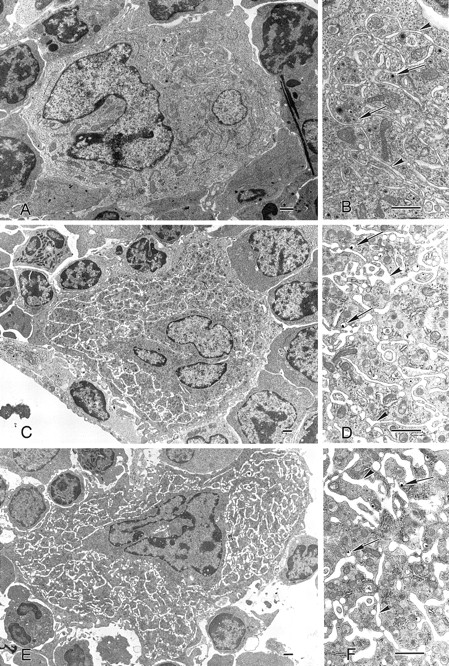
To determine if a functional TPO or c-mpl gene was required for the formation of normal megakaryocytes and platelets, we analyzed the morphology of these cells in c-mpl-/- and TPO-/- mice by electron microscopy. As in the bone marrow from normal animals, megakaryocytes were found in close proximity to the bone marrow sinusoids in c-mpl-/- and TPO-/- mice. No obvious morphologic differences could be detected in megakaryocytes of c-mpl-/- and TPO-/- mice (Fig 1C, D, E, and F) compared with the controls (Fig 1A and B). Immature megakaryocytes without α-granules or platelet demarcation systems, as well as fully mature megakaryocytes, were easily recognized by their large size and nuclear contours in both knockout and control animals. Figure 1 shows fully mature megakaryocytes from c-mpl-/- mice (C and D) and TPO-/- mice (E and F). The peripheral cytoplasm of the megakaryocytes shows evidence of platelet formation as indicated by the extensive network of platelet demarcation channels (Fig 1A, C, and E). Higher magnifications of c-mpl-/- and TPO-/- megakaryocytes (Fig 1B, D, and F) revealed that the cytoplasm is fully partitioned by platelet demarcation channels (arrowheads) and contains α-granules with and without dense cores (arrows). Similarly, electron microscopic analysis of platelets from c-mpl-/- or TPO-/- mice did not reveal any abnormality as compared with wild-type platelets. Figure 2 shows nonactivated platelets from wild-type, c-mpl-/-, and TPO-/- mice as recognized by their uniform discoid shape. They appear to have a normal number and appearance of granules.
Megakaryocyte ultrastructure. Megakaryocytes from wild-type (A and B), c-mpl-/- (C and D), and TPO-/- (E and F) mice display multilobulated nuclei, and the cytoplasm contains normal α-granules, some of which display dense cores (B, D, and F, arrows). The demarcation membrane system appears normal (arrowheads). Bar = 1 μm.
Megakaryocyte ultrastructure. Megakaryocytes from wild-type (A and B), c-mpl-/- (C and D), and TPO-/- (E and F) mice display multilobulated nuclei, and the cytoplasm contains normal α-granules, some of which display dense cores (B, D, and F, arrows). The demarcation membrane system appears normal (arrowheads). Bar = 1 μm.
Platelet ultrastructure. High-magnification micrographs of unactivated circulating platelets from wild-type (WT), c-mpl-/-, and TPO-/- mice show that the cytoplasmic constituents display similar morphologic features including α-granules and marginal bands of microtubules.
Platelet ultrastructure. High-magnification micrographs of unactivated circulating platelets from wild-type (WT), c-mpl-/-, and TPO-/- mice show that the cytoplasmic constituents display similar morphologic features including α-granules and marginal bands of microtubules.
To determine if platelets from knockout mice were functionally different compared with platelets from normal mice, we first measured the ability to upregulate αIIB β3 (GP-IIb-IIIa) in response to platelet agonist stimulation. Platelets become activated when exposed to extracellular matrix or soluble factors such as thrombin or ADP. During activation, the platelet receptor αIIb β3 undergoes conformational changes enabling it to bind fibrinogen and vWF, leading to platelet aggregation and formation of a hemostatic plug in vivo, an important primary step in the arrest of bleeding. The expression of αIIb β3 at the platelet surface is therefore a direct measure of platelet activation. In this assay, stimulation of platelets from c-mpl-/- mice by ADP was not significantly different from that of wild-type platelets (Fig 3A). Similarly, ADP stimulation of platelets from TPO-/- mice led to an increase of 125I-fibrinogen binding equivalent to the increase observed in normal platelets (Fig 3B). The presence of TPO receptors on murine platelets has been demonstrated,21 and addition of exogenous TPO to platelets has been shown to potentiate fibrinogen binding induced by ADP or other known agonists, decreasing the EC50 by about twofold.12 13 Platelets from TPO-/- mice showed a comparable stimulation of ADP sensitivity in the presence of exogenous TPO (Fig 3B) and are therefore indistinguishable from wild-type platelets. Consistent with the absence of TPO receptors, this effect of TPO was not seen in platelets from c-mpl-/- mice (Fig 3A).
Fibrinogen binding stimulated by ADP. (A) Platelets from wild-type (WT) and c-mpl-/- mice with and without addition of exogenous TPO. (B) Platelets from WT and TPO-/- mice with and without addition of exogenous TPO.
Fibrinogen binding stimulated by ADP. (A) Platelets from wild-type (WT) and c-mpl-/- mice with and without addition of exogenous TPO. (B) Platelets from WT and TPO-/- mice with and without addition of exogenous TPO.
We next evaluated the ability of platelets from wild-type and knockout mice to interact with immobilized extracellular matrix proteins. Platelets isolated from TPO/c-mpl double-knockout mice showed normal adhesion to vWF and to collagen-coated plates compared with platelets from control littermates. Attachment of wild-type and knockout platelets was enhanced to the same extent by preactivation with ADP (Fig 4).
Adhesion of washed platelets from wild-type (WT) and TPO/c-mpl double-knockout mice to immobilized collagen and vWF with and without ADP activation. Platelet attachment was quantified by staining for the lysosomal enzyme hexoseaminidase.
Adhesion of washed platelets from wild-type (WT) and TPO/c-mpl double-knockout mice to immobilized collagen and vWF with and without ADP activation. Platelet attachment was quantified by staining for the lysosomal enzyme hexoseaminidase.
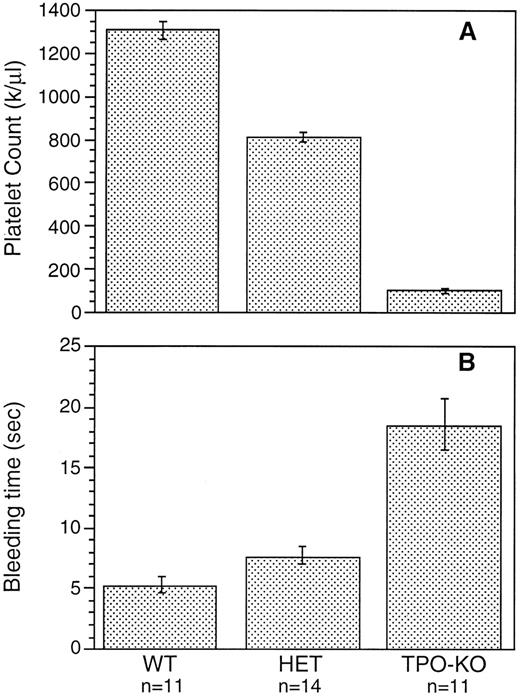
Although c-mpl-/- and TPO-/- mice have a reduced number of platelets, no spontaneous bleeding was observed in these animals. To determine whether the reduction in platelet number had a significant effect on bleeding, bleeding times were measured by a tail-cut technique. Figure 5 shows that bleeding in TPO-/- mice was prolonged threefold to fourfold and was slightly prolonged in TPO+/- mice compared with controls. A similar reduction was observed in c-mpl-/- mice (data not shown). This increase in bleeding time is most likely a reflection of the reduced platelet count, since no functional differences were observed in the in vitro studies between platelets from wild-type and knockout mice.
Bleeding time measured by tail cut in wild-type mice (WT), TPO heterozygous mice (HET), and TPO-/- mice (TPO-KO).
Bleeding time measured by tail cut in wild-type mice (WT), TPO heterozygous mice (HET), and TPO-/- mice (TPO-KO).
DISCUSSION
Preclinical studies point to TPO as the primary regulator of platelet production.22 Clearly, the dramatic increase in platelet counts observed when animals are injected with TPO can be attributed to a proliferative effect of TPO on megakaryocyte progenitors and to an increase in megakaryocyte size and ploidy.6,8,9,22,23 Similarly, the low levels of platelets observed in c-mpl-/- and TPO-/- mice are associated with a decrease in megakaryocyte progenitors and a reduction in megakaryocyte size and ploidy.16-18,24 25 However, it still remains to be established whether TPO is essential only for the regulation of platelet number or whether it is also required for the proper maturation of megakaryocytes and platelets produced in vivo.
Megakaryocytes and platelets that are functionally and morphologically equivalent to those present in the bone marrow or plasma can now be generated in cultures from bone marrow, fetal liver, or peripheral blood stem cells.10,11,14,15,26 TPO has both direct proliferative and differentiative effects on these progenitor cells26 and appears to be required for the formation of normal megakaryocytes and platelets in vitro.7 However, even if such cultures do contain a number of cell types capable of supplying a range of factors necessary for megakaryocytopoiesis, it is unlikely that these conditions will reproduce exactly the stromal environment in which megakaryocytes develop in vivo. This may explain why these in vitro results do not correlate with our observations of megakaryocyte and platelet formation in c-mpl-/- and TPO-/- mice. First, even though these mice are thrombocytopenic, with a 90% reduction in both megakaryocyte and platelet count, they are not subject to any spontaneous bleeding. Their bleeding time, the most useful measure of platelet function in vivo,27 is increased threefold to fourfold. In contrast, mice with a normal platelet count but impaired platelet function due to abnormal platelet granules, such as the muted, mocha, or cocoa mouse, have bleeding times prolonged up to 10-fold.28 29 Second, both megakaryocytes and platelets from mutant mice display an ultrastructural morphology similar to that of cells from normal animals. Finally, as measured by the increase in activated αIIb β3 in ADP-stimulated platelets or by adhesion to extracellular matrix proteins, these platelets are functionally the same as the controls. These results indicate that in vivo, TPO is not absolutely required for the formation of normal megakaryocytes and platelets, but is responsible for dramatically increasing their number. An elevated number of circulating platelets do not appear to be necessary for the survival of these mice in a well-protected environment, but is likely to provide a survival advantage to a wild animal.
Are other cytokines responsible for the basal level of megakaryocytopoiesis and thrombopoiesis present in TPO-/- and c-mpl-/- mice? Several soluble factors have been shown to have in vitro or in vivo megakaryocytopoietic activity1,2 and have been shown to be capable of exerting the effect in the absence of a functional TPO/c-mpl system.30 However, gene-targeted mice have been generated for most of these cytokines individually, and none seem to display abnormal megakaryocyte or platelet formation.31-33 It will be interesting to see if the combination of mice deficient in cytokines such as IL-3 or IL-11 and TPO-/- mice would affect platelet counts further or platelet function. The megakaryocytopoietic activity of these cytokines may become apparent only in the absence of a functional TPO/c-mpl system. However, it remains to be seen if the thrombocytopenia caused by the absence of any combination of soluble factor will be as dramatic as the thrombocytopenia observed in NF-E2 transcription factor–deficient mice.34 These mice display an increased number of highly abnormal megakaryocytes that are unable to form a normal membrane demarcation system and are unable to shed platelets into the circulation. The thrombocytopenia in NF-E2–deficient mice is therefore more profound than in TPO-/- or c-mpl-/- mice, and most of them die at birth from excessive bleeding. As observed for other blood cell lineages,35 the various stages of megakaryocyte and platelet formation appear to be absolutely dependent on the activity of transcription factors. It is possible that cytokines such as TPO influence the number of megakaryocytes and platelets produced by acting on the amount or activity of specific transcription factors, and that in the absence of TPO, a basal level of the required transcription factors is responsible for the production of a basal level of megakaryocytes and platelets.
ACKNOWLEDGMENT
The authors thank Joanne Mathias and Linda Loverro for helping with the animals, David Wood for assistance in preparing the figures, and Daniel Friend for helpful advice.
Address reprint requests to Frederic J. de Sauvage, PhD, Genentech Inc, 460 Pt San Bruno Blvd, South San Francisco, CA 94080.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal