Abstract
Somatic gene therapy of hemoglobinopathies depends initially on the demonstration of safe, efficient gene transfer and long-term, high-level expression of the transferred human β-globin gene in animal models. We have used a β-globin gene/β-locus control region retroviral vector containing several modifications to optimize gene transfer and expression in a mouse transplant model. In this report we show that transplantation of β-globin–transduced hematopoietic cells into lethally irradiated mice leads to the continued presence of the gene up to 8 months posttransplantation. The transferred human β-globin gene is detected in 3 of 5 mice surviving long term (>4 months) transplanted with bone marrow cells transduced with high-titer virus. Southern blotting confirms the presence of the unrearranged 5.1-kb human β-globin gene-containing provirus in 2 of these mice. In addition, long-term expression of the transferred gene is seen in 2 mice at levels of 5% and 20% that of endogenous murine β-globin at 6 and 8 months posttransplantation. We further document stem cell transduction by the successful transfer and high-level expression of the human β-globin gene from mice transduced 9 months earlier into irradiated secondary recipient mice. These results demonstrate high-level, long-term somatic human β-globin gene transfer into the hematopoietic stem cells of an animal for the first time, and suggest the potential feasibility of a retroviral gene therapy approach to sickle cell disease and the β thalassemias.
PREREQUISITES for successful somatic gene therapy of β-thalassemia and sickle cell disease include safe and efficient gene transfer as well as long-term and high-level expression of the transferred gene. Retroviral vectors when used with appropriate packaging cell lines have demonstrated safety and are able to efficiently transfer genes such as the human multiple drug resistance gene (MDR-1)1-3 and the neomycin resistance gene (neoR)4 into hematopoietic cells. Progress in β-globin gene transfer has been difficult, primarily because of low titer vectors and poor expression of the transferred gene.5-7 The landmark discovery of the β-globin locus control region (LCR) and data from transgenic mice incorporating these sequences seemed to hold the solution for many prior problems.8-12 However, initial vectors incorporating these sequences exhibited frequent rearrangements on transmission of the provirus, resulting in low β-globin expression using in vivo models.13 14 To date there has been no report of sustained long-term, high-level β-globin expression in an in vivo animal model.
Recently, several studies have shown more stable proviral transmission, using β-globin constructs modified to remove splice signals and/or optimizing the organization of the LCR derivatives.15-17 In this report we show for the first time that, using one of these modified β-globin retroviral vectors in a mouse transplant model, long-term, high-level expression of a transferred human β-globin gene is possible. In addition, the transduced gene is capable of being transferred to secondary transplant recipients with continued expression, consistent with stem cell transduction. Therefore, our findings represent progress toward the ultimate goal of somatic human β-globin gene therapy for sickle cell disease and β-thalassemia.
MATERIALS AND METHODS
Retroviral Vector
The structure of the retroviral vector p141 is shown in Fig 1.15 The backbone of the vector derives from the LXSN vector, which contains an extended packaging signal.18 A deletion in the 3′ LTR results in self-inactivation. The neoR gene, used as a selectable marker, is driven by the PGK promoter. The β-globin gene is inserted in reverse orientation with deletions of the 3′ enhancer and of a 372-bp region in intron 2. LCR elements HS2, HS3, and HS4 are incorporated upstream of the β-globin gene.19 Mutations in potential splicing and polyadenylation signals were added for increased stability of proviral transmission.15
A diagram of the p141 vector. The 5′ long terminal repeat (LTR) and 3′ deleted long terminal repeat (Δ LTR) are indicated. Ψ+ represents the extended packaging signal. The β-globin gene is shown in reverse orientation with exons (▧), introns (▨), the deleted 3′ enhancer (⊠), and the 372-bp deletion in intron 2 (Δ Rsa I). LCR elements HS234 (▪) and the neomycin resistance gene (NEO) with the PGK promoter (▨) are indicated.
A diagram of the p141 vector. The 5′ long terminal repeat (LTR) and 3′ deleted long terminal repeat (Δ LTR) are indicated. Ψ+ represents the extended packaging signal. The β-globin gene is shown in reverse orientation with exons (▧), introns (▨), the deleted 3′ enhancer (⊠), and the 372-bp deletion in intron 2 (Δ Rsa I). LCR elements HS234 (▪) and the neomycin resistance gene (NEO) with the PGK promoter (▨) are indicated.
Preparation of Viral Producer Lines
β-Globin and N2 producer lines were prepared by transfecting GP+E86 ecotropic packaging cells20 with the retroviral vectors p141 and N2 using the calcium phosphate coprecipitation method and were then selected in medium containing 600 μg of G418 per mL, as described previously.21 Culture supernatants were titered for viral production using G418 resistance of target 3T3 cells, as described.20 22 Clones with the highest titers were maintained as viral producer lines.
Hematopoietic Cell Transduction and Transplantation
Bone marrow (BM) cells were obtained from femurs and tibias of 10- to 12-week-old C57BL/6J male donor mice (Jackson Laboratories, Bar Harbor, ME) 48 hours after they had received one dose of 5-fluorouracil (150 mg/kg intraperitoneally [i.p.]). Fetal liver (FL) cells were obtained from day 14.5 fetuses as described.23 Cell aliquots, 5 × 107, were preincubated in T175 flasks (Nunc, Glostrup, Denmark) containing 80 mL α minimum essential medium (αMEM) with 20% fetal calf serum (FCS), 1% penicillin/streptomycin solution (GIBCO, Grand Island, NY), murine interleukin-3 (IL-3, 50 to 100 U/mL; Intergen Co, Purchase, NY), human IL-6 (200 U/mL), and rat stem cell factor (100 ng/mL; a gift of Amgen, Thousand Oaks, CA) for 24 hours at 37°C. Aliquots of BM or FL (5 × 106 to 107 cells) were then cocultured on 100-mm plates containing either semi-confluent GP+E86 β-producers or N2 producers. Cocultures were incubated in αMEM with 20% FCS, 1% penicillin/streptomycin solution, murine IL-3 (50 to 100 U/mL), IL-6 (200 U/mL), and stem cell factor (100 ng/mL) with the addition of polybrene 6 μg/mL or protamine 5 μg/mL (Sigma) for 24 to 48 hours. BM or FL cells were obtained from the coculture plates by gentle pipetting. C57BL/6J female recipient mice were gamma irradiated with 1,100 rad given in two doses 3 hours apart. Mice were injected via tail vein with either 1 to 2 × 106 β-transduced cells or 1 to 2 × 106 N2-transduced cells.
Analysis of Transplanted Mice
Polymerase chain reaction (PCR). DNA was prepared from day 12 spleen colonies and peripheral blood at various time points using the Iso-Quick Kit (Microprobe, Garden Grove, CA), as described previously.24 PCR reactions for β-globin, neoR, mouse platelet-derived growth factor (PDGF ) B receptor, and Y chromosome sequences were performed. For β-globin, PCR primers 5′-TGGATCCTGAGAACTTCAGG-3′ (sense) and 5′-CACTGGTGGGGTGAATTCTT-3′ (antisense) were used which spanned the RsaI deletion in IVS-2, resulting in a 564-bp amplification fragment from the transferred human β-globin gene and a 936-bp fragment from normal human DNA. One-half microgram of DNA was amplified for 28 cycles; each cycle comprised 94°C denaturation for 60 seconds, 60°C primer annealing for 50 seconds, and 72°C extension for 75 seconds. NeoR and PDGF B and Y chromosome PCR reactions were performed as previously described.25 26 PCR analysis using primers for PDGF B was used as a control for DNA loading; Y chromosome analysis was used for reconstitution with donor cells (male donors to female recipients).
Southern blot analysis. Selected mice were killed 9 months posttransplantation and chromosomal DNA was extracted from blood, marrow, and spleens using a standard phenol extraction method. Approximately 10 μg of DNA was digested with Sac I and Xba I and standard Southern blotting was performed using a neoR probe (32P-labeled by random primer elongation).
Primer extension assays. Total RNA was extracted from 150 μL of peripheral blood obtained at various time points using a Micro RNA Isolation Kit (Stratagene La Jolla, CA). Quantitative primer extension assays were performed with 32P end-labeled primers specific for retroviral-derived human β-globin (5′-CTCCTCTTCAGGCGGCAATGAC-3′) and mouse βmaj globin (5′-TGATGTCTGTTTCTGGGGTTGTG-3′), with predicted extension products of 90 bp and 58 bp, respectively. Four micrograms of total RNA was used in each reaction comprising 80°C denaturation for 5 minutes, 52°C hybridization for 16 hours, and 42°C extension for 30 minutes. Reaction samples were then phenol extracted, precipitated, and run on a denaturing sequencing gel. RNA isolated from the blood of a transgenic mouse known to contain three copies of a human β-globin gene and to be expressing human β-globin27 was used as a positive control for primer extension. Radioactive bands were quantitated by phosphorimaging.
Secondary Transplantation
Marrow from sacrificed primary recipient mice was used to reconstitute lethally irradiated secondary recipients. One to 2 × 107 unmanipulated marrow cells were injected via tail vein per mouse. Peripheral blood analyses for gene transfer and expression were performed as for primary recipients.
RESULTS
Generation of Virus-Producing Lines
Two producer clones containing vector p141 with titers of 1 × 104 and 1 × 105 G418-resistant 3T3 colony-forming units (CFU)/mL, respectively, transferred intact copies of the proviral construct into 3T3 cells as assessed by Southern blotting (data not shown) and were used for transplantation experiments. N2 transfections yielded producer lines with intact transfer of the neoR gene and titers of 1 × 106 to 1 × 107 G418 resistant 3T3 CFU/mL.
Gene Transfer
At day 12 posttransplantation, mice were killed and CFU-spleen (CFU-S) assayed for the presence of the human β-globin and neoR genes by PCR (Table 1, Fig 2). Twenty-nine of 34 CFU-S were PCR positive when a 105 β-globin gene-containing producer was used, whereas only 1 of 6 colonies was positive with the 104 producer (Table 1). In mice transplanted with N2 transduced hematopoietic cells (titer of producer 5 × 106), 100% transduction was observed in each experiment. Y chromosome PCR analysis was positive in all transplanted mice (data not shown).
Analysis of CFU-S From Mice Transplanted With Retrovirally Transduced Hematopoietic Cells
| Exp. No. . | Titer . | Cell Source . | Cation . | No. of Mice . | No. of CFU-S . | β PCR+ Colonies . | % . |
|---|---|---|---|---|---|---|---|
| 1 | 105 | BM | PB | 2 | 12 | 10* | 83 |
| 2 | 104 | BM | PB | 1 | 6 | 1 | 17 |
| 3 | 105 | FL | PB | 2 | 11 | 8 | 73 |
| 4 | 105 | BM | PT | 2 | 11 | 11 | 100 |
| Exp. No. . | Titer . | Cell Source . | Cation . | No. of Mice . | No. of CFU-S . | β PCR+ Colonies . | % . |
|---|---|---|---|---|---|---|---|
| 1 | 105 | BM | PB | 2 | 12 | 10* | 83 |
| 2 | 104 | BM | PB | 1 | 6 | 1 | 17 |
| 3 | 105 | FL | PB | 2 | 11 | 8 | 73 |
| 4 | 105 | BM | PT | 2 | 11 | 11 | 100 |
Hematopoietic cells were obtained, preincubated with growth factors, and then cocultured with β-globin retroviral producer cells. Lethally irradiated recipient mice were injected with transduced cells and 1-2 mice were killed 12 days posttransplantation. CFU-S were counted and DNA extracted. PCR specific for the transferred β-globin gene was performed on 0.5 μg of DNA from individual colonies.
Abbreviations: Exp, experiment; PB, polybrene; PT, protamine.
β IVS-2 PCR of transduced mouse spleen colonies. Two recipient mice were killed 12 days after transplantation with human β-globin transduced marrow. DNA samples were obtained from individual spleen colonies and PCR analysis performed using primers specific for the β-globin IVS-2 region (upper panel). Lanes 1 through 10, samples from individual spleen colonies; lane 11, producer cell DNA; lane 12, normal human DNA; lane 13, a size marker, φX. Indicated on the right of the figure are the predicted PCR bands for normal human β-globin (936 bp) and vector-derived human β-globin which contains a 372-bp deletion in IVS-2 (564 bp). Eight of 10 spleen colonies are strongly PCR-positive, the other two are weakly positive. As a control for DNA loading, PCR analysis using PDGF B primers was performed on each CFU-S sample as described in Materials and Methods (lower panel). DNA loading was approximately equal, except for the sample in lane 9.
β IVS-2 PCR of transduced mouse spleen colonies. Two recipient mice were killed 12 days after transplantation with human β-globin transduced marrow. DNA samples were obtained from individual spleen colonies and PCR analysis performed using primers specific for the β-globin IVS-2 region (upper panel). Lanes 1 through 10, samples from individual spleen colonies; lane 11, producer cell DNA; lane 12, normal human DNA; lane 13, a size marker, φX. Indicated on the right of the figure are the predicted PCR bands for normal human β-globin (936 bp) and vector-derived human β-globin which contains a 372-bp deletion in IVS-2 (564 bp). Eight of 10 spleen colonies are strongly PCR-positive, the other two are weakly positive. As a control for DNA loading, PCR analysis using PDGF B primers was performed on each CFU-S sample as described in Materials and Methods (lower panel). DNA loading was approximately equal, except for the sample in lane 9.
DNA from peripheral blood of transplanted mice assayed by PCR for the presence of the human β-globin gene was positive in 40% to 60% of mice at time points less than 4 months posttransplantation when the 105 producer was used (Table 2). Long-term persistence of the transferred β-globin gene (assessed by PCR at time points from 4 to 8 months posttransplantation) is evident in 3 of 5 (60%) of mice from experiment 1 (Fig 3, Table 2), 7 of 7 (100%) of mice from experiment 4, and a single mouse from each of the other experiments (Table 2). The presence of a transduced gene more than 4 months posttransplantation in a mouse is suggestive of stem cell transduction.
Human β -Globin Gene Transfer and Expression in Peripheral Blood
| Exp. No. . | Total Mice . | Short Term (<4 mo) . | Long Term (>4 mo) . | ||||
|---|---|---|---|---|---|---|---|
| (titer, cell source, cation) . | . | Mice PCR+ . | Mice RNA+ . | Hu β/Mouse β . | Mice PCR+ . | Mice RNA+ . | Hu β/Mouse β . |
| 1 (105, BM, PB) | 5 | 3 | NA | — | 3 | 2 | 20%* |
| 5%* | |||||||
| 2 (104, BM, PB) | 9 | 1 | 0 | — | 1 | 0 | — |
| 3 (105, FL, PB) | 10 | 4 | 1 | 3% | 1 | 0 | — |
| 4 (105, BM, PT) | 9 | 9 | 0 | — | 7 | 0 | — |
| Exp. No. . | Total Mice . | Short Term (<4 mo) . | Long Term (>4 mo) . | ||||
|---|---|---|---|---|---|---|---|
| (titer, cell source, cation) . | . | Mice PCR+ . | Mice RNA+ . | Hu β/Mouse β . | Mice PCR+ . | Mice RNA+ . | Hu β/Mouse β . |
| 1 (105, BM, PB) | 5 | 3 | NA | — | 3 | 2 | 20%* |
| 5%* | |||||||
| 2 (104, BM, PB) | 9 | 1 | 0 | — | 1 | 0 | — |
| 3 (105, FL, PB) | 10 | 4 | 1 | 3% | 1 | 0 | — |
| 4 (105, BM, PT) | 9 | 9 | 0 | — | 7 | 0 | — |
Hematopoietic cells were obtained, preincubated with growth factors, and then cocultured with retroviral producer cells. Lethally irradiated recipient mice were injected with transduced cells and peripheral blood samples were analyzed for gene transfer by PCR and gene expression by primer extension at various time points as described in Materials and Methods.
Abbreviations: Exp, experiment; PB, polybrene; PT, protamine; NA, not available.
Levels stable at 6 months and 8 months posttransplantation. These are mice 3 and 4 in Fig 4.
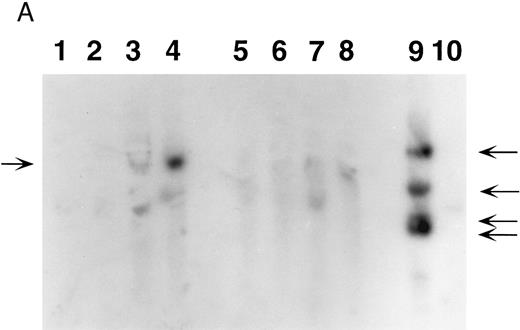
β IVS-2 PCR of 5-month peripheral blood samples. DNA samples were obtained from mice transplanted with human β-globin transduced marrow (β-mice) and from mice transplanted with neomycin resistance gene-transduced marrow (neoR mice). PCR analysis was performed using primers specific for the β-globin IVS-2 region (upper panel). Lane 1, a size marker (φX); lane 2, a normal human DNA sample; lanes 3 through 5 represent dilutions of producer cell DNA (lane 3, 100%; lane 4, 10%, lane 5, 1%); lanes 6 and 7, samples from two neoR mice; and lanes 8 through 12, samples from β-mice. Indicated at the left of the figure are the predicted PCR bands for human β-globin (936 bp) and vector-derived human β-globin (564 bp). Three of 5 mice (lanes 9, 11, and 12) are positive. As a control for DNA loading, PCR analysis using PDGF B primers was performed on each sample as described in Materials and Methods (lower panel). Apart from lane 10, DNA loading was approximately equal.
β IVS-2 PCR of 5-month peripheral blood samples. DNA samples were obtained from mice transplanted with human β-globin transduced marrow (β-mice) and from mice transplanted with neomycin resistance gene-transduced marrow (neoR mice). PCR analysis was performed using primers specific for the β-globin IVS-2 region (upper panel). Lane 1, a size marker (φX); lane 2, a normal human DNA sample; lanes 3 through 5 represent dilutions of producer cell DNA (lane 3, 100%; lane 4, 10%, lane 5, 1%); lanes 6 and 7, samples from two neoR mice; and lanes 8 through 12, samples from β-mice. Indicated at the left of the figure are the predicted PCR bands for human β-globin (936 bp) and vector-derived human β-globin (564 bp). Three of 5 mice (lanes 9, 11, and 12) are positive. As a control for DNA loading, PCR analysis using PDGF B primers was performed on each sample as described in Materials and Methods (lower panel). Apart from lane 10, DNA loading was approximately equal.
We assume that any single hematopoietic stem cell has only one copy of the retroviral vector containing the transferred human β-globin gene at the relatively low multiplicity of infection of virus per stem cell used (certainly much less than 1). With this assumption, we can estimate the percentage of cells containing the transferred human β-globin gene from the results shown in Fig 3. The intensity of the PCR signal in transduced mice lies between the 1:10 and 1:100 dilutions of producer cells. Because each producer cell contains three intact copies of the human β-globin gene vector by Southern blotting (Fig 4), we estimate that between 3% and 30% of the cells of the transduced mice contain the transferred globin gene, although this number reflects the DNA present in peripheral blood nucleated white blood cells, not red blood cells.
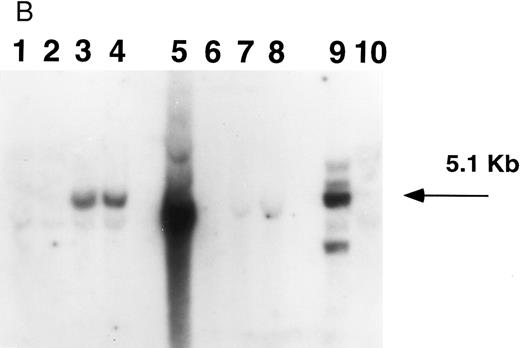
Southern blot analysis of mouse marrow. DNA obtained from marrow and spleens of experiment 1 mice was digested with Xba I (A) and Sac I (B) and probed with a neoR probe as described in Materials and Methods. In both figures, lanes 1 through 4 represent DNA samples obtained from BM of mice 1 through 4; lanes 5 through 8, DNA samples from corresponding spleens; lane 9, producer cell DNA; lane 10, DNA from normal mouse spleen. Mice 1 and 2 show no evidence of human β-globin gene expression while mice 3 and 4 do (Fig 5, Table 3). (A). The retroviral vector p141 contains a single Xba I restriction site, and this digest was used to determine the number of chromosomal integration sites. The arrows on the right indicate the four integration sites seen in the producer cells in lane 9. In mice 1 and 2, no significant bands are seen; mouse 3 has at least two integration sites, while mouse 4 has a single dominant integration site (indicated by the arrow on the left). (B). The LTR regions of p141 each contain an Sac I site, and this digest was used to determine the integrity of the transferred β-globin gene. Producer cells contain three intact copies and one truncated copy of the retroviral vector (intensity of 5.1-kb band is three times that of the lower band in lane 9). Mice 1 and 2 have no detectable provirus in BM, while mice 3 and 4 both have the intact 5.1-kb human β-globin gene-containing provirus (lanes 1 through 4). Twice as much DNA (10 μg) of spleen and marrow samples was loaded as was producer cell DNA (5 μg). By comparing the intensities of the single and the three copy bands from the producer line in lane 9 with those of the 5.1-kb bands in mice 3 and 4 (lanes 3 and 4) by phosphorimaging, we calculate that there are 0.53 and 0.64 copies of the human β-globin gene per cell in the marrow cells of these mice. The intense signal in spleen DNA in lane 5 of (B) is caused by contamination, and is not seen on repeat Southern blot analysis.
Southern blot analysis of mouse marrow. DNA obtained from marrow and spleens of experiment 1 mice was digested with Xba I (A) and Sac I (B) and probed with a neoR probe as described in Materials and Methods. In both figures, lanes 1 through 4 represent DNA samples obtained from BM of mice 1 through 4; lanes 5 through 8, DNA samples from corresponding spleens; lane 9, producer cell DNA; lane 10, DNA from normal mouse spleen. Mice 1 and 2 show no evidence of human β-globin gene expression while mice 3 and 4 do (Fig 5, Table 3). (A). The retroviral vector p141 contains a single Xba I restriction site, and this digest was used to determine the number of chromosomal integration sites. The arrows on the right indicate the four integration sites seen in the producer cells in lane 9. In mice 1 and 2, no significant bands are seen; mouse 3 has at least two integration sites, while mouse 4 has a single dominant integration site (indicated by the arrow on the left). (B). The LTR regions of p141 each contain an Sac I site, and this digest was used to determine the integrity of the transferred β-globin gene. Producer cells contain three intact copies and one truncated copy of the retroviral vector (intensity of 5.1-kb band is three times that of the lower band in lane 9). Mice 1 and 2 have no detectable provirus in BM, while mice 3 and 4 both have the intact 5.1-kb human β-globin gene-containing provirus (lanes 1 through 4). Twice as much DNA (10 μg) of spleen and marrow samples was loaded as was producer cell DNA (5 μg). By comparing the intensities of the single and the three copy bands from the producer line in lane 9 with those of the 5.1-kb bands in mice 3 and 4 (lanes 3 and 4) by phosphorimaging, we calculate that there are 0.53 and 0.64 copies of the human β-globin gene per cell in the marrow cells of these mice. The intense signal in spleen DNA in lane 5 of (B) is caused by contamination, and is not seen on repeat Southern blot analysis.
Southern blot analysis of selected mice from experiment 1 9 months posttransplantation shows that mouse 3 has at least two sites of chromosomal integration of the provirus, while mouse 4 appears to have a single dominant site, probably representing clonal (or oligoclonal reconstitution) (Fig 4A). The presence of the intact 5.1-kb human β-globin gene-containing provirus in the two mice (mice 3 and 4) expressing human β-globin is shown in the Southern blot in Fig 4B. We calculate that there are 0.53 and 0.64 copies of the human β-globin gene per cell in these mice, respectively (see legend to Fig 4B). There is variability in detecting proviral sequences in the spleens of mice 9 months posttransplantation (Fig 4). The spleen is not an important erythropoietic organ at this time as it is earlier in murine life. Human β-globin gene-transduced clones may no longer be detectable at this late time either because of their loss or dilution by nontransduced cells.
Human β-Globin Gene Expression
Primer extension assays performed on RNA extracted from peripheral blood samples obtained at various time points show that some of the PCR-positive mice are expressing human β-globin at high levels (>3% as much human β-globin mRNA as mouse β-globin mRNA) (Fig 5). In one mouse from experiment 1, human β-globin RNA is present at a level 20% that of endogenous mouse βmaj globin RNA at 6 and 8 months posttransplantation (Table 3). Two other mice, one from experiment 1 using BM cells showed 5% as much human β-globin as mouse expression at 8 months posttransplantation, while another, using fetal liver cells, showed 3% as much expression at 3 months which disappeared at 8 months.
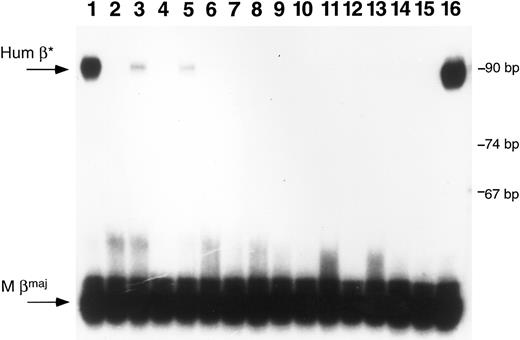
Primer extension analysis of RNA from peripheral blood samples. RNA samples were obtained from peripheral blood of mice after transplantation with transduced marrow or fetal liver cells. Primer extension analysis was performed using one primer specific for mouse β-globin major and one specific for human β-globin. Lanes 1 through 3 are samples from mice transplanted with β-globin transduced marrow; lanes 4 through 13 represent samples from mice transplanted with β-globin transduced fetal liver cells; lanes 14 and 15 are negative controls and lane 16 represents positive control RNA obtained from a mouse transgenic for multiple copies of the human β-globin gene.24 The arrows at the left of the figure indicate the predicted positions for human β-globin (Hum β) and mouse β-globin (M βmaj). Size markers are noted on the right side of the figure. Positive signals are seen in the mouse blood samples in lanes 1, 3, and 5. The RNA sample in lane 1 is from mouse 4 in Fig 4; the sample in lane 3 is from mouse 3 in Fig 4.
Primer extension analysis of RNA from peripheral blood samples. RNA samples were obtained from peripheral blood of mice after transplantation with transduced marrow or fetal liver cells. Primer extension analysis was performed using one primer specific for mouse β-globin major and one specific for human β-globin. Lanes 1 through 3 are samples from mice transplanted with β-globin transduced marrow; lanes 4 through 13 represent samples from mice transplanted with β-globin transduced fetal liver cells; lanes 14 and 15 are negative controls and lane 16 represents positive control RNA obtained from a mouse transgenic for multiple copies of the human β-globin gene.24 The arrows at the left of the figure indicate the predicted positions for human β-globin (Hum β) and mouse β-globin (M βmaj). Size markers are noted on the right side of the figure. Positive signals are seen in the mouse blood samples in lanes 1, 3, and 5. The RNA sample in lane 1 is from mouse 4 in Fig 4; the sample in lane 3 is from mouse 3 in Fig 4.
Human β -Globin Gene Transfer and Expression in Peripheral Blood of Primary and Secondary Recipient Mice
| Animal . | Recipient . | β-Globin . | Primer Extension . | Human β/Mouse βmaj (%) . |
|---|---|---|---|---|
| . | . | PCR . | . | . |
| Mouse 1 | Primary | + | − | − |
| 1A | Secondary | − | − | − |
| 1B | Secondary | − | − | − |
| Mouse 2 | Primary | − | − | − |
| 2A | Secondary | − | − | − |
| 2B | Secondary | − | − | − |
| Mouse 3 | Primary | + | + | 5 |
| 3A | Secondary | + | + | 8 |
| 3B | Secondary | + | + | 11 |
| Mouse 4 | Primary | + | + | 20 |
| 4A | Secondary | + | + | 3 |
| 4B | Secondary | + | + | 12 |
| Animal . | Recipient . | β-Globin . | Primer Extension . | Human β/Mouse βmaj (%) . |
|---|---|---|---|---|
| . | . | PCR . | . | . |
| Mouse 1 | Primary | + | − | − |
| 1A | Secondary | − | − | − |
| 1B | Secondary | − | − | − |
| Mouse 2 | Primary | − | − | − |
| 2A | Secondary | − | − | − |
| 2B | Secondary | − | − | − |
| Mouse 3 | Primary | + | + | 5 |
| 3A | Secondary | + | + | 8 |
| 3B | Secondary | + | + | 11 |
| Mouse 4 | Primary | + | + | 20 |
| 4A | Secondary | + | + | 3 |
| 4B | Secondary | + | + | 12 |
Four primary recipient mice from experiment 1 were killed 9 months posttransplantation and their marrow used to reconstitute secondary recipients. Peripheral blood samples of secondary recipients were analyzed for human β-globin gene transfer by PCR and gene expression by primer extension as described in Materials and Methods. Data are shown for each of the primary and secondary recipients. (+) indicates a signal for the human β-globin gene by PCR or mRNA by primer extension; (−) indicates no signal.
In experiment 4 (Table 2), the high-titer producer line was used after several additional passages with G418 selection; the 9 mice transduced with these producer cells were found to have a β-globin PCR signal but had no β-globin mRNA expression by primer extension. By Southern blot analysis, all of these mice were found to have a truncated copy of the β-globin retrovirus of the same size in all samples (data not shown). This was due to the presence of a smaller truncated retrovirus containing an intact NeoR gene, but only part of the human β-globin gene; this truncated vector was also seen initially in the producer line in low amounts following calcium phosphate transfection and was selected by repeated growth in G418 (Fig 4B, lane 9).
Secondary Transplants
Four primary recipient mice from experiment 1 were killed 9 months posttransplantation and their marrow used to reconstitute lethally irradiated secondary recipients (two recipients per primary mouse). Secondary recipients from two of three mice that were PCR-positive and expressing human β-globin RNA in primary mice continued to retain and express the transferred gene 6 weeks posttransplantation (Table 3, Fig 6). This is strong evidence of stem cell transduction. By contrast, PCR-negative primary recipients result in PCR-negative secondary recipients.
Primer extension of RNA samples from primary and secondary recipients. Mice from experiment 1 were killed and their marrow cells used to reconstitute lethally irradiated secondary recipients. Each successive group of 4 lanes represents RNA samples from a primary mouse BM, spleen, and peripheral blood of two secondary recipients. Lanes 1 through 4, samples originating from mouse 1; lanes 5 through 8, mouse 2; lanes 9 through 12, mouse 3; and lanes 13 through 16, mouse 4. Lane 17 represents RNA from a mouse transgenic for the human β-globin gene. The arrows at the left of the figure indicate the predicted positions for human β-globin (Hum β) and mouse β-globin (M βmaj).
Primer extension of RNA samples from primary and secondary recipients. Mice from experiment 1 were killed and their marrow cells used to reconstitute lethally irradiated secondary recipients. Each successive group of 4 lanes represents RNA samples from a primary mouse BM, spleen, and peripheral blood of two secondary recipients. Lanes 1 through 4, samples originating from mouse 1; lanes 5 through 8, mouse 2; lanes 9 through 12, mouse 3; and lanes 13 through 16, mouse 4. Lane 17 represents RNA from a mouse transgenic for the human β-globin gene. The arrows at the left of the figure indicate the predicted positions for human β-globin (Hum β) and mouse β-globin (M βmaj).
DISCUSSION
We have used a producer line containing a unique retroviral vector to transfer and express a human β-globin gene in a mouse transplant model. This retroviral vector has a human β-globin gene modified to remove potential splicing and polyadenylation signals, incorporates locus control region core elements (HS2, HS3, and HS4), and has been shown to transduce mouse erythroleukemia cells and mouse marrow cells in culture without rearrangement previously.15 We now show that this vector in an ecotropic producer line is capable of high-level unrearranged gene transfer in mice both short-term and long-term. Evidence for short-term gene transfer is provided by both CFU-S assays (Table 1) and PCR from peripheral blood of mice (Table 2). In experiment 4, Table 2, G418 selection of producer cells led to the preferential growth of cells containing a smaller retrovirus initially present in the calcium phosphate–selected clone. We have other producer clones now available which do not contain this contaminant.
The high level of stem cell transduction achieved in these experiments is consistent with results we have previously reported using the human MDR gene and a similar transduction protocol.1,24 From the data in Tables 2 and 3, it is clear that the retroviral producer line with the higher titer, 105 as compared with 104, is much more efficient in both the transfer and expression of the human β-globin gene in mice, as expected. Fetal liver cells were less transducible with the human β-globin gene than the MDR gene.24 Using marrow cells and intact high-titer virus (experiment 1), 3 of 5 mice were transduced with the human β-globin gene short-term and all 3 retained the gene long-term. We show long-term human β-globin gene transfer by PCR and Southern blot analysis 6 to 9 months posttransplantation (Table 2, Figs 3 and 4). At these late times, it is presumed that persistent gene transfer is caused by stem cell transduction. To further document stem cell transduction, we also show successful transfer of the human β-globin gene from mice transduced 9 months earlier into irradiated secondary recipient mice (Table 3). In a previous study comparable short-term transfer of the human β-globin gene was obtained using a different vector; long-term gene transfer (>4 months) was reported in only 4 of 51 mice.14 No conclusive evidence for stem cell transduction was shown in the previous study.14
More importantly, we now show both short- and long-term expression of the transferred human β-globin gene in mice, as assessed by primer extension (Table 2, Fig 5). Long-term, high-level (>3% human β as compared with mouse β-globin) expression was achieved in 2 of 3 mice transduced using BM cells (experiment 1, Table 2). One mouse shows 20% as high human β-globin expression as mouse β-globin expression. This high level of expression appears to be the result of high-level human β-globin gene expression of one clone that is predominantly represented in the marrow of this mouse at 8 months. This level of normal human β-globin gene expression, if achieved consistently in a patient with sickle cell disease or β-thalassemia, would be expected to ameliorate, if not cure, the disease.28,29 In addition, marrow transferred into irradiated secondary recipients from two of three primary mice expressing β-globin mRNA also leads to persistence of human β-globin gene expression, confirming expression of the transferred gene in stem cells (Table 3, Fig 6). Others have reported extinction of demonstrable human β-globin gene expression at these late times, although smaller numbers of mice were analyzed in that report (M. Sadelain, personal communication). In another report, there were no mice demonstrating long-term, high-level expression.14
In summary, long-term expression in mice transplanted with BM cells transduced with intact high-titer virus occurred in 2 of 3 mice. Our experiments are the first to show high-level, long-term expression of the human β-globin gene in vivo.
There is a great deal of variability in the level of human β-globin gene transfer and expression in these murine experiments, even with the higher titer producer line. In the two high human β-globin gene-expressing mice (Fig 5), there is also no correlation between the copy numbers (0.53 and 0.64) (Fig 4) and the levels of expression which are 5% and 20% that of mouse β-globin. These differences in copy number versus expression in our experiments are most likely due to differences in expression of the human β-globin gene inserted in different chromosomal positions (position effects), as well as the small number of stem cell clones active in the oligoclonal reconstitution in this ablated mouse model. In other mice, we were unable to detect the inserted human β-globin gene by Southern blotting (mice in lanes 1 and 2, Fig 4). In these latter mice, we cannot distinguish between position effects and potential gene rearrangements as the cause of undetectable β-globin gene expression since there is inadequate gene transfer. In contrast to previous reports,14 we have chosen to express our results in actual measured globin RNA expression ratios without attempting any normalization on a per-gene basis. Although such a normalization (ie, one proviral copy per transduced cell v two endogenous copies, and only a fraction of erythroid cells contain a provirus) would obviously bring the calculated expression levels to even higher levels, we believe that the exact measurement of the fraction of erythroid cells transduced is not quantitative in such studies and will consequently bias the analysis.
In our studies, the presence of the β-globin LCR elements does not appear to confer site-independent chromosomal integration on the transferred human β-globin gene, as has been reported in transgenic mice.9,30 Our results add to recent evidence that the β-LCR functions primarily as a strong erythroid-specific enhancer in retroviral constructs.13,15,16 The properties of “copy number-dependence, position-independence,” as originally assigned to LCR function,9 are best observed in transgenic animal experiments with large genomic fragments integrated as tandem repeats. With single copies of the β-LCR, this position independence of expression is variable.31 In addition, the μ-LCR components HS 2, 3, and 4 used in our experiments may not be optimal for conferring consistent high-level expression on a single-copy retroviral integrant; other β-LCR elements may be more optimal.
Before somatic human β-globin gene therapy can be attempted, it will be necessary to have more consistent high-level gene transfer and expression of the human β-globin gene in reconstituting stem cell clones by either: (1) increasing the efficiency of gene transfer and expression, (2) identifying other constructs that will permit position-independent expression, and/or (3) selecting transduced cells coexpressing a drug resistance gene such as MDR either in vivo, or in vitro by preselecting cells transduced with a cell-surface marker such as MDR or CD24.24 32 Constructs with (1) modified LCR components, (2) nuclear localization signals to transduce nondividing cells, (3) stem cell targeting strategies, (4) more effective cytokine combinations in transduction, and/or (5) other promoters and enhancers may be required to accomplish this. However, despite the limitations, in our studies, the finding of unrearranged human β-globin gene transfer and the occasional high level of human β-globin gene expression long-term, as well as evidence of hematopoietic stem cell transduction for the first time in animals, provide the basis for using this and related β-globin retroviral producer lines to further improve this gene transfer system in preclinical studies. In addition, these studies provide an impetus for considering that human globin retroviral gene transfer into human hematopoietic stem cells may eventually be a feasible approach to the treatment of sickle cell disease and β-thalassemia.
ACKNOWLEDGMENT
We thank Vivian Hayashi for her technical assistance.
Supported by Public Health Service Grants from the National Institutes of Health (NIH) (DK-25274, HL-28381, HL-48345, HL-48374, and HL-55435). H.R. was supported by an NIH Hematology Training Grant (DK-07373).
Address reprint requests to Arthur Bank, MD, Columbia University, HHSC 16-1602, 701 W 168th St, New York, NY 10032.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal