Abstract
The aminothiol, amifostine (Ethyol; U.S. Bioscience, West Conshohocken, PA), is a cytoprotective agent that ameliorates the toxicities of anticancer therapy. In vitro, amifostine promotes the formation and survival of primitive hematopoietic progenitors derived from myelodysplastic bone marrow (BM) specimens. To evaluate the hematological effects of amifostine, 18 patients with myelodysplastic syndrome (MDS) and one or more refractory cytopenias received treatment with amifostine in a Phase I/II study. Four cohorts received intravenous treatment with 100, 200, or 400 mg/m2 amifostine three times a week, or 740 mg/m2 weekly for three consecutive weeks followed by 2 weeks observation. Nonresponding patients received a second course of therapy at the next higher dose level depending upon drug tolerance. Bone marrow (BM) progenitor growth was assessed before treatment and after day 21. Diagnoses included refractory anemia (7), refractory anemia with ringed sideroblasts (5), refractory anemia with excess blasts (RAEB) (4), and RAEB-in transformation (RAEB-t) (2). Single- or multi-lineage hematologic responses occurred in 15 patients (83%) treated with the three-times-a-week dose schedule. Fourteen patients had a 50% or greater increase in absolute neutrophil count with amifostine treatment (range, 426 to 11,348/μL). Platelet count increased in 6 (43%) of 14 patients with thrombocytopenia (absolute increase, 16,000 to 110,000/μL), and 5 of 15 red blood cell transfusion-dependent patients had a 50% of greater reduction in transfusion needs. Assayable hematopoietic progenitors increased in 13 of 15 evaluable patients; including CFU-GEMM (12), BFU-E (8), and CFU-GM (6). Amifostine doses less than or equal to 200 mg/m2 were well tolerated, whereas grade II nausea, vomiting, and fatigue was limiting at higher doses. Three patients with excess blasts before enrollment experienced an increase in BM blast percentage and two patients had evolution to acute leukemia that persisted after treatment withdrawal. We conclude that amifostine administered at doses ≤200 mg/m2 three times a week is well tolerated and has hematologic activity in patients with MDS.
EFFECTIVE TREATMENT alternatives for patients with myelodysplastic syndrome (MDS) remain limited.1 The cytopenias that complicate these disorders reflect a diminished responsiveness of bone marrow (BM) progenitors to normal trophic signals, leading to premature progenitor loss and ineffective hematopoiesis.2-4 Supportive measures such as blood product transfusions offer interim improvement, but the majority of patients succumb to infectious or bleeding complications, iron overload, or leukemic transformation. Because of the generally advanced age of the population affected, few patients are candidates for allogeneic BM transplantation or aggressive cytoreductive therapy. In recent years, recombinant hematopoietic cytokines have shown considerable therapeutic potential. At supraphysiologic concentrations these agents enhance the survival and maturation of myelodysplastic progenitors.12 In clinical trials, both granulocyte- (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) promote granulopoiesis and improve neutrophil counts in the majority of patients.156 However, the hematopoietic activity of these cytokines is largely restricted to the granulocyte series and few patients experience amelioration of anemia or thrombocytopenia. Erythropoietin and the multilineage growth factor, interleukein-3 (IL-3), have only limited single agent effectiveness in MDS.7-11 When combined with G- or GM-CSF, erythropoietin may augment the erythropoietic response in selected patients with suboptimal endogenous growth factor response.1213
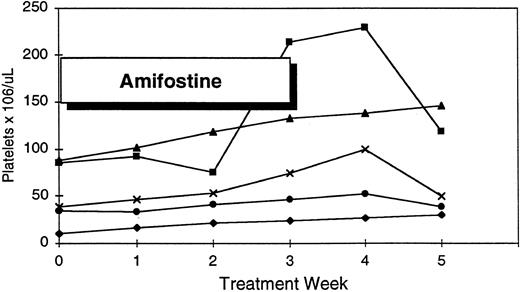
Platelet response to amifostine in five patients with pretreatment thrombocytopenia.
Platelet response to amifostine in five patients with pretreatment thrombocytopenia.
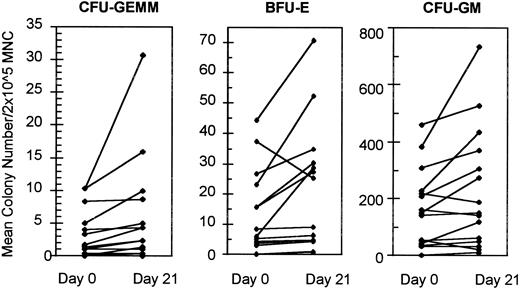
Comparison of BM progenitor recovery before treatment with amifostine and after day 21.
Comparison of BM progenitor recovery before treatment with amifostine and after day 21.
Clinical and Hematologic Features in Patients Treated With Ethyol
| Patient . | Age . | Sex . | FAB . | Karyotype . | % Abnormal . | Pretreatment . | Postethyol Treatment . | Platelets . | % BM Blasts . | Ethyol Dose . | Tx . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | . | . | Category . | . | Metaphases . | ANC/μL . | Hgb (g/dL) . | RBC . | Platelets . | . | ANC/μL . | Hgb (g/dL) . | RBC . | (/μL) . | Pre/Post . | (final dose) . | Duration . |
| . | . | . | . | . | Pre/Post . | . | (retic %) . | Trnsfns . | (/μL) . | . | . | (retic %) . | Trnsfns . | . | . | . | (wk) . |
| . | . | . | . | . | . | . | . | (no. units) . | . | . | . | . | . | . | . | . | . |
| 101 | 79 | M | RARS | 47,xy,+8 | 15/5 | 2,968 | 7.9 (0.2) | 2 q2 wk | 216,000 | 5,387 | 11.4 (2.6) | 2 q5 wk | 231,000 | ≤1/≤1 | 100 (200) | 45 | |
| 102 | 57 | F | RAEB-t | 42,xx,der (3), −7,−8,del(12) (p12),−15,−17 | 100/NE | 938 | 6.3 (2.3) | 2 q2-3 wk | 32,000 | 1,440 | 6.5 (2.3) | 2 q 2-3wk | 33,000 | 8/16 | 100 (200) | 10 | |
| 103 | 73 | M | RAEB | 46,xy | 0/0 | 288 | 8.2 (0.2) | 2 q2 wk | 13,000 | 714 | 5.7 (1.7) | 2 q2 wk | 28,000 | 7/9 | 100 (200) | 20 | |
| 201 | 63 | F | RARS | 46,xx | 0/0 | 2,115 | 9.2 (0.1) | 2 q2 wk | 282,000 | 3,650 | 8.0 (3.9) | 2 q2 wk | 377,000 | 1.4/2 | 200 (400) | 10 | |
| 202 | 75 | M | RAEB-t | 47,xy,+11 | 100/95 | 650 | 9.3 (1.4) | 2 q2 wk | 90,000 | 1,462 | 8.4 (5.5) | 2 q4 wk | 146,000 | 10/32 | 200 (200) | 10 | |
| 203 | 67 | M | RA | 46,xy | 0/0 | 4,756 | 9.7 (1.8) | 2 q2 wk | 49,000 | 8,789 | 10.7 (1.8) | 2 q3 wk | 52,000 | 0/1 | 200 (400) | 15 | |
| 301 | 78 | F | RARS | 46,xx | 0/NE | 496 | 11.3 (1.2) | None | 119,000 | 2,356 | 12.4 (4.7) | None | 229,000 | 0/ND | 400 (400) | 10 | |
| 302 | 78 | M | RA | 47,xy,+8 | 100/100 | 2,442 | 11.2 (3.7) | None | 58,000 | 3,990 | 11.8 (6.0) | None | 100,000 | 2.5/2.0 | 400 (200) | 15 | |
| 303 | 75 | M | RARS | 46,xy | 0/0 | 897 | 8.6 (0.8) | 2 q6 wk | 214,000 | 1,734 | 10.2 (1.4) | 2 q8 wk | 228,000 | 1/1 | 400 (200) | 15 | |
| 304 | 75 | M | RA | 48,xy, del (7) (p13; p15), +14,+19 | 76/100 | 1,770 | 9.8 (2.7) | 2 q8 wk | 34,000 | 1,938 | 10.5 (3.0) | 2 q8 wk | 52,000 | 1/1 | 400 (400) | 10 | |
| 401 | 72 | M | RA | 46,xy | 0/0 | 540 | 7.4 (1.0) | 2 q2 wk | 10,000 | 975 | 9.0 (2.3) | 2 q4 wk | 8,000 | ≤1/0 | 740 (200) | 20 | |
| 402 | 76 | M | RARS | 46,xy, del (20) (q11.20) | 50/50 | 3,844 | 11.1 (0.2) | 2 q5 wk | 185,000 | 6,162 | 10.3 (0.7) | 2 q5 wk | 220,000 | 1/1 | 740 (200) | 15 | |
| 403 | 66 | M | RA | 46,xy | 0/0 | 36 | 9.5 (0.4) | 3 q2 wk | 87,000 | 403 | 9.5 (0.6) | 2 q4 wk | 108,000 | 1/1 | 740 (200) | 10 | |
| 501 | 76 | M | RAEB | 39-41,xy,+2,−7,−10,−14, −15,−17, −18,−20,−21, −22,+mar | 100/87 | 88 | 8.0 (0.5) | 3 q3 wk | 5,000 | 100 | 8.1 (1.0) | 3 q3 wk | 9,000 | 1/1 | 200 (200) | 10 | |
| 502 | 72 | M | RAEB | 45,x,−y, +8 (p23), del(11) (q21) | 100/NE | 576 | 9.8 (1.0) | 2 q2 wk | 132,000 | 11,928 | 10.4 (1.0) | 2 q2 wk | 200,000 | 18/ND | 200 (200) | 5 | |
| 503 | 68 | F | RA | 47-70,xx,−5,−18,+22, +2mar | 100/100 | 195 | 9.3 (0.3) | 3 q3 wk | 11,000 | 220 | 9.7 (1.1) | 3 q2-3 wk | 17,000 | 1.1/3.4 | 200 (200) | 10 | |
| 504 | 75 | M | RA | 45,x,−y | 100/100 | 576 | 11.9 (1.1) | None | 46,000 | 828 | 12.4 (5.0) | None | 49,000 | 1.5/1 | 200 (200) | 10 | |
| 505 | 66 | F | RAEB | 47,xx,del(5) (q15),+8 | 100/NE | 630 | 9.1 (0.1) | 2 q2 wk | 68,000 | 1,190 | 8.5 (0.2) | 2 q2 wk | 71,000 | 5.5/43 | 200 (200) | 5 | |
| Abbreviations: Hgb, hemoglobin; NE, not evaluable or inadequate metaphases; RBC Trnsfns, number and frequency of red blood cell transfusions; q, every. | |||||||||||||||||
| Patient . | Age . | Sex . | FAB . | Karyotype . | % Abnormal . | Pretreatment . | Postethyol Treatment . | Platelets . | % BM Blasts . | Ethyol Dose . | Tx . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | . | . | Category . | . | Metaphases . | ANC/μL . | Hgb (g/dL) . | RBC . | Platelets . | . | ANC/μL . | Hgb (g/dL) . | RBC . | (/μL) . | Pre/Post . | (final dose) . | Duration . |
| . | . | . | . | . | Pre/Post . | . | (retic %) . | Trnsfns . | (/μL) . | . | . | (retic %) . | Trnsfns . | . | . | . | (wk) . |
| . | . | . | . | . | . | . | . | (no. units) . | . | . | . | . | . | . | . | . | . |
| 101 | 79 | M | RARS | 47,xy,+8 | 15/5 | 2,968 | 7.9 (0.2) | 2 q2 wk | 216,000 | 5,387 | 11.4 (2.6) | 2 q5 wk | 231,000 | ≤1/≤1 | 100 (200) | 45 | |
| 102 | 57 | F | RAEB-t | 42,xx,der (3), −7,−8,del(12) (p12),−15,−17 | 100/NE | 938 | 6.3 (2.3) | 2 q2-3 wk | 32,000 | 1,440 | 6.5 (2.3) | 2 q 2-3wk | 33,000 | 8/16 | 100 (200) | 10 | |
| 103 | 73 | M | RAEB | 46,xy | 0/0 | 288 | 8.2 (0.2) | 2 q2 wk | 13,000 | 714 | 5.7 (1.7) | 2 q2 wk | 28,000 | 7/9 | 100 (200) | 20 | |
| 201 | 63 | F | RARS | 46,xx | 0/0 | 2,115 | 9.2 (0.1) | 2 q2 wk | 282,000 | 3,650 | 8.0 (3.9) | 2 q2 wk | 377,000 | 1.4/2 | 200 (400) | 10 | |
| 202 | 75 | M | RAEB-t | 47,xy,+11 | 100/95 | 650 | 9.3 (1.4) | 2 q2 wk | 90,000 | 1,462 | 8.4 (5.5) | 2 q4 wk | 146,000 | 10/32 | 200 (200) | 10 | |
| 203 | 67 | M | RA | 46,xy | 0/0 | 4,756 | 9.7 (1.8) | 2 q2 wk | 49,000 | 8,789 | 10.7 (1.8) | 2 q3 wk | 52,000 | 0/1 | 200 (400) | 15 | |
| 301 | 78 | F | RARS | 46,xx | 0/NE | 496 | 11.3 (1.2) | None | 119,000 | 2,356 | 12.4 (4.7) | None | 229,000 | 0/ND | 400 (400) | 10 | |
| 302 | 78 | M | RA | 47,xy,+8 | 100/100 | 2,442 | 11.2 (3.7) | None | 58,000 | 3,990 | 11.8 (6.0) | None | 100,000 | 2.5/2.0 | 400 (200) | 15 | |
| 303 | 75 | M | RARS | 46,xy | 0/0 | 897 | 8.6 (0.8) | 2 q6 wk | 214,000 | 1,734 | 10.2 (1.4) | 2 q8 wk | 228,000 | 1/1 | 400 (200) | 15 | |
| 304 | 75 | M | RA | 48,xy, del (7) (p13; p15), +14,+19 | 76/100 | 1,770 | 9.8 (2.7) | 2 q8 wk | 34,000 | 1,938 | 10.5 (3.0) | 2 q8 wk | 52,000 | 1/1 | 400 (400) | 10 | |
| 401 | 72 | M | RA | 46,xy | 0/0 | 540 | 7.4 (1.0) | 2 q2 wk | 10,000 | 975 | 9.0 (2.3) | 2 q4 wk | 8,000 | ≤1/0 | 740 (200) | 20 | |
| 402 | 76 | M | RARS | 46,xy, del (20) (q11.20) | 50/50 | 3,844 | 11.1 (0.2) | 2 q5 wk | 185,000 | 6,162 | 10.3 (0.7) | 2 q5 wk | 220,000 | 1/1 | 740 (200) | 15 | |
| 403 | 66 | M | RA | 46,xy | 0/0 | 36 | 9.5 (0.4) | 3 q2 wk | 87,000 | 403 | 9.5 (0.6) | 2 q4 wk | 108,000 | 1/1 | 740 (200) | 10 | |
| 501 | 76 | M | RAEB | 39-41,xy,+2,−7,−10,−14, −15,−17, −18,−20,−21, −22,+mar | 100/87 | 88 | 8.0 (0.5) | 3 q3 wk | 5,000 | 100 | 8.1 (1.0) | 3 q3 wk | 9,000 | 1/1 | 200 (200) | 10 | |
| 502 | 72 | M | RAEB | 45,x,−y, +8 (p23), del(11) (q21) | 100/NE | 576 | 9.8 (1.0) | 2 q2 wk | 132,000 | 11,928 | 10.4 (1.0) | 2 q2 wk | 200,000 | 18/ND | 200 (200) | 5 | |
| 503 | 68 | F | RA | 47-70,xx,−5,−18,+22, +2mar | 100/100 | 195 | 9.3 (0.3) | 3 q3 wk | 11,000 | 220 | 9.7 (1.1) | 3 q2-3 wk | 17,000 | 1.1/3.4 | 200 (200) | 10 | |
| 504 | 75 | M | RA | 45,x,−y | 100/100 | 576 | 11.9 (1.1) | None | 46,000 | 828 | 12.4 (5.0) | None | 49,000 | 1.5/1 | 200 (200) | 10 | |
| 505 | 66 | F | RAEB | 47,xx,del(5) (q15),+8 | 100/NE | 630 | 9.1 (0.1) | 2 q2 wk | 68,000 | 1,190 | 8.5 (0.2) | 2 q2 wk | 71,000 | 5.5/43 | 200 (200) | 5 | |
| Abbreviations: Hgb, hemoglobin; NE, not evaluable or inadequate metaphases; RBC Trnsfns, number and frequency of red blood cell transfusions; q, every. | |||||||||||||||||
Hematologic Response to Amifostine
| . | No. Evaluable . | No. of Responders . | Net Change . |
|---|---|---|---|
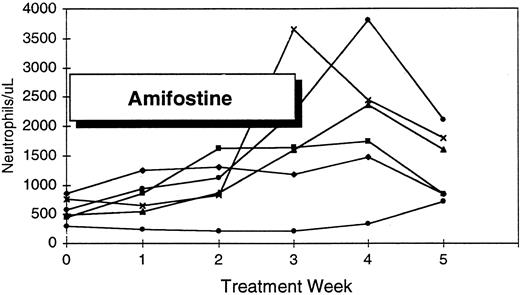
| ANC ≥50% increase | 18 | 14 (78%) | [426-11,348/μL] |
| Pretreatment ANC <1,000/μL | 12 | 9 (75%) | |
| Pretreatment ANC >1,000/μL | 6 | 5 | |
| Platelets ≥50% increase* | 14 | 6 (43%) | [16-110,000/μL] |
| Pretreatment <50,000/μL* | 8 | 2 | |
| Pretreatment >50,000/μL | 6 | 4 | |
| Reticulocytes ≥50% increase | 16 | 8 (50%) | [1.3-4.1] |
| RBC transfusions ≥50% reduction | 15 | 5 (33%) |
| . | No. Evaluable . | No. of Responders . | Net Change . |
|---|---|---|---|
| ANC ≥50% increase | 18 | 14 (78%) | [426-11,348/μL] |
| Pretreatment ANC <1,000/μL | 12 | 9 (75%) | |
| Pretreatment ANC >1,000/μL | 6 | 5 | |
| Platelets ≥50% increase* | 14 | 6 (43%) | [16-110,000/μL] |
| Pretreatment <50,000/μL* | 8 | 2 | |
| Pretreatment >50,000/μL | 6 | 4 | |
| Reticulocytes ≥50% increase | 16 | 8 (50%) | [1.3-4.1] |
| RBC transfusions ≥50% reduction | 15 | 5 (33%) |
Exceeding a 15,000/μL platelet increment and ≥50% of baseline.
Grade 1-2 Toxicities by Amifostine Dose (mg/m2)
| Toxicitiy . | 100 . | 200 . | 400 . | 740 . |
|---|---|---|---|---|
| No. of patients | 3 | 17 | 6 | 3 |
| Nausea | − | 1 | 4 | 3 |
| Vomiting | − | − | 3 | 3 |
| Fatigue | − | − | 1 | − |
| Rash + fever | − | − | 1 | − |
| Dose reduction | − | − | 2 | 3 |
| Hypotension | − | − | − | 1 |
| Autoimmune hemolytic anemia | − | 1 | − | − |
| Toxicitiy . | 100 . | 200 . | 400 . | 740 . |
|---|---|---|---|---|
| No. of patients | 3 | 17 | 6 | 3 |
| Nausea | − | 1 | 4 | 3 |
| Vomiting | − | − | 3 | 3 |
| Fatigue | − | − | 1 | − |
| Rash + fever | − | − | 1 | − |
| Dose reduction | − | − | 2 | 3 |
| Hypotension | − | − | − | 1 |
| Autoimmune hemolytic anemia | − | 1 | − | − |
Address reprint requests to Alan F. List, MD, Director, Bone Marrow Transplant Program, The Arizona Cancer Center, 1515 N Campbell Ave, Room 3947, PO Box 245024, Tucson, AZ 85724-5024.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal