Abstract
Lym-2 is a murine monoclonal antibody (MoAb) directed towards a human class II molecule variant reactive with both normal and neoplastic human B lymphocytes. Previous studies have shown that signals transmitted by class II molecules that stimulate normal lymphocytes can be inhibitory for B-cell lymphoma growth by signaling activation-induced cell death. Therefore, we sought to evaluate the effects of nonconjugated murine Lym-2 and a human-mouse chimeric Lym-2 (chCLL-1; with murine variable regions and human constant regions) MoAb on the growth of various human lymphomas by using both in vitro and in vivo assays. Cell lines derived from Burkitt's lymphomas, diffuse large cell B-cell lymphomas, anaplastic large-cell lymphomas, and Epstein-Barr virus–induced B-cell lymphomas were incubated with Lym-2 or chCLL-1 in vitro, and effects on proliferation were determined by [3H]-thymidine incorporation. The effects of Lym-2 in vitro were also compared with those of Lym-1, which is a similar MoAb that has been evaluated clinically. After immobilization, which enhances crosslinking of the MoAbs, both Lym-2 and chCLL-1 were capable of directly inhibiting the growth of various lymphoma lines in vitro. These human lymphomas were then transferred into mice with severe combined immunodeficiency to evaluate the efficacy of these MoAbs in vivo. Treatment with either murine Lym-2 or the chimeric chCLL-1 were significantly effective in improving the survival of tumor-bearing mice. These results indicate that stimulation by nonconjugated chCLL-1 may offer a biological approach to the treatment of various human lymphomas.
CANCER IMMUNOTHERAPY that uses monoclonal antibodies (MoAbs) against cell surface antigens on lymphoid malignancies has shown promising results in clinical trials (anti-idiotype,1,2 CAMPATH-13 ). There are a variety of effector mechanisms by which MoAbs may kill tumor cells including complement-mediated lysis or antibody-dependent cellular cytotoxicity (ADCC). The effects of B-cell–reactive MoAbs, such as anti-CD21, anti-CD23, or anti-CD24 on B-cell tumors in vivo are presumably by ADCC.4 However, because target molecules on lymphoid cells can also be involved in signal transduction, direct effects of MoAbs on lymphoid malignancies may also contribute to tumor growth inhibition.5,6 MoAbs can theoretically target specific receptors on the cell surface that can regulate the growth of the neoplastic cells. We and others have observed in both B- and T-cell malignancies that antitumor effects (growth arrest with or without apoptosis) often result when malignant cells are exposed to stimuli that activate normal lymphocytes.7-11 Anti-IgM, anti-CD19, anti-CD40, and anti-class II major histocompatibility complex MoAbs have been shown to exert direct inhibitory effects on human B-cell lymphoma growth both in vitro and in vivo.8,9,11 12
Lym-2 is a mouse IgG1 MoAb directed against a human class II molecule variant and is reactive with a cell surface protein present on both normal and transformed human B-lymphocytes.13 It appears to be B-cell specific as it has not been detected on other cell types either normal or neoplastic.13 Lym-2 is strongly reactive with the majority of B-cell–derived malignancies including the majority of chronic lymphocytic leukemias and malignant cell lines derived from large-cell lymphomas, Burkitt's lymphomas, and B-cell acute lymphoblastic leukemia.13 Therefore, we wished to determine the effects of nonconjugated Lym-2 on the growth of various human lymphomas both in vitro and in vivo, because stimulation with Lym-2, which is directed against the human class II molecule variant, may induce “activation-induced cell death.” Because Lym-2 is of murine origin and would be likely to elicit human-antimouse antibodies with clinical use, a human-mouse chimeric Lym-2, designated chCLL-1, was produced and also examined for its antitumor effects. We report here that stimulation by either murine Lym-2 or chCLL-1 inhibits the growth of several human B-cell lymphomas and an anaplastic large-cell lymphoma line both in vitro and in vivo.
MATERIALS AND METHODS
Mice. C.B-17 severe combined immune deficiency (SCID) mice were obtained from the Animal Production Area (National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD) and were not used until they had reached 6 to 8 weeks of age. SCID mice were kept under specific pathogen-free conditions at all times. The mice were housed in microisolator cages and all food, water, and bedding were autoclaved before use. SCID mice received trimethoprim/sulfamethoxazole (40 mg trimethoprim and 200 mg sulfamethoxazole per 320 mL) in their drinking water.
Antibodies. Lym-2 (mouse IgG1) and Lym-1 (mouse IgG2a) were purchased from DAMON Biotech (Needham Heights, MA). Antihuman CD40 (m3 hybridoma, mouse IgG1 antibody) was provided by Immunex (Seattle, WA). Mouse IgG1 myeloma protein was purchased from Cappel (West Chester, PA).
The human-mouse chimeric CLL-1 (chCLL-1) antibody was generated by genetic engineering methods. The variable regions were cloned and the chimeric antibody fusion genes were constructed as described previously.14
Tumor cell lines. RL and DB are cell lines obtained from patients with diffuse large-cell lymphomas of B-cell origin.10 TU-2C and CHIM-62 are Epstein-Barr virus (EBV)-induced B-cell lymphoma cell lines obtained from SCID mice that received human peripheral blood (huPBL) from EBV-seropositive donors.12 The development of human EBV-induced B-cell lymphomas has been previously shown to occur in these huPBL-SCID chimeras.15,16 Raji and Daudi are human Burkitt's lymphoma cell lines.12 Michel and Karpas 299 are human anaplastic large-cell lymphoma (ALCL) cell lines kindly provided by Dr Hans J. Gruss (Immunex).17
In vivo experiments. All mice received 20 μL of antiasialo GM1 (ASGM1; Wako Chemicals, Richmond, VA) by intravenous injection 1 day before tumor transfer to reduce host natural killer cells.18 RL, TU-2C, Raji, or Michel tumor cells (5 × 106/0.5 mL) were then administered by intraperitoneal (IP) injection. SCID recipients then received either 2 μg of Lym-2, anti-CD40, chCLL-1, or mouse IgG1 myeloma protein in 0.1 mL Hanks' balanced salt solution (HBSS) IP every other day for 20 days for a total of 10 injections starting at various time points (day 2 to 7). Tumor-bearing mice were then monitored for tumor development and progression. Moribund mice were euthanized and all mice were necropsied for evidence of tumor. Nonparametric (Wilcoxon rank-sum test) analysis was performed to determine if the groups differed significantly (P < .05). All experiments had eight mice or more per group.
Proliferation assay. The effect of Lym-2 and Lym-1 on B-cell lymphoma growth in vitro was determined by [3H]-thymidine incorporation.12 The cell lines were split 24 hours before the assays were performed. Cells were resuspended in culture medium with 5% fetal bovine serum to a concentration of 1 × 105/mL, and 100 μL was plated in the 96-well flat-bottom microtiter plates (Corning Glass Works, Corning, NY) already containing 100 μL of appropriately diluted reagents. For immobilization of the antibodies, 100 μL of goat-antimouse IgG1 (for Lym-2), goat-antihuman Ig (for ChCLL -1), or goat-antimouse IgG2a (for Lym-1; Fisher-Science, Pittsburgh, PA) at a concentration of 25 μg/mL was added into the wells of a 96-well flat-bottom microtiter plate and incubated at 4°C for 24 hours. After washing with HBSS twice, the wells then received Lym-2, chCLL-1, Lym-1, or control mouse IgG1 myeloma protein at various concentrations and were allowed to incubate for 2 hours at 37°C followed by an addition of cell suspension. Seventy-two hours later, 1 μCi of [3H]-thymidine/well (specific activity, 6.7 μCi/mmol; New England Nuclear Research Products, Boston, MA) was added for the final 24 hours of culture. Cultures were harvested onto glass fiber filters (Wallac Oy, Turku, Finland) with a Cell Harvesting System (Cambridge Technology, Cambridge, MA), and [3H]-thymidine uptake was assayed by liquid scintillation counting on an LKB beta plate reader (LKB Instruments, Turku, Finland). Each experiment was performed 4 to 6 times with a representative experiment being shown. To determine the effect of crosslinking, further experiments were done with the uncoated plates under the same experimental conditions. Values are presented as a percentage of control Ig values. Cell viability was assessed using the trypan blue exclusion method (Life Technology, Grand Island, NY). A Student's t-test was used to determine if the values differed significantly.
RESULTS
Surface expression of the antigen recognized by Lym-2 on various human B-cell lymphomas. Surface expression of the antigen recognized by Lym-2 and Lym-1 was examined by flow cytometry in RL and DB, human diffuse large-cell lymphoma lines; TU-2C and CHIM-62, EBV-induced human B-cell lymphoma cell lines; Raji and Daudi, human Burkitt's lymphoma cell lines; and Michel and Karpas 299, ALCL cell lines. Lym-2 bound to the surface of all of these lymphomas (Table 1). This is in agreement with a precious report characterizing expression of Lym-2 on various cell lines.13 Numerous cell lines (eg, RAMOS, MOLT-4, K562, HL-60, NALM-1, and V266) were found to be negative for Lym-2 staining.13 Surprisingly, even the ALCL lines were positive for Lym-2 despite the previous report showing that Lym-2 was exclusively restricted to tumors of B-cell origin.13
Reactivity of Lym-2 With Human Lymphoma Cell Lines by Flow Cytometry
| Cell Line . | Histological Subtype . | Lym-2 . | Lym-1 . |
|---|---|---|---|
| RL | diffuse large cell | + | + |
| DB | diffuse large cell | + | + |
| TU-2C | EBV-induced B-cell | + | + |
| CHIM-62 | EBV-induced B-cell | + | + |
| Raji | Burkitt's | + | ++ |
| Daudi | Burkitt's | + | + |
| Michel | anaplastic large cell | ++ | ++ |
| Karpas 229 | anaplastic large cell | + | + |
| Cell Line . | Histological Subtype . | Lym-2 . | Lym-1 . |
|---|---|---|---|
| RL | diffuse large cell | + | + |
| DB | diffuse large cell | + | + |
| TU-2C | EBV-induced B-cell | + | + |
| CHIM-62 | EBV-induced B-cell | + | + |
| Raji | Burkitt's | + | ++ |
| Daudi | Burkitt's | + | + |
| Michel | anaplastic large cell | ++ | ++ |
| Karpas 229 | anaplastic large cell | + | + |
+, represents reactivity; ++, for strong reactivity.
Effects of Lym-2 and chCLL-1 on human lymphoma proliferation in vitro. We next ascertained the effects of Lym-2 and chCLL-1 on the proliferative potential of these various B-cell lymphomas in vitro. Inhibitory effects of crosslinked Lym-2 on various human lymphomas were determined by [3H]-thymidine incorporation assays (Fig 1). It has been shown that the prior immobilization of antibodies may markedly enhance their inhibitory effects on B-cell lymphomas.10,12 In our study, no significant inhibitory or stimulatory effects were observed when cells were incubated with soluble Lym-2 over a range of concentrations when compared with an isotype-matched control antibody. However, immobilized Lym-2 significantly (P < .05) inhibited the proliferation, compared with immobilized control antibody alone, of RL, DB, TU-2C, Raji, Daudi, and Michel cell lines (Fig 1). Dose response studies indicated that the optimal dose range of Lym-2 was between 1 and 10 μg/mL for the various cell lines. Incubation with immobilized Lym-2 also resulted in similar growth inhibition of other Lym-2–reactive cell lines such as CHIM-62 and the ALCL line, Karpas 299 (data not shown). When Lym-2 was compared with chCLL-1 by using the two ALCL lines, it was found that both MoAbs were similar in their ability to inhibit the proliferation of the two lines in vitro (Fig 2). Crosslinking of chCLL-1 with a goat-antihuman Ig necessitated the use of ALCL lines to compare chCLL-1 with Lym-2 because the goat-antihuman Ig inhibited the proliferation of the surface Ig+ B-cell lymphoma lines19 (data not shown). However, the data indicate that chCLL-1 still retains the inhibitory properties of the native antibody with regard to effects on neoplastic cell growth in vitro. We then assessed cell viability by using the trypan blue exclusion method. Incubation with immobilized Lym-2 at 1 μg/mL for 2 days resulted in a modest but significant decrease (P < .05) of cell viability in the Raji cell lines compared with untreated cells (from 74% viable to 42% in the treated group). Thus, Lym-2 was capable of inducing activation-induced growth inhibition and cell death of various human B lymphoma cell lines in vitro.
Effect of immobilized Lym-2 on the proliferation of various B-cell lymphoma cell lines. Lymphoma cell lines were incubated with Lym-2 or mIgG1 with immobilization by prior coating plates by goat-antimouse IgG1. Three days later, effects on growth were evaluated by incorporation of [3H]-thymidine. Soluble Lym-2 exerted no effect on proliferation of these lymphoma cell lines (data not shown). However, immobilized Lym-2 significantly inhibited the proliferation of (A) RL, (B) TU-2C, (C) Raji, (D) Michel, (E) Daudi, and (F ) DB cell lines (P < .05) with optimal inhibition of thymidine incorporation (39% to 65%) occurring at 1 μg/mL of Lym-2.
Effect of immobilized Lym-2 on the proliferation of various B-cell lymphoma cell lines. Lymphoma cell lines were incubated with Lym-2 or mIgG1 with immobilization by prior coating plates by goat-antimouse IgG1. Three days later, effects on growth were evaluated by incorporation of [3H]-thymidine. Soluble Lym-2 exerted no effect on proliferation of these lymphoma cell lines (data not shown). However, immobilized Lym-2 significantly inhibited the proliferation of (A) RL, (B) TU-2C, (C) Raji, (D) Michel, (E) Daudi, and (F ) DB cell lines (P < .05) with optimal inhibition of thymidine incorporation (39% to 65%) occurring at 1 μg/mL of Lym-2.
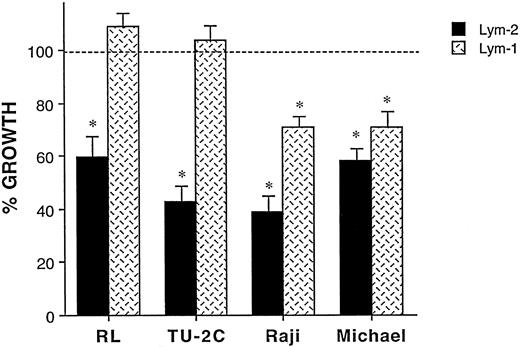
Comparison of the in vitro effects of Lym-2 versus Lym-1 on various B-cell lymphoma cell lines. Briefly, lymphoma cell lines were incubated with 1 μg/mL of antibodies that were previously immobilized to the plates with goat-antimouse IgG1 (for Lym-2) or goat-antimouse IgG2a (for Lym-1). Data are presented as percent of growth compared with crosslinked control mouse IgG1 or mouse IgG2a, and are representative of three experiments. Lym-1 inhibited the Raji Burkitt's cell line and Michel anaplastic cell line but not the RL diffuse large cell line nor the TU-2C EBV-induced cell line.
Comparison of the in vitro effects of Lym-2 versus Lym-1 on various B-cell lymphoma cell lines. Briefly, lymphoma cell lines were incubated with 1 μg/mL of antibodies that were previously immobilized to the plates with goat-antimouse IgG1 (for Lym-2) or goat-antimouse IgG2a (for Lym-1). Data are presented as percent of growth compared with crosslinked control mouse IgG1 or mouse IgG2a, and are representative of three experiments. Lym-1 inhibited the Raji Burkitt's cell line and Michel anaplastic cell line but not the RL diffuse large cell line nor the TU-2C EBV-induced cell line.
The comparison of inhibitory effects of Lym-2 and Lym-1 MoAb on human lymphoma cell lines. We then compared the in vitro inhibitory effects of Lym-2 and Lym-1 on these lymphomas (Fig 3). Several clinical in vitro studies of Lym-1 have also indicated antitumor activity against lymphoma cell lines.20-22 With prior immobilization, Lym-1 slightly inhibited Raji (Burkitt's lymphoma) and Michel (ALCL) cell lines (29% and 30% inhibition, respectively). However, neither RL (diffuse large-cell lymphoma) nor the TU-2C EBV-induced cell lymphoma lines were inhibited with Lym-1, even with prior immobilization. With Raji and Michel cell lines, the inhibitory effects of Lym-2 were consistently greater than Lym-1 (63% versus 29% inhibition with Raji and 42% versus 30% inhibition with Michel; Fig 3). However, the effects on the Michel cell line were not statistically significant. Thus, under these conditions the Lym-2 MoAb appears as efficacious, if not superior to Lym-1, in inducing growth arrest of several human lymphoma cell lines in vitro.
Comparison of the in vitro effects of Lym-2 with chCLL-1 using ALCL cell lines. In these experiments 1 μg/mL of antibody was used with Karpas and Michel ALCL lines being assayed. Lym-2 was immobilized by using a goat-antimouse Ig and chCLL-1 was immobilized by using a goat-antihuman Ig. The data are presented as percent of growth as a percent of control crosslinked mouse or human Ig growth. Representative of two experiments.
Comparison of the in vitro effects of Lym-2 with chCLL-1 using ALCL cell lines. In these experiments 1 μg/mL of antibody was used with Karpas and Michel ALCL lines being assayed. Lym-2 was immobilized by using a goat-antimouse Ig and chCLL-1 was immobilized by using a goat-antihuman Ig. The data are presented as percent of growth as a percent of control crosslinked mouse or human Ig growth. Representative of two experiments.
Antitumor effects of Lym-2 treatment on SCID mice bearing human lymphoma cell lines. Because Lym-2 was inhibitory for B-cell lymphoma growth in vitro, we determined if Lym-2 would be equally efficacious in the treatment of these lymphomas in vivo. Lym-2 was administered to mice bearing the RL diffuse large-cell lymphoma or the ALCL line, Michel. Treatment was initiated 3 days after tumor inoculation. Treatment with Lym-2 resulted in significant increases in survival with both tumors (Fig 4). We then compared the in vivo effects of Lym-2 (mouse IgG1) with another isotype-matched binding MoAb. We have previously shown that treatment with an anti-CD40 MoAb (mouse IgG1) improves the survival of SCID mice bearing the Raji lymphoma cell line12; therefore, Lym-2 was compared with anti-CD40 in vivo. Raji lymphoma cells (5 × 106) were inoculated by IP injection to SCID mice, and the mice were treated with Lym-2, anti-CD40, or an isotype control starting on day 2 after inoculation of Raji. Mice were then monitored for effects on survival, and moribund mice were euthanized. Survival of Raji-bearing mice was also significantly improved by treatment with Lym-2 starting on day 2 (P < .05), yielding comparable results with anti-CD40 (Table 2).
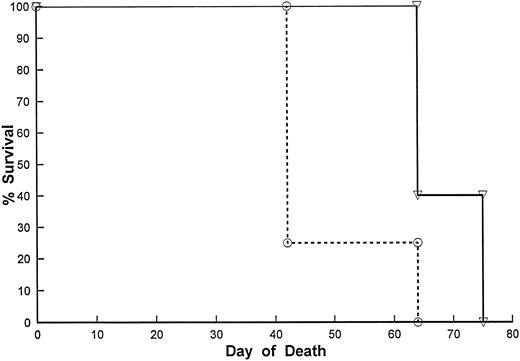
Effect of Lym-2 administration on the survival of SCID mice bearing RL lymphoma cells. SCID mice received RL human B-cell lymphomas as described in Materials and Methods. Seven days after IP tumor inoculation of 5 × 106 cells, the mice were injected with Lym-2 (▿) (2 μg), or mouse IgG1 (○) (2 μg) IP every other day for 20 days for a total of 10 injections. The mice were then monitored for tumor growth and survival. Moribund mice were euthanized when they showed evidence of extensive tumor burden. Lym-2 treatment significantly (P < .05) improved survival of RL-bearing mice compared with the isotype control.
Effect of Lym-2 administration on the survival of SCID mice bearing RL lymphoma cells. SCID mice received RL human B-cell lymphomas as described in Materials and Methods. Seven days after IP tumor inoculation of 5 × 106 cells, the mice were injected with Lym-2 (▿) (2 μg), or mouse IgG1 (○) (2 μg) IP every other day for 20 days for a total of 10 injections. The mice were then monitored for tumor growth and survival. Moribund mice were euthanized when they showed evidence of extensive tumor burden. Lym-2 treatment significantly (P < .05) improved survival of RL-bearing mice compared with the isotype control.
Effect of chCCL-1 on Survival in Mice Bearing Human Lymphoma
| Experiment Tumor . | Initiation of Therapy . | Treatment . | Mean DOD . | Range . |
|---|---|---|---|---|
| Michel (ALCL) | 3 d | msIgG1 | 31 | 30-32 |
| Lym-2 | 38 | 31-60 | ||
| Raji (Burkitt's) | 3 d | msIgG1 | 39 | 28-45 |
| Anti-CD40 | 72 | 57-91* | ||
| Lym-2 | 72 | 37-91* | ||
| RL (Diffuse large cell) | 3 d | msIgG1 | 70 | 58-81 |
| 3 d | Lym-2 | 98 | 75-220* | |
| Daudi (Burkitt's) | 3 d | msIgG1 | 37 | 34-50 |
| 3 d | chCLL-1 | 84 | 40-104* |
| Experiment Tumor . | Initiation of Therapy . | Treatment . | Mean DOD . | Range . |
|---|---|---|---|---|
| Michel (ALCL) | 3 d | msIgG1 | 31 | 30-32 |
| Lym-2 | 38 | 31-60 | ||
| Raji (Burkitt's) | 3 d | msIgG1 | 39 | 28-45 |
| Anti-CD40 | 72 | 57-91* | ||
| Lym-2 | 72 | 37-91* | ||
| RL (Diffuse large cell) | 3 d | msIgG1 | 70 | 58-81 |
| 3 d | Lym-2 | 98 | 75-220* | |
| Daudi (Burkitt's) | 3 d | msIgG1 | 37 | 34-50 |
| 3 d | chCLL-1 | 84 | 40-104* |
Mice were treated with 2 μg of Lym-2, anti-CD40, chCCL-1, or msIgG1 as described in the Materials and Methods.
Abbreviations: DOD, day of death; ALCL, anaplastic large-cell lymphoma.
P < .01.
P < .005.
Human-mouse chCLL-1 is also efficacious against human B-cell lymphomas in vivo. The chCLL-1 antibody was examined for its effects in SCID mice bearing Daudi Burkitt's lymphoma cells. Treatment with 2 μg of chCLL-1 given every other day significantly (P < .05) promoted the survival of the recipients (Table 2) indicating that the chimeric molecule still retains in vivo antitumor properties. We then compared the in vivo efficacy of Lym-2 and chCLL-1 against the RL diffuse large-cell lymphoma cell line. The results show that chCLL-1 was as efficacious as, if not more than, Lym-2 in promoting the survival of SCID mice bearing the RL B-cell lymphoma line (Fig 5). When treatment was not initiated until day 10 after tumor injection no significant effects were detected. However, the data indicate that chCLL-1 retains comparable antitumor activities with Lym-2 in vivo.
Effect of Lym-2 and ChCLL-1 treatment on the survival of RL bearing SCID mice. SCID mice received RL B cell lymphomas as described in Materials and Methods. Two days after IP tumor inoculation of 5 × 106 cells, the mice were injected with Lym-2 (▿) (2 μg), chCLL-1-γ (□) (2 μg), or mouse IgG1 (○) (2 μg) IP every other day for 20 days for a total of 10 injections. The mice were then monitored for tumor growth and survival. Lym-2 treatment and ChCLL-1 treatment significantly (P < .05) improved the survival of tumor-bearing mice compared with the isotype control. There were no significant differences in survival between Lym-2– and ChCLL-1–treated recipients.
Effect of Lym-2 and ChCLL-1 treatment on the survival of RL bearing SCID mice. SCID mice received RL B cell lymphomas as described in Materials and Methods. Two days after IP tumor inoculation of 5 × 106 cells, the mice were injected with Lym-2 (▿) (2 μg), chCLL-1-γ (□) (2 μg), or mouse IgG1 (○) (2 μg) IP every other day for 20 days for a total of 10 injections. The mice were then monitored for tumor growth and survival. Lym-2 treatment and ChCLL-1 treatment significantly (P < .05) improved the survival of tumor-bearing mice compared with the isotype control. There were no significant differences in survival between Lym-2– and ChCLL-1–treated recipients.
DISCUSSION
We report here that stimulation via Lym-2 directly inhibits the growth of a variety of human lymphoma cell lines; including diffuse large-cell, EBV-induced and Burkitt's B-cell lymphoma, as well as ALCL lines. Lym-2 was first described as an antibody against human B-lymphocytes and B-cell–derived tumors.13 Additionally, although it was characterized as a potential immunodiagnostic and immunotherapeutic reagent, there have been no reports concerning its biological effects. To be a successful therapeutic MoAb, it should possess (1) high binding reactivity with a wide spectrum of human tumors and (2) high avidity constants and a large number of antibody binding sites. The antigen recognized by Lym-2 is highly expressed on various B-lymphocyte–derived tumor cells with high tumor specificity and increased activity of binding for lymphoma cells compared with normal B-lymphocytes.13 In our study, even the ALCL lines were positive for Lym-2 and were inhibited by incubation with Lym-2. This is in contrast with other B lymphocyte-specific antibodies that are currently used for lymphomas. Thus, Lym-2 appears to have a broad spectrum of binding to leukemias and lymphomas of B-cell origin. In the present study, Lym-2 markedly improved the survival of lymphoma-bearing SCID mice. Some of these mice were disease free after 100 to 110 days without visible tumors, suggesting that a cure of the disease may be achieved (Table 2). As an isotype-matched antibody for in vivo comparison, an anti-CD40 MoAb, which has already been shown to improve the survival of the lymphoma-bearing SCID mice, was used.12 In these studies, the treatment with Lym-2 yielded comparable results as anti-CD40 (Table 2). Interestingly, although it has been shown to be directed against a human leukocyte antigen-DR (HLA-DR) variant, extensive typing has shown that Lym-2 was exclusively B-cell specific.13
Despite the large number of B-cell specific MoAbs described thus far, only few MoAbs with immunotherapeutic potential have been identified. Many of the clinical trials to date have been undertaken based on the ability of the MoAb to induce complement-mediated lysis or ADCC or by using immunoconjugated forms of MoAb.20,21,22 More recently, researchers have focused more on the signal transduction events induced by direct binding of MoAbs to target molecules on T and B cells. Although many of these molecules are involved in the growth of normal cells, stimulation of tumor cells often results in inhibition of cell growth. These physiological effector mechanisms could be potent and should be examined in detail to exploit them fully for clinical intervention. Because Lym-2 is a murine IgG1 isotype, it is most likely that, in addition to its direct inhibitory effects, ADCC may contribute to the antitumor effects of this MoAb in vivo.23
Because Lym-2 is of murine origin, a potential problem with clinical application is the occurrence of neutralizing human-antimouse antibodies. The availability of a human-mouse chimeric derivative may obviate that problem. The results showing that the chCLL-1 is as effective as, if not better, than Lym-2, indicate that the antitumor properties of Lym-2 were retained by the chimeric antibody. Therefore, these results suggest that nonconjugated MoAbs, such as the chCLL-1 may be of potential use clinically in the treatment of a variety of human B-cell lymphomas and leukemias.
Lym-1 was generated in the same period as Lym-2, and its radioimmunoconjugated form is currently used clinically for the treatment of various lymphoid malignancies with promising results.21,22 A phase I clinical trial of Lym-1 serotherapy, which only resulted in minor responses, did show a correlation between the response and the number of infiltrating T cells in biopsied lymphoma specimens.20 In our results comparing the in vitro effects of Lym-2 with Lym-1, the inhibitory effects of Lym-2 were consistently greater than those of Lym-1 (Fig 2). In addition, Lym-2 was shown to have a broader B-cell reactivity than Lym-1 because, unlike Lym-1, it is also positive with the majority of chronic lymphocytic leukemia patients.13 This suggests that Lym-2, and particularly the human-mouse chCLL-1, may find an additional role in the treatment of B-cell malignancies, even in a nonconjugated form.
We show here that immobilized Lym-2 markedly inhibited in vitro proliferation of these lymphoma cells, although incubation with soluble Lym-2 had no inhibitory effect on the lymphoma cell lines (Fig 2). Cross-linking of anti-IgM and CD40 antibodies has been shown to markedly enhance the inhibitory effect of these antibodies on the growth of human B-cell lymphomas,5,12 and it appears that Lym-2 also needs to be immobilized for optimal inhibition in vitro. However, once the human lymphomas were transferred into SCID mice, both Lym-2 and chCLL-1 showed significant antitumor effects. Because Fc receptors are expressed on various types of cells, improved crosslinking of Lym-2 and chCLL-1 may be achieved in vivo as well as the elicitation of ADCC, although the mechanism underlying the increased therapeutic efficacy seen in vivo still remains to be elucidated. These results also suggest that nonconjugated Lym-2 may be directly capable of mediating growth inhibition in vivo. Earlier studies showed that engagement of the surface antigen receptor can induce a suicide pathway in various types of transformed lymphoid cells by apoptosis,23 cell cycle arrest,9 necrosis,18 or a combination of the above.8 9 Preliminary data indicate that incubation with Lym-2 induces a percentage of lymphoma cells to undergo apoptosis (data not shown). However, the underlying mechanisms of the antitumor effects still remain unclear, because only a small percentage of cells were detected undergoing apoptosis. Experiments are ongoing to delineate further the antitumor effects of Lym-2. It will also be of interest to compare the antiproliferative effects of Lym-2 with other class II–specific antibodies, although the apparent B-cell restriction of Lym-2 makes it an attractive alternative over other pan-class II antibodies.
ACKNOWLEDGMENT
The authors gratefully acknowledge the expert technical assistance provided by Ms Kelli T. Czarra, Vasavi Reddy, and the Clinical Monitoring Laboratory. We thank Ms Laura Knott for outstanding secretarial services.
The content of the publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Institute of Health Publication No. 86-23, 1985).
Address reprint requests to William J. Murphy, PhD, Intramural Research Support Program, SAIC Frederick, NCI-FCRDC, Bldg 567, Room 210, Frederick, MD 21702-1201.

![Fig. 1. Effect of immobilized Lym-2 on the proliferation of various B-cell lymphoma cell lines. Lymphoma cell lines were incubated with Lym-2 or mIgG1 with immobilization by prior coating plates by goat-antimouse IgG1. Three days later, effects on growth were evaluated by incorporation of [3H]-thymidine. Soluble Lym-2 exerted no effect on proliferation of these lymphoma cell lines (data not shown). However, immobilized Lym-2 significantly inhibited the proliferation of (A) RL, (B) TU-2C, (C) Raji, (D) Michel, (E) Daudi, and (F ) DB cell lines (P < .05) with optimal inhibition of thymidine incorporation (39% to 65%) occurring at 1 μg/mL of Lym-2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3160/4/m_bl_0029f1.jpeg?Expires=1766165969&Signature=AneYEXJg3vovLpUvQ19i6e5rKlvShwLZGVS279OIu~KTr9NZFkSmNrmj3wMRNa1~lJvUXlBkaGHCtawz1M7-dKFl2591Mf~J4Wc6USKTAEHIkRqhuYtnkd6VeV83QJNoAvYWc7tA7hQD31wTYDUejj8sro3cs~whubshB9EIXOK3er9JSI5j30vkCep3ma0xWksTOw6jz5j14jcR~likSvpaNFCFP4ybhObfRgEzSWEyqQah63-emSfCl8e4g0bc-r2Fn22OjD2Dfh5nR5LLrl83bPk7rNvF0F4z-NAIJ1jGaEqCcXpBVo1Of-FSmLByAFt3JHEE8w-SlxY5dUbCOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal