To the Editor:
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hematopoietic disease characterized by abnormal red blood cells with complement-mediated hemolysis associated with a somatic mutation in a totipotent hematopoietic stem cell. Different cytopenias raise the question of the relation between aplastic anemia (AA) and PNH.1 The biochemical defect underlying PNH is a deficiency in the biosynthesis of the glycosylphosphatidylinositol (GPI) anchor which is caused by a somatic mutation of the PIG-A gene localized to the X chromosome. In one case of PNH, we show the efficiency of long term granulocyte colony-stimulating factor (G-CSF ) administration to correct severe neutropenia with granulocytes of the abnormal clone.
CASE REPORT
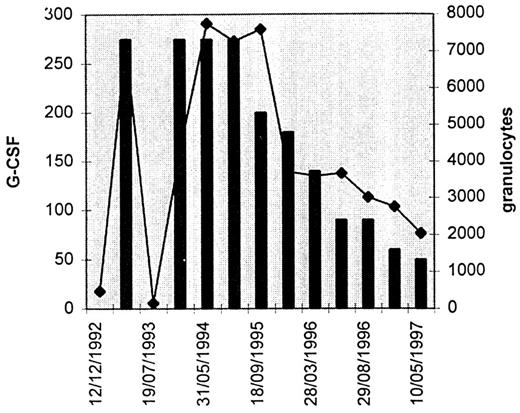
The diagnosis of PNH was established in October 1987 in a 16-year-old girl admitted for pancytopenia without splenomegalia: hemoglobin concentration was 66 g/L; mean corpuscular volume, 97 FL; reticulocyte count 130,000/μL; white blood cell (WBC) count, 2,000/μL, 29% of which were neutrophils; platelet count, 49,000/μL. Acid hemolysis test and sucrose lysis test were positive. Hemosiderinuria was detected. Bone marrow examination show no abnormal infiltration. Therapy with androgens was successful: reduced abdominal pain, episodes and anemia corrected (hemoglobin [Hb] level, 124 g/L); platelets were 243,000/μL whereas neutrophils remained below 1,000/μL. In May 1990, treatment was stopped because of virilism. From May 1990 to December 1992, minimal infectious problems resulted in hemolytic episodes and blood transfusions. At this date, while the neutrophil count decreased to less than 500/μL and a perianal abscess led to surgical treatment, the temperature was 40°C and antibiotic treatment was ineffective. On account of the severity of this infectious complication, G-CSF (Neupogen; Roche, Neuilly, France) was administred at a dose of 5 μg/kg subcutaneously once daily (275 μg). One week later, the neutrophil count was 7,000 and the patient had recovered. The growth factor treatment was then stopped. In July 1993, the neutrophil count was less than 100/μL and surgery was again necessary because of a perineal abscess. G-CSF treatment was restarted and 20 days later the neutrophil count was 2,500/μL. Karyotype control was normal. Bone marrow culture show a weak number of colony-forming unit-granulocyte macrophage (CFU-GM) colonies with a normal cluster/colony ratio. Since this date, the patient has benefitted from G-CSF treatment at a decreasing rate, which is now 60 μg per day (Fig 1). The result is dramatic: since July 1993, no blood transfusions have been required. From time to time, viral infections of the respiratory upper tracts with rhinorrhea, sneezing, and nasal congestion are observed. Rare hemolytic episodes are responsible for a moderate anemia without requiring transfusion. In January 1997, the hemoglobin level was 91 g/L, the neutrophil count was 2,750, and the platelet count 110,000. Flow cytometric studies have confirmed the absence of GPI-linked proteins: PNH affected cells represented about 50% of erythrocytes as determined using CD55 or CD59 monoclonal antibodies, 20% of lymphocytes (CD48), 95% of monocytes (CD14), and 98% of neutrophils (CD16 and CD66b). CD16 expression was observed at intermediate level whereas CD66b expression was abolished on all neutrophils. (Fig 2).
Variations in the number of granulocytes (microliters) in relation to G-CSF administered (micrograms per day). Dramatic increases in the number of granulocytes was observed when G-CSF was administered.
Variations in the number of granulocytes (microliters) in relation to G-CSF administered (micrograms per day). Dramatic increases in the number of granulocytes was observed when G-CSF was administered.
(A) Flow cytometry study of polymorphonuclear cells from a normal volunteer. Coexpression of GPI-linked proteins is detected with a CD16 monoclonal antibody (clone 3G8, P.cy5 labeled Immunotech France [Marseille], horizontal axis) and a CD66b monoclonal antibody (clone 80H3, FITC labeled Immunotech, vertical axis). (B) Flow cytometry study of polymorphonuclear cells from the patient treated with G-CSF. Normal coexpression of both CD16 and CD66b is observed in only 2% of the cells. The abnormal clone, which constitutes 98% of the population is characterized by a low expression of CD 16 without expression of CD66b.
(A) Flow cytometry study of polymorphonuclear cells from a normal volunteer. Coexpression of GPI-linked proteins is detected with a CD16 monoclonal antibody (clone 3G8, P.cy5 labeled Immunotech France [Marseille], horizontal axis) and a CD66b monoclonal antibody (clone 80H3, FITC labeled Immunotech, vertical axis). (B) Flow cytometry study of polymorphonuclear cells from the patient treated with G-CSF. Normal coexpression of both CD16 and CD66b is observed in only 2% of the cells. The abnormal clone, which constitutes 98% of the population is characterized by a low expression of CD 16 without expression of CD66b.
DISCUSSION
To our knowledge, the use of G-CSF treatment alone in typical cases of PNH over prolonged periods has not been examined. Webb and Bundtzen have reported the effect of GM-CSF on PNH.2 A 69-year-old white woman thought to have a myelodysplastic syndrome was treated with GM-CSF and had an adverse reaction resembling capillary leak syndrome. Later, the diagnosis of PNH was established. Nimomiya et al3 reported the effect of G-CSF in two patients during three clinical courses (2 μg/kg/d) with a transitory enhancement of the WBC polynuclear count. Schubert et al4 treated a female PNH patient with severe thrombocytopenia, anemia, and granulopenia with G-CSF associated with cyclosporine. A trilineage response was observed with a significant increase of normal granulocytes and monocytes within 8 weeks.4 Lastly, Jin et al5 present evidence for the emergence of GPI-positive blood cells after treatment by antithymocyte globulin and G-CSF in a case of myelodysplasia associated with an AA PNH clone. At present, despite better understanding of the biochemical defect underlaying PNH, treatment remains supportive. Bone marrow transplantation remains a candidate therapy although the risks clearly limit its usefulness. In addition, the possibility of spontaneous remission and long-term survival argues against the use of bone marrow transplantation.1 In our case, HLA studies suggested the possibility of a sibling allogenic bone marrow transplantation. For the moment, the patient refuses this option. Growth factor treatment therefore appears as an obvious alternative. However, it raises different questions concerning the risk of acute transformation or medullary depletion leading to AA. If one considers that the PNH clone is of malignant origin it is clear that G-CSF treatment poses the risk of proliferation of this clone. On the other hand, because there is no clear evidence to suggest that the PNH clone is malignant, G-CSF treatment could be considered without risk in this respect.

![Fig. 2. (A) Flow cytometry study of polymorphonuclear cells from a normal volunteer. Coexpression of GPI-linked proteins is detected with a CD16 monoclonal antibody (clone 3G8, P.cy5 labeled Immunotech France [Marseille], horizontal axis) and a CD66b monoclonal antibody (clone 80H3, FITC labeled Immunotech, vertical axis). (B) Flow cytometry study of polymorphonuclear cells from the patient treated with G-CSF. Normal coexpression of both CD16 and CD66b is observed in only 2% of the cells. The abnormal clone, which constitutes 98% of the population is characterized by a low expression of CD 16 without expression of CD66b.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2841/3/m_bl_0044f2.jpeg?Expires=1767815522&Signature=YRiK3Ffxe3fJGcxssJ52gIu6PtVY6eOKz3qCiNYU8-FicmGxL0X6jZaFmMXP7nsZkVwlaR85QbVniqUBjJJ1wQ8X8li8~NtX~0duxsuDdYfxckrpPnceY05i6wD-Hd78pZamJ5UK-pkFf-TkxIxBj3s-T4UuB5hX6uGW02Ng3fnzd4QDYeMPmeg3GLEhPo8YhTvgw5gEDTET4HonP8eILIM0qdOymRpJs3tQ~DoXmeZ9T8oD4YPt7pE-dDfp~drZiR9zBHVSsrrZ5rhcdAnbeCu1LSwgsolZgiTiwhgsobv66ao~QkWLf-yhlCnCEkajuPo8SW4iL~dJsEy5lmanIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal