Abstract
Kaposi's sarcoma–associated herpesvirus (KSHV) is a newly discovered herpes virus found in all forms of Kaposi's sarcoma (KS) including KS among immunosuppressed transplant patients. It is unknown whether this virus is transmitted by organ transplantation or is reactivated during immunosuppression among those patients infected before transplantation. To investigate the risk of KSHV transmission during organ transplantation, we conducted a case-control study of transplant recipients with and without KS matched to their respective donors. Sera were collected at time of transplantation and tested in a randomized and blinded fashion using four KSHV serologic assays testing for antibodies to both latent and lytic phase antigens. Ten (91%) of 11 organ recipients who developed KS were seropositive prior to transplantation by one or more of the assays compared with two (12%) of 17 control organ recipients (OR = 75, 95% CI = 4.7, 3500). KS cases were more likely to have been born in southern Italy where KS is endemic than the recipient controls or either donor group. Only four (36%) of 11 donors to case patients and three (18%) of 17 donors to control patients were seropositive (P = .38, two-tailed Fisher's exact test). KSHV transmission could not be ruled out for the single KS patient seronegative at transplantation and clear evidence for organ-related transmission was found for another KS patient outside of the case-control study. Antibodies to KSHV are detectable in the sera from most transplant recipients before initiation of immunosuppressive treatment suggesting that KS among immunosuppressed transplant patients is primarily due to virus reactivation. KSHV transmission, however, from an infected allograft can occur, and our study reports the first documented case of person-to-person transmission of KSHV.
A NEWLY DISCOVERED human herpes virus, Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8,1,2 is found in all forms of Kaposi's sarcoma (KS).3-8 Both nested polymerase chain reaction (PCR) examination of peripheral blood mononuclear cells9,10 and serologic studies of acquired immunodeficiency syndrome (AIDS)-KS patients11 12 show that KSHV infection precedes onset of KS, suggesting an etiologic role for KSHV in KS.
Among allograft recipients who develop KS, the appearance and development of KS lesions is highly related to immunosuppressive treatment.13-15 In contrast to other KS groups such as AIDS-KS patients, posttransplant KS patients represent a highly informative group because of strict control of initiation, duration, and extent of immunosuppression. Complete KS remission is often achieved following cessation of immunosuppressive therapy.13-15 This, together with serologic evidence showing that KS development is independent of duration of infection suggests that KSHV is under strict immunologic control.12 However, it is unknown whether KS results from KSHV reactivation in patients infected before transplantation or if de novo KSHV transmission occurs during transplantation from infected allografts. We used four serologic assays designed to detect antibodies to KSHV-related lytic and latent antigens to investigate the role of KSHV in posttransplantation KS.
MATERIALS AND METHODS
Study design and patients.We conducted a case-control study using organ transplant recipients matched to their organ donors. The case group consisted of 11 transplant recipients diagnosed with KS subsequent to transplantation and their 11 donors, all known to be clinically free of KS at time of donation. All KS specimens were confirmed by histologic examination. Seventeen controls were consecutively selected from among those recipients known to be clinically free of KS 2 years after transplantation and matched to their respective donors. Both case and control patients were referred to the Northern Italian Transplant Unit, Hospital Policlinico in Milan, Italy between January 1989 and December 1994. All subjects were human immunodeficiency virus (HIV) seronegative. One patient received a heart transplant (case pair no. 11) and the rest kidney transplants. None received immunosuppressive drugs or blood transfusions during the 5 or 2 months, respectively, preceding transplantation. Transfusion history was available for 9 of the 11 patients; 2 patients never received blood transfusions, 5 had received one to four, and 2 had received five or more lifetime blood transfusions. Immunosuppressive therapy, which included Cyclosporin A and prednisone, began at the time of transplantation. One additional transplant KS patient was recruited after completion of the case-control study (Fig 1) and was not included in the analysis.
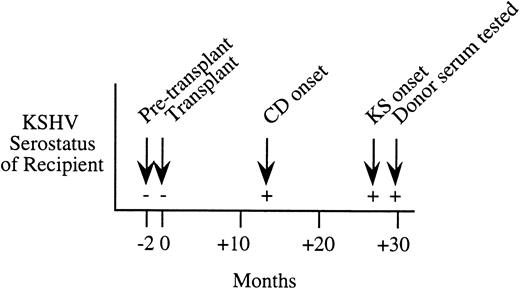
Serial serologic results for a Northern Italian female kidney transplant patient who developed KS 27 months after transplantation. This patient was KSHV seronegative before transplantation, but seroconverted at 13 months after transplantation with onset of Castleman's disease (CD). Serum from the living-related donor was seropositive at 30 months after transplantation.
Serial serologic results for a Northern Italian female kidney transplant patient who developed KS 27 months after transplantation. This patient was KSHV seronegative before transplantation, but seroconverted at 13 months after transplantation with onset of Castleman's disease (CD). Serum from the living-related donor was seropositive at 30 months after transplantation.
Cell lines and cultures.The BC-1 cell line was established from a primary effusion lymphoma (PEL) and is dually infected with KSHV and EBV.16 KSHV-positive and EBV-negative BCP-1 cells were derived from mononuclear cells of an HIV seronegative PEL patient.12 The KSHV positive, EBV negative BCBL-1 cell line was established from a PEL.17 Ramos and Raji are lymphoblastoid B-cell lines negative and positive for EBV, respectively, and are from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured with RPMI-1640 (GIBCO, BRL, Grand Island, NY) supplemented with 10% or 20% fetal calf serum.
Serologic assays.To detect the presence of antibodies to KSHV-related antigens, we used four serologic assays: immunofluorescence assay (IFA),12 western blot assay for latent nuclear antigen (WB LNA),11 immunoperoxidase assay (IPA), and western blot for recombinant open reading frame 65.2 capsid protein (WB ORF 65).18 The basis for the IFA, WB LNA, and IPA assays is antibody reactivity to a KSHV latency-associated nuclear antigen (LANA) encoded by the KSHV ORF73 gene.19 IFA was performed on KSHV infected BCP-1 cells using sera diluted at 1:160 and the reaction was revealed with a mixture of fluorescein isothiocyanate (FITC)-labeled goat antisera specific for human IgG (Molecular Probes, Eugene, OR) and human IgM (Sigma, St Louis, MO) diluted at 1:100, as previously reported.2 12
WB LNA was performed on nuclear preparations of BC-1 protein extracts as previously described.11 The sera were tested at a dilution of 1:100 and antibody reactivity was demonstrated with alkaline phosphatase-conjugated goat antiserum specific for human IgG, IgM, and IgA at a dilution of 1:5,000 and developed with NBT/BCIP (GIBCO-BRL). Serum reacting to both p226 and p234 was scored as positive.11
IPA was performed on acetone-fixed cytospins of unstimulated, KSHV-infected BCBL-1 using sera diluted at 1:10,000. The reaction was revealed by a peroxidase-labeled rabbit antiserum specific for human IgG (Dako, Glostrup, Denmark) diluted at 1:100, followed by tyramide signal amplification (DuPont/NEN, Boston, MA) and development with diaminobenzidine (DAB, Sigma). Patient sera and peroxidase-labeled rabbit antihuman IgG were diluted in 0.05M TBS (TRIS-buffered saline, pH 7.2) containing 1% bovine serum albumin (BSA) (fraction V, Sigma Chemical) and 0.001% Nonidet P-40 (Sigma Chemical). The BCBL-1 IPA is essentially identical to previously reported IFA assays12 20 and represents a technical modification of this test. All positive sera were examined by IPA using Raji and Ramos Burkitt's lymphoma cell lines. None showed evidence of nuclear staining cross-reactivity. IFA and IPA were considered positive when clear-cut nuclear staining was observed.
The KSHV open reading frame (ORF ) 65.2, was expressed in bacteria and purified using the Xpress System Protein Purification (Invitrogen Corp, San Diego, CA) and immunoblotted as previously described.18
Sera from KS patients and blood donors previously shown to be seropositive and seronegative, respectively, were included as internal controls. All sera were coded and blindly tested by IFA, WB LNA, and WB ORF 65 in New York and IPA in Milan. Results were evaluated for each assay independently before breaking the code.
Statistical analysis.Epi Info, version 6 (USD, Stone Mountain, GA) was used to calculate odds ratios and exact 95% confidence intervals (CI) and Fisher's exact test (FET) of significance of association in a 2 × 2 table.
RESULTS
Eleven organ allograft case recipients who developed KS were compared with 17 control recipients who remained free of KS for at least 2 years after transplantation. Organ recipient KS cases and controls did not differ significantly with respect to sex or mean age (Table 1); however, KS cases were more likely to have been born in Southern Italy than either the controls (OR = 13, 95% CI = 1.1, 670) or the donors supplying the allograft (P = .04, two-tailed FET). Among the organ donors, there were no significant differences with respect to age, sex, or place of birth between donors and KS patients or donors and controls. The median duration between transplantation and KS onset for cases was 7 months (range, 5 to 23 months).
Demographic Characteristics of Matched Cases and Controls
| . | Cases . | Controls . | ||
|---|---|---|---|---|
| . | Donor . | Recipient . | Donor . | Recipient . |
| . | (n = 11) . | (n = 11) . | (n = 17) . | (n = 17) . |
| Male | 7 (64%) | 6 (55%) | 12 (71%) | 10 (58.8%) |
| Mean age | 38 | 43 | 30 | 42 |
| Place of birth | ||||
| Northern Italy | 11 (100%) | 6 (55%) | 16 (94%) | 16 (94%) |
| Southern Italy | 0 | 5 (45%) | 0 | 1 (6%) |
| Non-Italian | 0 | 0 | 1 (6%) | 0 |
| KSHV seropositive* | 4 (36%) | 10 (91%) | 3 (18%) | 2 (12%) |
| . | Cases . | Controls . | ||
|---|---|---|---|---|
| . | Donor . | Recipient . | Donor . | Recipient . |
| . | (n = 11) . | (n = 11) . | (n = 17) . | (n = 17) . |
| Male | 7 (64%) | 6 (55%) | 12 (71%) | 10 (58.8%) |
| Mean age | 38 | 43 | 30 | 42 |
| Place of birth | ||||
| Northern Italy | 11 (100%) | 6 (55%) | 16 (94%) | 16 (94%) |
| Southern Italy | 0 | 5 (45%) | 0 | 1 (6%) |
| Non-Italian | 0 | 0 | 1 (6%) | 0 |
| KSHV seropositive* | 4 (36%) | 10 (91%) | 3 (18%) | 2 (12%) |
Serologic testing was performed on serum collected before organ transplantation; seropositive by one or more assays.
Serologic analyses.Ten (91%) of the 11 KS cases were KSHV positive by one or more KSHV antigen assays before receipt of the allograft compared with only two (12%) of 17 controls (OR = 75, 95% CI = 4.7, 3500). Among the donors, four (36%) of the 11 organ donors to patients who developed KS were seropositive compared with three (18%) of the 17 who donated organs to control patients (P = .38, 2-tailed FET). Eight (73%) of the eleven KS patients were positive by two or more of the assays, whereas the only one KSHV seropositive control patient was positive by two assays (WB ORF 65 and IFA) and the other was only positive by IFA. Of the four assays, 64% of the KS patients were IFA seropositive at time of transplantation compared with 73% for the WB LNA, IPA, and WB ORF 65 tests.
Only one case was potentially at risk for de novo infection from the allograft. This patient (case pair no. 9, Table 2) was seronegative at transplantation, received an organ from a donor seropositive by the IFA and IPA tests, and developed KS 3 months later. Follow-up serology was not available. The remaining three case patients who received allografts from seropositive donors (case pairs nos. 3, 5, and 6, Table 2) were seropositive before transplantation. Three control patients (control pair nos. 1, 6, and 14) were seronegative at transplantation, but received an allograft from a seropositive donor. Follow-up serology was available for one of the three, which was tested by IPA, IFA, and ORF 65 WB; 18 months after organ transplantation, this recipient was KSHV negative by all three assays (control pair no. 1). Although sera from the other two pairs are not available to determine if the individuals seroconverted to KSHV positivity, these patients have remained free of KS during 2 years of follow-up. Two additional patients (control pair nos. 5 and 17) were seropositive at transplantation, but did not develop KS for over 2 years after transplantation.
Serologic Results of Matched Cases and Controls
| Pair No. . | Donor . | Transplant KS . | Months to KS . | Pair No. . | Donor . | Transplant Control . |
|---|---|---|---|---|---|---|
| . | . | (Recipient) . | . | . | . | (Recipient) . |
| 1. | − | + | 9 | 1. | + | − |
| 2. | − | + | 7 | 2. | − | − |
| 3. | + | + | 5 | 3. | − | − |
| 4. | − | + | 6 | 4. | − | − |
| 5. | + | + | 10 | 5. | − | + |
| 6. | + | + | 12 | 6. | + | − |
| 7. | − | + | 3 | 7. | − | − |
| 8. | − | + | 3 | 8. | − | − |
| 9. | + | − | 11 | 9. | − | − |
| 10. | − | + | 23 | 10. | − | − |
| 11. | − | + | 7 | 11. | − | − |
| 12. | − | − | ||||
| 13. | − | − | ||||
| 14. | + | − | ||||
| 15. | − | − | ||||
| 16. | − | − | ||||
| 17. | − | + |
| Pair No. . | Donor . | Transplant KS . | Months to KS . | Pair No. . | Donor . | Transplant Control . |
|---|---|---|---|---|---|---|
| . | . | (Recipient) . | . | . | . | (Recipient) . |
| 1. | − | + | 9 | 1. | + | − |
| 2. | − | + | 7 | 2. | − | − |
| 3. | + | + | 5 | 3. | − | − |
| 4. | − | + | 6 | 4. | − | − |
| 5. | + | + | 10 | 5. | − | + |
| 6. | + | + | 12 | 6. | + | − |
| 7. | − | + | 3 | 7. | − | − |
| 8. | − | + | 3 | 8. | − | − |
| 9. | + | − | 11 | 9. | − | − |
| 10. | − | + | 23 | 10. | − | − |
| 11. | − | + | 7 | 11. | − | − |
| 12. | − | − | ||||
| 13. | − | − | ||||
| 14. | + | − | ||||
| 15. | − | − | ||||
| 16. | − | − | ||||
| 17. | − | + |
Serologic testing was performed on serum collected before organ transplantation; seropositive by one or more assays.
While the case-control study found only one KS patient-donor pair where primary infection from the organ donor was possible, we have subsequently identified a posttransplantation patient who was likely to have been infected during transplantation. This 24-year old female kidney transplant patient from northern Italy developed histologically confirmed Castleman's disease and KS 13 and 27 months, respectively, after receipt of the donor organ. Serum samples taken 2 months before and after transplantation show strong evidence of seroconversion occurring at the time of transplantation (see Fig 1). The patient had no history of blood transfusion before or after transplantation. While sera taken before and at transplantation were negative by IPA, IFA, and WB ORF65, the patient became persistently seropositive by IPA and WB ORF65 at 13 months after transplantation and by IFA at 27 months. The organ donor was a living-related northern Italian male who was KSHV seropositive by IPA, IFA, and WB ORF65 assays.
DISCUSSION
This is the first study to show the presence of antibodies in posttransplant KS patients and to examine the risk of KSHV transmission through organ allograft transplantation. Using four different immunoassays to detect antibodies to KSHV lytic and latent nuclear antigens, we found that 91% of Italian organ recipients who developed posttransplantation KS were KSHV seropositive before organ allograft. These results suggest that most Italian transplant patients who develop KS are infected with the virus before onset of immunosuppression, and their KS is not due to infection from the allograft.
There were four donor-recipient pairs, including one KS patient-donor pair, in which a seronegative recipient received an organ allograft from a seropositive donor. Follow-up serology was available for only one of these recipients (control pair no. 1), who was found to be KSHV seronegative. It is not possible to know whether or not the remaining three seronegative recipients became KSHV infected, although one patient subsequently developed KS and was presumably infected by the allograft. Even in this case, caution is warranted, as it is possible that the seronegativity of this patient at transplantation could have been due to test insensitivity. Because previous evaluations have found these assays to individually be 80% to 90% sensitive, we have used a combination of both latent and lytic antigen assays to evaluate seropositivity. These assays were collectively 91% sensitive in detecting KSHV infection among the transplant KS patients. Our study may have underestimated the infection rate among donors and transplant control patients due to test false negativity, although the sensitivity of the combined assays suggests that this is unlikely to significantly alter these results.
Although most KS patients were KSHV seropositive before transplantation, none showed clinical manifestations of KS until immunosuppressive therapy was begun, suggesting that posttransplant KS is caused by virus reactivation. This may be manifested as replication of the virus or, as occurs with Epstein-Barr virus,21 expression of cell transforming antigens that would otherwise be detected by immune surveillance. These findings are supported by a longitudinal examination of KSHV seroconversion rates, which suggests that development of KS may be independent of the duration of infection.12 Not all KSHV seropositive patients undergoing immunosuppression would be expected to develop KS and two of the control recipients were KSHV seropositive before transplantation, but did not develop KS during 2 years of follow-up. The rapid onset of tumor formation in KS patients (median, 7 months) after onset of immunosuppression shows the critical role of immune surveillance in preventing KS among those infected with KSHV.
It has previously been shown that the seroprevalence of KSHV antibodies to latent antigens in low risk populations is very low.12,20 Lennette et al22 used whole cells treated with phorbol esters in an IFA assay to induce lytic phase viral antigens and found higher rates of seropositivity in various low risk populations. However, use of KSHV-infected cell lines at low serum dilution (eg, 1:10) results in nonspecific cross-reactivity that can result in false-positive reactions and may account for these discordant results.12,20 This was circumvented in our study by use of IFA serum dilutions (>1:160), which eliminates nonspecific cross-reactivity12 and use of a truncated recombinant lytic antigen, which is not cross-reactive with other common herpes virus lytic antigens.18
Seroconversion for one KS patient (Fig 1), identified after completion of the case-control study, provides the first documentation of KSHV person-to-person transmission. Serial serum results strongly suggest that the patient became KSHV infected by the organ allograft, providing direct evidence that de novo infection from transplantation can occur. While our study indicated that transmission is uncommon, serologic screening of donors for KSHV infection may be necessary, especially in geographic areas with high seroprevalence of KSHV infection.
ACKNOWLEDGMENT
The authors are indebted to Yuan Chang for helpful advice and discussion and Luciana Ottoni, Katia Brambilla, Nicoletta Cagni, and Wei Zheng for their skillful technical assistance.
Supported by Public Health Service Grant No. CA67391 from the National Cancer Institute, Bethesda, MD to P.S.M., by VIII° AIDS Project (N 9306-46), Instituto Superiore di Sanità, Roma, Italy, and by Research Project ANLAIDS, Sezione Lombarda, Milano, Italy.
C.P. and S.J.O. contributed equally to this work.
Address reprint requests to Carlo Parravicini, MD, Department of Pathology, Luigi Sacco Hospital, Via G.B. Grassi 74, 20157 Milano, Italy and Patrick S. Moore, MD, MPH, P&S 14-442, Department of Pathology, Columbia University, 630 W 168 St, New York, NY 10032.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal