Abstract
Uveal melanoma is the most common intraocular malignancy in adults and results in the death of 50% of the patients. Plasminogen activators (PA) are believed to facilitate tumor metastasis by promoting invasion of tissue barriers. The present study explored the possibility of preventing the metastasis of intraocular melanomas by disrupting plasminogen activator function through gene transfer. A replication-deficient adenovirus vector was used for the in vivo transfer of plasminogen activator inhibitor type 1 (PAI-1) cDNA. Intraocular injection of an adenovirus vector (AdCMV-PAI-1) expressing plasminogen activator inhibitor-1 resulted in: (1) the transduction of more than 95% of human and murine uveal melanoma cells in the eyes of nude mice; (2) a 50% reduction in the number of animals developing liver metastases; and (3) a 78% reduction in the metastatic tumor burden in animals that eventually developed metastases. In other experiments intravenous injections of AdCMV-PAI-1 resulted in transduction of normal liver cells and culminated in a sharp reduction in the incidence of metastases and a significant prolongation of host survival. The results support the feasibility of disruption of PA function through gene transfer as a therapeutic strategy for preventing metastases and prolonging host survival.
MELANOMA OF THE uveal tract is the most common intraocular malignancy in adults with an incidence of 7 cases per million adults per year.1 The 5-year survival rate for uveal melanoma is 75% and is comparable to cutaneous melanoma.2 Unlike cutaneous melanoma which can metastasize by lymphatic and hematogenous routes to multiple organs including the lungs and lymph nodes, uveal melanoma spreads by the hematogenous route and preferentially localizes in the liver.3 Metastatic disease of the liver remains the leading cause of death in uveal melanoma patients, with up to 95% of uveal melanoma patients having liver metastases at the time of death.4 Regardless of the treatment modality, at least 30% of uveal melanoma patients will develop metastases within 10 years of successful control of the primary intraocular neoplasm.5 Thus, prevention of liver metastases is of paramount importance in the management of intraocular melanomas.
The metastatic spread of a primary tumor to distant organs involves a series of predictable processes that include tumor cell detachment from the primary tumor, migration and invasion through the basement membranes of blood and lymph vessels, embolization, arrest and binding to vascular endothelium at secondary organs, extravasation, and invasion of the secondary organ. Tumor cells are known to elaborate a variety of proteolytic enzymes such as plasminogen activators, collagenase IV, stromelysin, and various cathepsins that degrade extracellular matrices and promote intravasation and extravasation.6,7 Of the various proteases associated with tumor invasion and metastasis, plasminogen activators have received the most attention.6,7 Two types of plasminogen activators, tissue plasminogen activator (tPA) and urokinase type activator (uPA), have been implicated in the metastasis of various categories of tumors, including melanomas.8-10 In the case of human uveal melanoma, the presence of uPA on primary intraocular melanomas correlates closely with metastatic disease and poor prognosis.11 Both uPA and tPA are irreversibly inhibited by the serpin, plasminogen activator inhibitor type-1 (PAI-1).12
The feasibility of altering tumor metastasis by disrupting PA function with PAI-1 was recently demonstrated in murine models of prostate carcinoma and intraocular melanoma.13,14 In vitro transfection of human prostate carcinoma cells with human PAI-1 cDNA resulted in reduced growth of primary tumors, a partial inhibition of tumor associated angiogenesis, and reduced numbers of liver and lung metastases in athymic nude mice. Similar treatment of B16 murine melanoma cells resulted in a sharp reduction in the formation of spontaneous metastases following intraocular tumor transplantation.14 Likewise, in vitro transfection with PAI-2 cDNA inhibited spontaneous metastasis of human melanoma cells transplanted to scid mice.15 However, mice engineered to overexpress human PAI-1 in multiple organs developed pulmonary metastases at the same rate as wild-type mice in a model of B16 murine melanoma.16 Thus, it appears that PAI-1 can be effective in controlling metastases depending on the type and location of the primary tumor. The next logical step in evaluating the therapeutic feasibility of PAI-1 is to employ a tumor model in a clinically relevant setting in which PAI-1 expression is manipulated after a primary tumor has been established.
Replication-defective adenoviruses have been recognized as efficient vectors for gene transfer into a broad spectrum of cell and tissue types, both in vitro and in vivo.17 Although recombinant adenoviruses display a strong predilection for gene transfer into the liver following intravenous injection, they localize to a relatively small region following direct injection into numerous other organs.18,19 Intraocular gene transfer with adenovirus vectors has been previously reported, with gene expression persisting for more than 100 days in some cases.20-24 Adenovirus vectors are therefore attractive candidates for gene targeting in the treatment of uveal melanoma. In this study we used adenovirus-mediated PAI-1 gene transfer to inhibit PA function as a means of preventing the metastasis of human and murine uveal melanomas in a nude mouse model.
MATERIALS AND METHODS
Uveal melanoma cells.OCM-1 human uveal melanoma cell line was generously provided by Dr June Kan-Mitchell (University of California, San Diego, CA). The origin and characteristics of OCM-1 melanoma cells have been described previously.25 OCM-1 cells were cultured in Ham's F-12 medium containing 10% heat-inactivated fetal calf serum, 1% L-glutamine, 1% sodium pyruvate, 1% nonessential amino acids, 1% HEPES buffer, and 1% antibiotic-antimycotic solution.
The 99E-1 tumor cell line was derived from a choroidal/retinal pigmented epithelial ocular tumor that arose in a transgenic FVB/N mouse bearing the SV40 oncogene.26 This tumor cell line expresses SV40 T antigen, as well as melanoma-associated antigens.26 The primary tumors that developed in the original transgenic hosts were a mixture of choroidal melanomas and retinal pigment epithelial carcinomas based on characteristic morphology and their elaboration of multilaminated basement membranes.26 99E-1 cells were cultured in Dulbecco's modified Eagle's medium containing the same supplements described above.
Intraocular tumor transplantation.A modified quantitative technique for the intracameral (IC) tumor transplantation of precise numbers of tumor cells into the anterior segment of the mouse eye has been described previously.27 Mice were deeply anesthetized with 0.66 mg of ketamine hydrochloride (Vetalar; Parke, Davis, and Co, Detroit, MI) given intramuscularly. Tumor cells (105/5 μL) were inoculated into the anterior chamber using a 0.1-mL Hamilton syringe (Hamilton Co, Reno, NV) fitted with a 35-gauge glass needle. Eyes were examined two to three times per week and the tumor growth scored as described previously.27
Mice.Female athymic nude BALB/c (H-2d) mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were incorporated into experiments when 8 to 10 weeks of age.
Generation of replication defective adenovirus vectors.Recombinant adenovirus vectors containing cDNA encoding either β-galactosidase cDNA (AdCMV-βGal) or PAI-1 (AdCMV-PAI-1) were prepared as described previously.28 The plasmid for construction of the recombinant adenovirus AdCMV-PAI-1 was generated by ligating the 1.5-kb EcoRI βglII fragment of pPAI-1 RBR,29 containing the entire coding region of human PAI-1, into EcoRI BamHI digested pAC CMVpLPA to produce pAC AdCMV-PAI-1. Generation of AdCMV-PAI-1 virus by cotransfection with pJM17 into 293 cells was as previously described.28 Viruses were characterized, propagated and purified by CsCl density gradient centrifugation as described.28
In vivo gene transfer with adenovirus vector.5 × 107 plaque forming units (PFU) of either AdCMV-PAI-1 or AdCMV-βGal were inoculated either IC or intravenously (IV) into BALB/c nude mice at varying times after intraocular tumor transplantation. For IC injections, inocula containing 5 × 107 PFU in 5 μL of balanced salt solution were inoculated using the same technique used for IC tumor transplantation. For IV injections, 5 × 107 PFU of either AdCMV-PAI-1 or AdCMV-βGal were suspended in 100 μL of balanced salt solution and injected into the lateral tail veins using a 30-gauge needle mounted on a tuberculin syringe.
Histochemistry.A murine IgG1 antihuman PAI-1 monoclonal antibody (MoAb, Product #380; American Diagnostica, Greenwich, CT) was used to detect human PAI-1 expression in paraffin-embedded eyes using an immunoperoxidase procedure described elsewhere.26 For β-galactosidase staining, frozen sections of tumor-containing eyes were fixed at room temperature in 0.5% glutaraldehyde freshly prepared in phosphate-buffered saline (PBS) for 15 minutes and extensively washed in PBS. β-Galactosidase activity was detected by immersing the sections into 5-bromo-4-chloro-3-indolyl β-galactopyranoside (X-Gal) staining solution (35 mmol/L K4Fe(CN)6 /35 mmol/L K3Fe(CN)6 /1 mmol/L MgCl2 /1 mg of X-Gal per mL [Boehringer Mannheim Corp, Indianapolis, IN]) for 15 hours at 37°C.
Assessment of hepatic metastases.Human uveal melanoma displays a strong predilection to metastasize to the liver, and up to 95% of the patients who die from uveal melanoma have liver metastases.4 Both OCM-1 and 99E-1 uveal melanoma cell lines preferentially metastasize to the livers following IC transplantation in nude mice thereby mimicking the metastatic behavior of the human counterpart.26,30 Liver metastases of uveal melanomas are readily demonstrable by histopathologic examination of the liver and were scored on day 49 as previously described.31 Severity of metastases was scored as: clear (0 = no discernible foci); minimal involvement (1+ = metastatic tumors involved less than 10% of the liver); moderate (2+ = metastatic tumors involved 10% to 25% of the liver); or extensive (3+ = metastatic tumor mass involved ≥ 25% of the liver). Data were also reported as “incidence of metastases” which is defined as the percentage of animals harboring one or more liver metastases.
99E-1 tumor cells express abundant quantities of SV40 large T antigen,26 which we used as a marker for quantifying the total liver tumor burden in individual mice at day 49. Individual livers were homogenized in 0.5% Triton X-100 (Sigma Chemical Co, St Louis, MO)/0.1 mol/L Tris-HCl pH 8.0 (Sigma Chemical Co). The level of SV40 T-antigen in the supernatant of homogenized liver was measured by double monoclonal antibody (MoAb) sandwich enzyme-linked immunosorbent assay (ELISA). Fifty microliters of primary purified mouse anti-SV40 T-antigen MoAb (2 μg/mL; PAb 419; described by Harlow et al32 ) were coated onto enhanced protein binding microplates (Baxter Diagnostics Inc, McGraw Park, IL) and incubated overnight at 4°C. Each well was blocked with 200 μL of PBS containing 20% fetal bovine serum by incubating at room temperature for 2 hours. Recombinant SV40 T-antigen (Molecular Biology Resources, Milwaukee, WI) and samples were added (50 μL per well) and incubated at 37°C for 1 hour. Biotin-conjugated mouse anti-SV40 T-antigen secondary MoAb (PAb 108; PharMingen, San Diego, CA) was added (50 μL per well at 1 μg/mL) and incubated at 37°C for 30 minutes. Peroxidase-conjugated streptavidin (Jackson ImmunoResearch Lab, Inc, West Grove, PA) was added to each well (75 μL/well; at 0.5 μg/mL) and incubated at 37°C for 25 minutes. Plates were washed with PBS containing 0.05% Tween 20 (Sigma Chemical Co) between each step. Finally, the assay was developed by the addition of 100 μL of 1 mg/mL ABTS (Sigma Chemical Co) and 0.003% H2O2 at room temperature for 5 to 20 minutes. Plates were read with a Thermomax microplate reader (Molecular Devices, Menlo Park, CA) at OD 405/490 nm. Results are expressed as nanograms of SV40 T antigen/liver.
Assessment of PA secretion by tumor cell lines.The secretion of PA by tumor cells was assessed by both zymographic and chromogenic assays.14 Confluent cell cultures were washed with Hanks' balanced salt solution (HBSS) and cultured for an additional 24 hours in serum-free medium supplemented with 0.2% lactalbumin hydrolysate. Conditioned media were collected, centrifuged, and filtered through 0.2-μm filters. For zymographic analyses, samples of conditioned media (1 mg protein/mL) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in gels containing bovine fibrinogen with or without plasminogen.14 Plasminogen-free fibrinogen was used to control for plasminogen-independent fibrinolysis. After electrophoresis, gels were incubated overnight at 37°C in 100 mmol/L glycine, pH 8.0. Gels were then stained in 0.1% amido black in 10% acetic acid and 15% isopropanol for 24 hours and destained in a mixture of acetic acid (10%) and methanol (20%) for 4 hours. Lysis appeared as a transparent band against a background of darkly stained fibrinogen. Molecular weight values were determined using 50 μg of marker proteins. tPA activity was quantified by radial diffusion in fibrin-agarose (1%) clots as previously described.33 Human two-chain tPA was used as a standard and the tPA activity of the conditioned media was calculated in international units (IU) of tPA/mg protein/mL. The presence of tPA was confirmed in neutralization assays in which conditioned media were preincubated with either goat antihuman tPA or goat antihuman uPA antibody (American Diagnostica, Inc) before addition to radial diffusion assays.
PA activity was also confirmed by a chromogenic assay.34 Conditioned media and commercial preparations of uPA and tPA were incubated with human glu-plasminogen (2.5 mg mL−1) and a plasmin-specific amidolytic substrate, Spectrozyme PL (H-D-norleucyl-hexahydrotyrosyl-lysine-p-nitroanilide diacetate salt) (0.3 mmol/L), in 0.1 mol/L Tris-HCl and 0.1% Tween 80 (pH 8.0). Reactions were performed in 96-well microtiter plates at 37°C for 60 minutes, and the release of free p-nitroaniline from the chromogenic substrate was measured on a microtiter plate reader (Thermomax; Molecular Devices) at 405 nm. The activity of conditioned medium samples was determined by comparison with a standard curve generated by using serial dilutions of tPA and is expressed as IU of PA/mg protein/mL. The plasminogen dependence of PA was determined by omitting glu-plasminogen from the chromogenic assay reactions.
Quantification of serum levels of PAI-1 and PAI-1 secreted by tumor cells in vitro.Plasma samples from AdCMV-PAI-1–treated mice and –untreated mice were collected and the levels of human PAI-1 were quantified by capture ELISA35 using two MoAbs (MA-7D4 and MA-7F5) directed against different epitopes on human PAI-1.36 Purified PAI-1 (American Diagnostica Inc) was used to generate a standard curve. The detection limit of the ELISA is 50 pg PAI-1/mL. The assay detects latent, as well as active human PAI-1 and PAI-1 complex, but does not detect PAI-2.
Both tumor cell lines were examined for the presence of secreted and intracellular PAI-1. Conditioned medium was removed from cell cultures (see above) and the cell monolayers washed three times in HBSS. Cell lysates were prepared by treating the cell monolayers with 0.5% Triton X-100 in 0.1 mol/L Tris (pH 8.1), followed by centrifugation at 10,000g for 15 minutes. The supernatant was serially diluted and tested for the presence of PAI-1 using the previously described ELISA.
RESULTS
The OCM-1 and 99E-1 tumor cell lines used in these studies were selected because they arose by in situ transformation within the eyes of a human subject and a transgenic mouse, respectively. Both cell lines form tumors within the eyes of nude mice and spontaneously metastasize to the liver and thereby mimic the metastatic behavior of human uveal melanomas. In the present study, we found that OCM-1 cell cultures produced tPA (20 IU/mg conditioned culture medium) but no detectable uPA or PAI-1 (<5.0 ng/mL conditioned culture medium). 99E-1 cell cultures also produced tPA (12 IU/mg protein) and no detectable uPA or PAI-1. Thus, there is an excess of active PA present in the extracellular environment of both of these tumor cells.
The present studies were performed in nude mice, rather than immunocompetent mice, to eliminate any confounding effects of T-cell–dependent immunological responses to the human histocompatibility antigens on OCM-1 cells and the highly immunogenic SV40 large T antigen expressed by 99E-1 cells. There are also blunted immune responses to the adenovirus vector preparation, host tissues transduced by the vector and to the foreign protein products expressed by the vector.
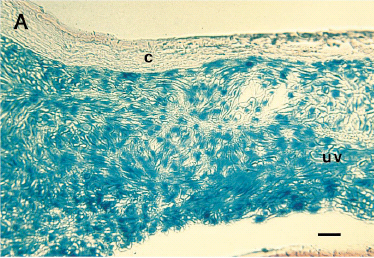
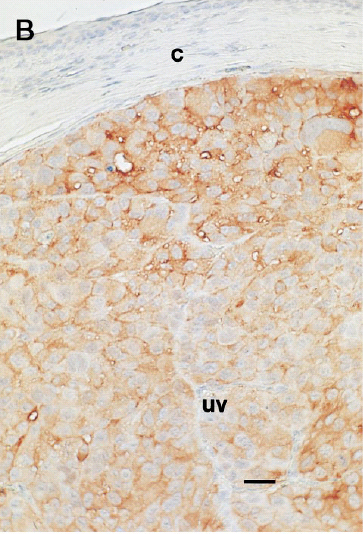
In situ transduction of intraocular melanomas.To establish that gene transfer could be achieved in the eye, recombinant adenovirus containing the beta-galactosidase reporter gene (AdCMV-βGal) was inoculated IC into intraocular melanoma-bearing mice (5 × 107 PFU/eye). Histochemical analysis of tumor-bearing eyes revealed that >95% of the murine and human intraocular melanoma cells displayed β-galactosidase activity (Fig 1). Although faint β-galactosidase staining was also observed in normal ocular tissues as previously reported by others,20-24 the most intense staining was found in the tumor. No staining was detected in the posterior regions of the eye, including the retina and choroid. The efficacy of AdCMV-PAI-1–mediated transduction was examined following IC injection of AdCMV-PAI-1 into intraocular melanoma-bearing mice on day 7 post tumor transplantation. Successful gene transfer and expression of the PAI-1 gene product was confirmed by immunohistological analysis of the intraocular tumors and revealed that >95% of the melanoma cells stained strongly with a monoclonal antibody directed against PAI-1 (Fig 1). A similar high rate of transduction was found with the 99E-1 murine uveal melanoma cell line (data not shown) and is most likely due to the high multiplicity of infection (estimated to be 10 to 100 PFU/cell) produced by the adenovirus vector preparation (data not shown). By contrast, similar immunohistological examination of OCM-1 and 99E-1 tumors that were not subjected to adenovirus transduction failed to detect constitutive expression of PAI-1 (data not shown).
Successful intraocular gene transfer using replication-deficient adenovirus vector in a nude mouse model. (A) β-Galactosidase reporter gene expression in intraocular human uveal melanomas treated in oculi with AdCMV-βGal vector. (B) PAI-1 antigen expression in human uveal melanoma treated in oculi with AdCMV-PAI-1 vector. A murine IgG1 antihuman PAI-1 MoAb was used to detect human PAI-1 expression in paraffin-embedded eyes using an immunoperoxidase procedure described elsewhere. (C) Absence of immunohistochemical staining in OCM-1 melanomas treated with AdCMV-PAI-1 and exposed to peroxidase-conjugated secondary antibody and substrate. c, cornea; uv, uveal melanoma. Bar = 80 μm.
Successful intraocular gene transfer using replication-deficient adenovirus vector in a nude mouse model. (A) β-Galactosidase reporter gene expression in intraocular human uveal melanomas treated in oculi with AdCMV-βGal vector. (B) PAI-1 antigen expression in human uveal melanoma treated in oculi with AdCMV-PAI-1 vector. A murine IgG1 antihuman PAI-1 MoAb was used to detect human PAI-1 expression in paraffin-embedded eyes using an immunoperoxidase procedure described elsewhere. (C) Absence of immunohistochemical staining in OCM-1 melanomas treated with AdCMV-PAI-1 and exposed to peroxidase-conjugated secondary antibody and substrate. c, cornea; uv, uveal melanoma. Bar = 80 μm.
Efficacy of intraocular gene transfer in preventing spontaneous metastasis of intraocular melanomas.The effect of AdCMV-PAI-1 gene transfer in preventing spontaneous metastasis of human uveal melanoma cells was evaluated by single and multiple IC inoculations of AdCMV-PAI-1. As shown in Table 1, OCM-1 human uveal melanomas and 99E-1 murine melanomas consistently produced histologically discernible metastases in approximately two-thirds of the untreated hosts at day 49 post tumor implantation. A single IC inoculation of AdCMV-PAI-1 resulted in a reduced incidence of metastases that approached but did not reach statistical significance (Table 1). However, two IC injections of AdCMV-PAI-1 produced sharp, statistically significant reductions in both the frequency and severity of liver metastases of both the human and murine uveal melanomas (Table 1). The antimetastatic effect of AdCMV-PAI-1 was not directly attributable to the adenovirus vector or virus-mediated cytolysis of tumor cells since mice treated with the AdCMV-βGal control vector were not significantly different from untreated controls.
Effect of Adenovirus-Mediated Gene Transfer on Incidence and Severity of Liver Metastases in Athymic Nude Mice Bearing Intraocular Melanomas
| Tumor . | Treatment . | Liver Metastases . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Incidence . | P . | Severity of Metastatic Foci . | P . | . | . | ||
| . | . | . | . | 1+ . | 2+ . | 3+ . | . | . | . |
| OCM-1 | None | 14/24 (58%) | — | 1 | 5 | 8 | — | ||
| OCM-1 | AdCMV-PAI-1 (1× IC)* | 6/19 (31%) | >.05† | 2 | 2 | 2 | .06‡ | ||
| OCM-1 | None | 14/19 (74%) | — | 3 | 5 | 6 | — | ||
| OCM-1 | AdCMV-βGal (2× IC) | 14/20 (70%) | >.05 | 2 | 4 | 8 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (2× IC) | 6/20 (30%) | .01 | 2 | 3 | 1 | .005 | ||
| OCM-1 | None | 7/10 (70%) | — | 2 | 1 | 4 | — | ||
| OCM-1 | AdCMV-βGal (2× IV) | 7/10 (70%) | >.05 | 2 | 2 | 3 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (2× IV) | 5/10 (50%) | >.05 | 2 | 1 | 2 | >.05 | ||
| OCM-1 | None | 13/20 (65%) | — | 3 | 2 | 8 | — | ||
| OCM-1 | AdCMV-βGal (3× IV) | 14/20 (70%) | >.05 | 2 | 6 | 6 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (3× IV) | 5/20 (25%) | .02 | 3 | 1 | 1 | .003 | ||
| 99E1 | None | 6/10 (60%) | — | 3 | 3 | 0 | — | ||
| 99E1 | AdCMV-PAI-1 (1× IC) | 3/10 (30%) | >.05 | 2 | 1 | 0 | >.05 | ||
| 99E1 | None | 14/19 (74%) | — | 3 | 2 | 9 | — | ||
| 99E1 | AdCMV-βGAL | 13/20 (65%) | >.05 | 1 | 3 | 9 | >.05 | ||
| 99E1 | AdCMV-PAI-1 (2× IC) | 6/20 (30%) | .01 | 4 | 0 | 2 | .002 | ||
| Tumor . | Treatment . | Liver Metastases . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Incidence . | P . | Severity of Metastatic Foci . | P . | . | . | ||
| . | . | . | . | 1+ . | 2+ . | 3+ . | . | . | . |
| OCM-1 | None | 14/24 (58%) | — | 1 | 5 | 8 | — | ||
| OCM-1 | AdCMV-PAI-1 (1× IC)* | 6/19 (31%) | >.05† | 2 | 2 | 2 | .06‡ | ||
| OCM-1 | None | 14/19 (74%) | — | 3 | 5 | 6 | — | ||
| OCM-1 | AdCMV-βGal (2× IC) | 14/20 (70%) | >.05 | 2 | 4 | 8 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (2× IC) | 6/20 (30%) | .01 | 2 | 3 | 1 | .005 | ||
| OCM-1 | None | 7/10 (70%) | — | 2 | 1 | 4 | — | ||
| OCM-1 | AdCMV-βGal (2× IV) | 7/10 (70%) | >.05 | 2 | 2 | 3 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (2× IV) | 5/10 (50%) | >.05 | 2 | 1 | 2 | >.05 | ||
| OCM-1 | None | 13/20 (65%) | — | 3 | 2 | 8 | — | ||
| OCM-1 | AdCMV-βGal (3× IV) | 14/20 (70%) | >.05 | 2 | 6 | 6 | >.05 | ||
| OCM-1 | AdCMV-PAI-1 (3× IV) | 5/20 (25%) | .02 | 3 | 1 | 1 | .003 | ||
| 99E1 | None | 6/10 (60%) | — | 3 | 3 | 0 | — | ||
| 99E1 | AdCMV-PAI-1 (1× IC) | 3/10 (30%) | >.05 | 2 | 1 | 0 | >.05 | ||
| 99E1 | None | 14/19 (74%) | — | 3 | 2 | 9 | — | ||
| 99E1 | AdCMV-βGAL | 13/20 (65%) | >.05 | 1 | 3 | 9 | >.05 | ||
| 99E1 | AdCMV-PAI-1 (2× IC) | 6/20 (30%) | .01 | 4 | 0 | 2 | .002 | ||
AdCMV-PAI-1 or AdCMV-βGal were inoculated IC either on day 7 or days 7 and 9. IV inocula containing either AdCMV-βGal or AdCMV-PAI-1 were administered on days 21 and 28 or on days 21, 28, and 35. Mice were euthanized on day 49 and liver metastases were evaluated histopathologically by three independent observers.
Determined by chi square analysis
Determined by Cochran-Mantel-Haenszel test
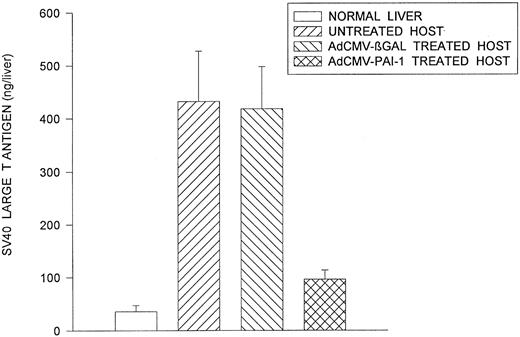
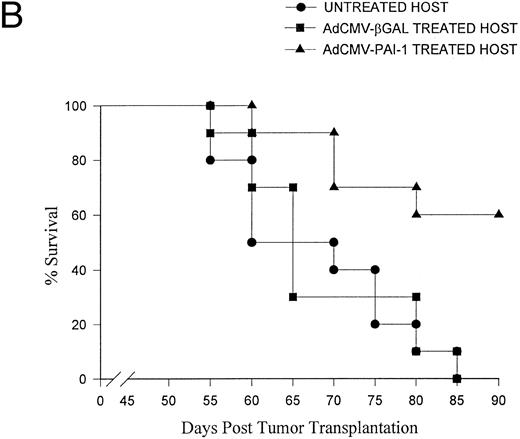
The 99E-1 murine uveal melanoma cell line was derived from a uveal tumor that arose in the eye of a transgenic FVB/n mouse bearing the SV40 oncogene.26 As a result, the 99E-1 tumor cells express abundant quantities of SV40 large T antigen26 which we used as a marker for quantifying the total liver tumor burden in individual mice. Quantitative analysis of liver metastases by capture ELISA for SV40 T antigen indicated that intraocular transfer of the PAI-1 gene produced a 78% reduction in the burden of liver metastases compared to control groups at day 49 (Fig 2). In similar experiments, two IC inoculations of AdCMV-PAI-1 resulted in a significant prolongation of host survival (Fig 3). As in the previous experiments, the antimetastatic effects of AdCMV-PAI-1 were produced by PAI-1 and not the adenovirus vector since mice treated with AdCMV-βGal were not significantly different from untreated control mice.
Reduced metastatic tumor burden in intraocular melanoma-bearing mice receiving AdCMV-PAI-1 gene transfer. 99E1 murine uveal melanoma cells were transplanted IC into BALB/c nude mice on day 0. On days 7 and 9, mice received IC inocula containing either AdCMV-PAI-1 or AdCMV-βGal. Mice were euthanized on day 49 and their livers removed. The metastatic tumor mass in the liver was quantified by a capture ELISA. Results are expressed as mean ± SEM. There were 10 animals in each group. AdCMV-PAI-1 group was significantly different (P = .001) from untreated and AdCMV-βGal–treated groups (Student's t-test).
Reduced metastatic tumor burden in intraocular melanoma-bearing mice receiving AdCMV-PAI-1 gene transfer. 99E1 murine uveal melanoma cells were transplanted IC into BALB/c nude mice on day 0. On days 7 and 9, mice received IC inocula containing either AdCMV-PAI-1 or AdCMV-βGal. Mice were euthanized on day 49 and their livers removed. The metastatic tumor mass in the liver was quantified by a capture ELISA. Results are expressed as mean ± SEM. There were 10 animals in each group. AdCMV-PAI-1 group was significantly different (P = .001) from untreated and AdCMV-βGal–treated groups (Student's t-test).
Effect of AdCMV-PAI-1 gene transfer on survival of intraocular melanoma-bearing mice. BALB/c nude mice bearing intraocular OCM-1 human uveal melanomas were treated with: (A) two IC inoculations of either AdCMV-βGal or AdCMV-PAI-1 on days 7 and 9 post IC tumor inoculation or (B) three IV inoculations of either AdCMV-PAI-1 or AdCMV-βGal on days 21, 28, and 35 post IC tumor inoculation. There were 10 mice per group. AdCMV-PAI-1 treated groups were significantly different from untreated controls in both experiments (A, P = .001 and B, P = .007) using Wilcoxon's test.
Effect of AdCMV-PAI-1 gene transfer on survival of intraocular melanoma-bearing mice. BALB/c nude mice bearing intraocular OCM-1 human uveal melanomas were treated with: (A) two IC inoculations of either AdCMV-βGal or AdCMV-PAI-1 on days 7 and 9 post IC tumor inoculation or (B) three IV inoculations of either AdCMV-PAI-1 or AdCMV-βGal on days 21, 28, and 35 post IC tumor inoculation. There were 10 mice per group. AdCMV-PAI-1 treated groups were significantly different from untreated controls in both experiments (A, P = .001 and B, P = .007) using Wilcoxon's test.
Effect of intravenous gene transfer on metastasis of intraocular melanomas.An important advantage of adenovirus-mediated gene transfer in the context of uveal melanoma is the propensity of recombinant adenovirus to target the liver following IV injection.18 19 Gene transfer into the liver was confirmed by histochemical analysis of livers of mice injected IV with AdCMV-βGal (data not shown). The antimetastatic effects of PAI-1 gene transfer were evaluated by IV injection of either AdCMV-PAI-1 or AdCMV-βGal into mice bearing either human or murine intraocular melanomas. Two and three IV injections of AdCMV-PAI-1 resulted in 28% and 66% reductions in incidence of liver metastases, respectively (Table 1). Moreover, the antimetastatic effect of IC and IV AdCMV-PAI-1 gene transfer was reflected by a significant prolongation of host survival (Fig 3).
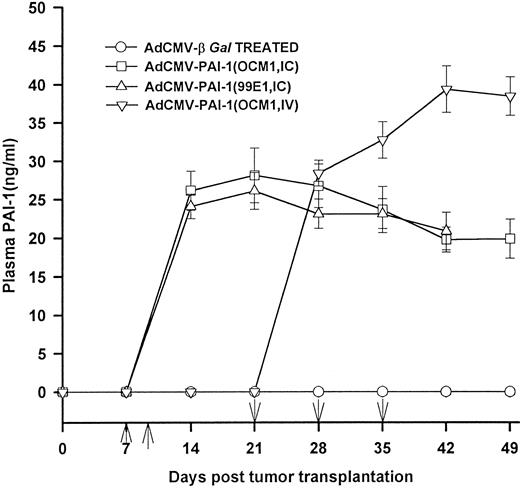
Local and systemic effects of AdCMV-PAI-1 gene transfer.Plasminogen activators play a crucial role in angiogenesis and it is possible that AdCMV-PAI-1 treatment interfered with the vascularization and growth of the primary tumors. However, this probably did not contribute to the antimetastatic effect in hosts treated by IC inoculation of AdCMV-PAI-1 since OCM-1 tumor-bearing eyes in mice treated with two IC inoculations of AdCMV-PAI-1 were not significantly smaller than untreated controls or AdCMV-βGal–treated mice at the termination of the experiment (mean weight of AdCMV-PAI-1–treated tumor eyes = 0.26 ± 0.14 g [N = 28]; mean weight of untreated tumor-bearing eyes = 0.31 ± 0.12 g [N = 28]; mean weight of AdCMV-βGal-treated eyes = 0.23 ± 0.09 g [N = 10]; P > .05). Likewise, the antimetastatic effect of intravenous gene transfer was probably not due to the effect of circulating PAI-1 on the primary intraocular tumors; there was no difference in the sizes of the primary intraocular OCM-1 tumors in mice that received AdCMV-PAI-1 given IV compared to untreated controls and mice treated with AdCMV-βGal (mean weight of AdCMV-PAI-1–treated tumor eyes = 0.28 ± 0.11 g [N = 10]; mean weight of untreated tumor-bearing eyes = 0.31 ± 0.12 g [N = 10]; mean weight of AdCMV-βGal = 0.33 ± 0.09 g [N = 10]; P > .05). In both the IV-treated and IC-treated hosts, PAI-1 might act by either impairing extravasation of blood-borne melanoma cells or by inhibiting the vascularization and subsequent growth of the tumors following metastasis to the liver since the plasma levels of PAI-1 rose sharply after either IV or IC administration of AdCMV-PAI-1 (Fig 4). Plasma PAI-1 levels remained between 25 and 40 ng/mL throughout the 49-day study period, which was markedly higher than baseline levels of murine PAI-1 (ie, 6 ng/mL).16
Human PAI-1 plasma levels in mice treated with AdCMV-PAI-1 gene transfer. BALB/c nude mice bearing intraocular OCM-1 human uveal melanomas were treated with either two IC inoculations of either AdCMV-βGal or AdCMV-PAI-1 on days 7 and 9 post IC tumor inoculation or three IV inoculations of either AdCMV-PAI-1 or AdCMV-βGal on days 21, 28, and 35 post IC tumor inoculation. BALB/c nude mice bearing intraocular 99E1 melanoma were treated with two IC injections of AdCMV-PAI-1 given on days 7 and 9. Plasma was collected and the levels of human PAI-1 were quantified by capture ELISA. Results are expressed as mean ± SEM. There were 10 animals per group. ↑ = days of IC injections; ↓ = days of IV injections
Human PAI-1 plasma levels in mice treated with AdCMV-PAI-1 gene transfer. BALB/c nude mice bearing intraocular OCM-1 human uveal melanomas were treated with either two IC inoculations of either AdCMV-βGal or AdCMV-PAI-1 on days 7 and 9 post IC tumor inoculation or three IV inoculations of either AdCMV-PAI-1 or AdCMV-βGal on days 21, 28, and 35 post IC tumor inoculation. BALB/c nude mice bearing intraocular 99E1 melanoma were treated with two IC injections of AdCMV-PAI-1 given on days 7 and 9. Plasma was collected and the levels of human PAI-1 were quantified by capture ELISA. Results are expressed as mean ± SEM. There were 10 animals per group. ↑ = days of IC injections; ↓ = days of IV injections
DISCUSSION
Plasminogen activators have been implicated in the invasion and metastasis of several categories of human tumors, including uveal melanoma.11 However, the precise role of PAI-1 in limiting metastases is unclear. Eitzman et al16 reported that transgenic mice overexpressing murine PAI-1 in multiple tissues, including the lung, were no more resistant to pulmonary metastases of B16 murine melanoma than wild-type control mice. By contrast, human prostate carcinoma cells transfected in vitro with human PAI-1 cDNA displayed reduced growth and metastasis compared to nontransfected parental tumor cells following subcutaneous transplantation in nude mice.13 In a similar study, Alizadeh et al14 transfected B16 murine melanoma cells with human PAI-1 cDNA and observed a significant reduction in metastases produced by either IV or IC tumor transplantation. The present study extends these observations and supports the feasibility of gene transfer in a clinically relevant setting. The results show the extraordinary efficiency of in situ transduction with adenovirus vectors since more than 95% of the intraocular melanoma cells expressed the transgene after IC gene transfer. Moreover, intraocular gene transfer had a demonstrable biologic effect resulting in significant reductions in both the number of animals that developed metastases and the metastatic tumor burdens in those hosts.
Results from intravenous gene transfer experiments suggest that this route offers a less traumatic and perhaps more efficient method for targeted gene therapy. Intravenous injection of AdCMV-PAI-1 resulted in significant reductions in the incidence and severity of metastatic liver lesions. More importantly, hosts treated via IV gene transfer survived significantly longer than untreated controls and mice treated with β-Gal vector. Although all of the control mice died of metastases, more than 50% of the AdCMV-PAI-1–treated hosts were alive at the termination of the experiment on day 90. Although multiple IV injections were needed to produce optimal antimetastatic effects, the virus dose was only 5 × 107 PFU and thus, approximately 40 times less than has been used by others to transfer genes into the livers of mice.19 We selected this relatively low dose for IV injections since preliminary studies indicated that this dose was highly efficient in transducing the intraocular tumors and accordingly, we wished to use the same dose for IV gene transfer so that we could compare the results from the IV injected groups with IC injected groups.
PAI-1 gene transfer might inhibit the metastatic cascade in multiple ways since plasminogen activators influence several biological processes necessary for metastasis. Although plasminogen activators play a crucial role in angiogenesis and upregulation of PAI-1 might be expected to disrupt tumor neovascularization, we did not observe any clinically perceptible differences in the overall size of the intraocular melanomas among the various treated and control groups. Moreover, we did not detect any histopathologically discernible differences in the vascularization of the intraocular tumors among the various groups. The similarity in the intraocular tumor masses in the AdCMV-PAI-1 and AdCMV-βGal treated mice also rules out the possibility that the antimetastatic effect of intraocularly delivered AdCMV-PAI-1 was due to direct cytotoxic effects of the adenovirus vector. The failure of IV administered AdCMV-βGal to affect liver metastasis development or host survival also argues against any nonspecific effects of the adenovirus vector.
Although we have no direct proof, we favor the hypothesis that in both IC-treated and IV-treated hosts, circulating PAI-1 acted to impair intravasation and extravasation of melanoma cells, respectively. This conclusion is based on the previous observation that PAI-1 can inhibit PA bound to the cellular PA receptor and thereby disrupt protease-mediated invasion of extracellular matrices.9,37 PAI-1 might also contribute to the inhibition of metastasis by blocking tumor cell binding to the extracellular matrix. Stefansson and Lawrence have recently shown that PAI-1 binds to vitronectin, a constituent of extracellular matrices, and prevents integrin-dependent cell binding and migration through artificial extracellular matrices in vitro.38 Although both IC-treated and IV-treated mice showed elevated plasma levels of PAI-1, circumstantial evidence suggests that plasma-borne PAI-1 did not inhibit spontaneous metastasis of the intraocular tumors. Eitzman et al16 showed that transgenic mice engineered to overexpress the murine PAI-1 gene displayed elevated plasma levels of PAI-1, yet were no more resistant to intravenously induced lung colonization by B16 murine melanoma than their wild-type counterparts. By contrast, the capacity of B16 melanoma cells to metastasize spontaneously from the eye is profoundly inhibited if the tumor cells are transfected in vitro with human PAI-1 cDNA before intraocular transplantation.14 In the present study, about one-third of the IC-treated hosts developed liver metastases despite elevated levels of PAI-1 in the plasma. Moreover, immunohistological examination of the liver metastases in these hosts revealed that the metastatic tumor cells failed to express detectable PAI-1 thereby suggesting that the tumor cells' capacity to invade the liver coincided with their reduced expression of PAI-1. Collectively, the present results along with previous findings suggest that PAI-1 exerts its antimetastatic effects locally rather than systemically.
Although previous investigations have evaluated the effect of PAI-1 on metastasis, several important insights have emerged from the present study. First, unlike previous studies that used in vitro PAI-1 gene transfer, this study employed in vivo gene transfer into pre-existing primary tumors and thus, represents a clinically relevant model. We are unaware of any previous studies that have shown an antimetastatic effect following adenovirus-mediated gene transfer in vivo. Second, it is clear that a high efficiency of intratumoral gene transfer can be accomplished in situ with a single injection of adenovirus vector. Third, the tumor model used in this study was transplanted orthotopically and the metastatic behavior of the metastases mimics the human counterpart. Fourth, the adenovirus vector has a predilection to transduce cells of the liver, the target organ most commonly affected by uveal melanoma metastases. Fifth, gene transfer produced significant prolongation of host survival, the most critical parameter for gauging therapeutic success in tumor-bearing hosts.
One pitfall of adenovirus-mediated gene transfer is the potential for toxicity. The livers of all of the experimental animals were examined histopathologically for evidence of hepatotoxicity. Since uveal melanoma metastases produce liver necrosis, we could not evaluate adenovirus-mediated hepatotoxicity in mice with liver metastases. However, we did not observe any remarkable inflammatory or necrotic lesions in the livers of mice treated with either AdCMV-PAI-1 or AdCMV-βGal and found to be free of microscopically discernible liver metastases. Thus, adenovirus-mediated liver toxicity, if present, was insignificant.
The prospect of ameliorating a wide variety of genetic disorders and disease conditions through gene transfer was initially heralded with great optimism that has since waned.39 However, several characteristics of uveal melanoma make it particularly amenable to targeted gene therapy with adenovirus vectors. Uveal melanomas are often well-localized and readily accessible to direct inoculation. Moreover, the immune privilege of the eye may prevent, or at least greatly delay, immune-mediated elimination of the virus or cells expressing viral antigens.40 Gene expression in retinal cells can persist for more than 100 days after intraocular injection of adenovirus vectors.24 Second, melanoma cells are readily infected by adenovirus vectors. In vitro results show that all 9 of the human uveal melanoma cell lines that we examined could be transduced with AdCMV-PAI-1 (data not shown). The predilection of AdCMV-PAI-1 to target the liver suggests that IV inoculation of patients before surgery or during other maneuvers that might exacerbate blood-borne shedding of melanoma cells might, at the very least, serve as a prophylactic adjunct to conventional modalities. Finally, it should be mentioned that despite improved surgical and radiotherapeutic procedures, no therapeutic modalities have been shown to be effective in preventing metastases or improving the 5-year survival rate of uveal melanoma patients.4 5
ACKNOWLEDGMENT
The authors thank Jessamee Mellon, Jay Cooper, and Jason Niederkorn for excellent technical assistance. Dr Desire Collen (Center for Transgene Technology and Gene Therapy, Katholieke Universiteit Leuven, Leuven, Belgium) generously provided MA-7D4 and MA-7F5 antihuman PAI-1 MoAbs used in the ELISA. Dr June Kan-Mitchell kindly provided the OCM-1 human uveal melanoma cell line. The authors appreciate statistical advice provided by Dr Qin-Chang Cheng (Academic Computing Services, UT Southwestern Medical Center, Dallas, TX).
Supported by National Institutes of Health (Bethesda, MD) Grant CA30276 and an unrestricted grant from Research to Prevent Blindness, Inc, NY.
Address reprint requests to Jerry Y. Niederkorn, PhD, Department of Ophthalmology, U.T. Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75235-9057.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal