Abstract
Binding of interferon-α (IFN-α) to its receptor on hematopoietic cells activates the signal transducers and activators of transcription (Stat)- and insulin receptor substrate (IRS)-pathways, and regulates expression of antiproliferative and antiviral activities. However, it remains unknown whether these two pathways cooperate in the generation of IFN-α responses or function independently, and whether IRS-proteins transduce distinct downstream signals in response to IFNs or insulin/insulin-like growth factor (IGF )-1–mediated activation. Our data show that in response to IFN-α treatment, IRS-1 functions selectively as a docking protein for the SH2 domains of the p85 subunit of the PI 3′-kinase, but not the SH2 domain of Grb-2 which is engaged during insulin/IGF-1 signaling. In studies with THP-1 human myelomonocytic cells and 32D mouse myeloid cells, which are IRS-defective, we found that the IFN-α–regulated activation of Stat-1, Stat-2, and Stat-3 does not require the function of the IRS-system. Furthermore, THP-1 cells are responsive to the protective effect of IFN-α against vesicular stomatitis virus. Both 32D and THP-1 cells were resistant to the growth inhibitory effect of IFN-α, but this effect was not reversible by expression of IRS-1 or IRS-2 alone in 32D cells. Taken altogether these data show that: (1) The IRS-system transduces common and distinct signals in response to IFN-α or insulin/IGF-1 stimulation of hematopoietic cells. (2) The IRS-pathway operates separately from the Stat-pathway, and its function is not essential for the generation of the antiviral effect of IFN-α. (3) Neither the IRS- nor the Stat-pathways alone are sufficient to mediate the antiproliferative effects of IFN-α in hematopoietic cells, and additional signaling elements are required.
TYPE I INTERFERONS (IFNs)1 are pleiotropic cytokines that exhibit antiviral and antiproliferative effects in normal and neoplastic cells in vitro and in vivo.1,2 Although the mechanisms of action of IFNs have been the focus of extensive investigation by several laboratories, the precise cellular events that mediate their biological effects have not been fully elucidated. However, significant advances have been made in our understanding of the early signaling steps that occur during engagement of the multisubunit Type I IFN receptor (IFNR). Two Jak kinases, Tyk-2 and Jak-1, are constitutively associated with the α and β subunits of the IFNR, respectively.3-6 Binding of IFN-α to its receptor results in activation of these kinases3,4,7-10 and tyrosine phosphorylation of the α subunit11-14 and the βL form of the β subunit.12,14 Activation of Tyk-2 and Jak-1 engages multiple proteins to transduce IFN-α signals, including signal transducers and activators of transcription (Stat)-proteins (Stat-1, Stat-2, Stat-3),15-18 insulin receptor substrate (IRS)-proteins (IRS-1, IRS-2),19,20 and the vav proto-oncogene product (p95vav ).21 The tyrosine phosphorylation of various signaling components is a common event in the signaling pathways of all Type I IFNs (α, β, ω),12,19-21 suggesting that these cytokines use similar signaling mechanisms to mediate certain biological effects. However, differences in the signaling events elicited by Type I IFNs at the receptor level also exist,12-14 suggesting that certain pathways are selectively regulated by distinct IFN subtypes.
The IRS-signaling system mediates cell growth and metabolism during insulin/IGF-1 and interleukin-4 (IL-4) stimulation,22 and is involved in SV40 large T-antigen transformation.23 The best characterized member of the family, IRS-1, contains multiple tyrosine phoshorylation sites, that during insulin stimulation are phosphorylated and act as docking sites for the SH2 domains of the p85 regulatory subunit of the PI-3′ kinase, the adaptor protein Grb-2 that links it to the Ras signaling cascade, the SHP-2 phosphatase, and the Nck protein.24-27 The recently cloned IRS-2,28 also contains multiple phosphorylation sites that function in a similar manner to IRS-1. However, some differences in the phosphorylation motifs present in the two proteins exist, suggesting that in addition to common functions, each protein may also mediate some distinct signaling events.
Our previous studies19,20 have established that IFN-α induces tyrosine phosphorylation of IRS-1 and IRS-2 in hematopoietic cells, and the activation of the lipid19 and serine29 kinase activities of the PI 3′-kinase. However, several important questions have been generated from our original observations: Do other SH2-proteins that bind to IRS-1 during insulin/IGF-1 stimulation, also bind to the protein during IFN-α stimulation? Do IRS-proteins provide a link between Jak-kinases and IFN-α–regulated Stat-proteins in hematopoietic cells? Do IRS-proteins mediate signals essential for the antiviral effects of Type I IFNs? Do defects in the expression/function of the IRS-system correlate with resistance to the growth inhibitory effects of interferons in hematopoietic cells? In the present study we used different cell-systems to address these issues. Our data strongly suggest that common and distinct sites of IRS-1 are tyrosine phosphorylated during IFN-α and insulin stimulation, as evidenced by the differential binding of various SH2-elements to the protein in response to IFN-α- or insulin-induced phosphorylation. Furthermore, our results establish that the IRS-pathway is distinct from the Stat-pathway, which appears to be the major pathway that mediates the antiviral effects of IFN-α. Our data also demonstrate that the function of the IRS-pathway alone is not sufficient to elicit the antiproliferative effect of IFN-α in hematopoietic cells.
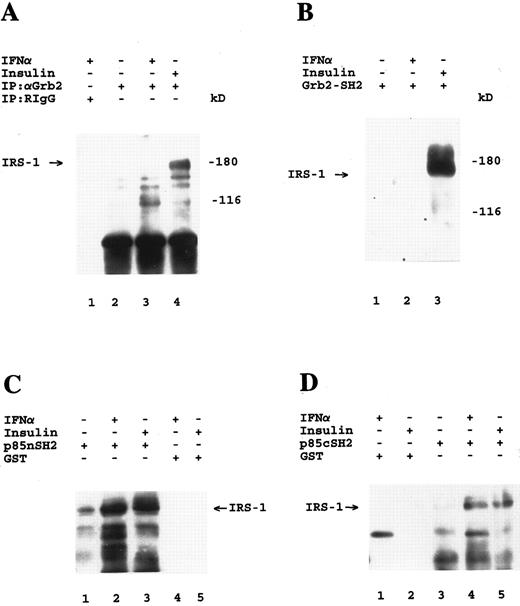
Association of Grb-2 with IRS-1 during insulin, but not IFN-α stimulation. Antiphosphotyrosine immunoblots are shown. (A) U-266 cells were incubated for 5 minutes at 37°C in the presence or absence of IFN-α or insulin as indicated. Cell lysates were immunoprecipitated with either control nonimmune rabbit Ig (RIgG) (lane 1) or a polyclonal antibody against Grb-2 (lanes 2 through 4). The identity of the 116-kD tyrosine phosphorylated protein seen in lanes 3 and 4 is unknown. (B) U-266 cells were serum-starved for 1 hour and were subsequently incubated for 5 minutes at 37°C in the absence (lane 1) or presence of IFN-α (lane 2) or insulin (lane 3). Cell lysates were bound to a GST fusion protein containing the SH2 domain of Grb-2, before SDS-PAGE analysis and immunoblotting. (C) U-266 cells were serum starved for 2 hours and were subsequently either not treated (lane 1) or treated with IFN-α (lanes 2 and 4) or insulin (lanes 3 and 5) for 5 minutes. Cell lysates were bound to either a GST fusion protein containing the nSH2 domain of p85 or to GST alone as indicated, before SDS-PAGE analysis and immunoblotting. (D) U-266 cells were serum starved for 1 hour and were subsequently either not treated (lane 3) or treated with IFN-α (lanes 1 and 4) or insulin (lanes 2 and 5) for 5 minutes. Cell lysates were bound to either a GST fusion protein containing the cSH2 domain of p85 or to GST alone as indicated, before SDS-PAGE analysis and immunoblotting.
Association of Grb-2 with IRS-1 during insulin, but not IFN-α stimulation. Antiphosphotyrosine immunoblots are shown. (A) U-266 cells were incubated for 5 minutes at 37°C in the presence or absence of IFN-α or insulin as indicated. Cell lysates were immunoprecipitated with either control nonimmune rabbit Ig (RIgG) (lane 1) or a polyclonal antibody against Grb-2 (lanes 2 through 4). The identity of the 116-kD tyrosine phosphorylated protein seen in lanes 3 and 4 is unknown. (B) U-266 cells were serum-starved for 1 hour and were subsequently incubated for 5 minutes at 37°C in the absence (lane 1) or presence of IFN-α (lane 2) or insulin (lane 3). Cell lysates were bound to a GST fusion protein containing the SH2 domain of Grb-2, before SDS-PAGE analysis and immunoblotting. (C) U-266 cells were serum starved for 2 hours and were subsequently either not treated (lane 1) or treated with IFN-α (lanes 2 and 4) or insulin (lanes 3 and 5) for 5 minutes. Cell lysates were bound to either a GST fusion protein containing the nSH2 domain of p85 or to GST alone as indicated, before SDS-PAGE analysis and immunoblotting. (D) U-266 cells were serum starved for 1 hour and were subsequently either not treated (lane 3) or treated with IFN-α (lanes 1 and 4) or insulin (lanes 2 and 5) for 5 minutes. Cell lysates were bound to either a GST fusion protein containing the cSH2 domain of p85 or to GST alone as indicated, before SDS-PAGE analysis and immunoblotting.
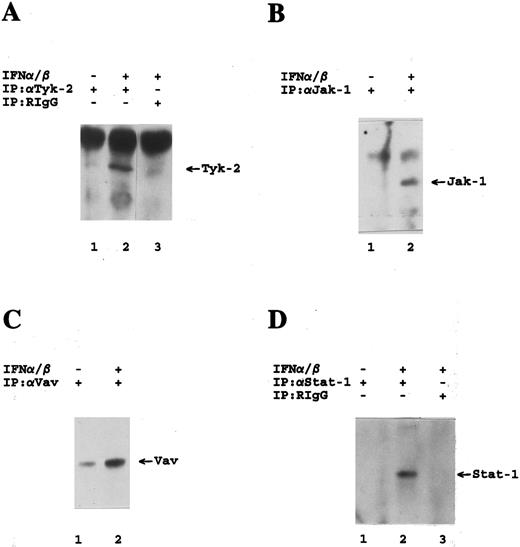
Tyrosine phosphorylation of IFN-α–signaling elements in cells lacking expression of IRS-proteins. Antiphosphotyrosine immunoblots are shown. (A) 32DIR cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with either an antibody against Tyk-2 (Santa Cruz) or nonimmune RIgG as indicated. (B) 32D cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with an antibody against Jak-1 as indicated. (C) 32D cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with an antibody against Vav as indicated. (D) 32D cells were incubated for 20 minutes at 37°C in the presence or absence or presence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with either an antibody against Stat-1 (Santa Cruz) or nonimmune RIgG as indicated.
Tyrosine phosphorylation of IFN-α–signaling elements in cells lacking expression of IRS-proteins. Antiphosphotyrosine immunoblots are shown. (A) 32DIR cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with either an antibody against Tyk-2 (Santa Cruz) or nonimmune RIgG as indicated. (B) 32D cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with an antibody against Jak-1 as indicated. (C) 32D cells were incubated for 5 minutes at 37°C in the presence or absence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with an antibody against Vav as indicated. (D) 32D cells were incubated for 20 minutes at 37°C in the presence or absence or presence of mouse IFNα/β as indicated, and cell lysates were immunoprecipitated with either an antibody against Stat-1 (Santa Cruz) or nonimmune RIgG as indicated.
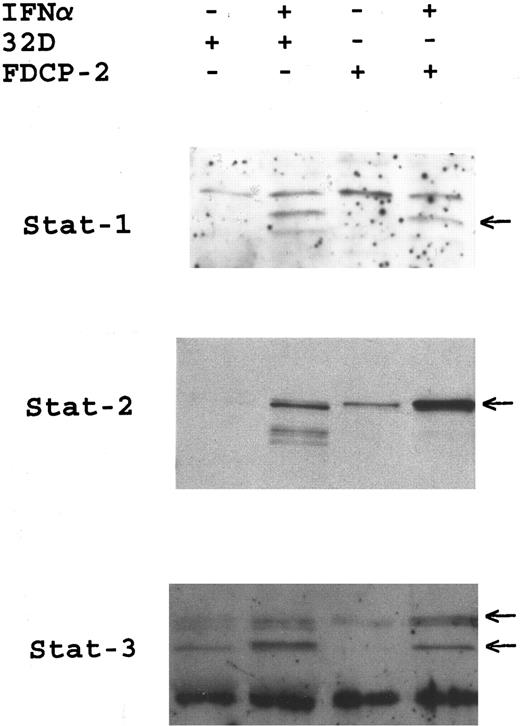
Identification of IFN-α–inducible Stat activation by GDAC. Actively growing 32D and FDCP-2 cells were incubated with or without IFNα for 15 minutes. Cytoplasmic extracts were prepared and analyzed for DNA-binding STAT complexes using GDAC. Eluates from genomic DNA were resolved by SDS-PAGE (7%) and after Western blotting, probed with antibodies to Stat-1, Stat-2, and Stat-3. IFN-α–induced Stat proteins are indicated by arrows.
Identification of IFN-α–inducible Stat activation by GDAC. Actively growing 32D and FDCP-2 cells were incubated with or without IFNα for 15 minutes. Cytoplasmic extracts were prepared and analyzed for DNA-binding STAT complexes using GDAC. Eluates from genomic DNA were resolved by SDS-PAGE (7%) and after Western blotting, probed with antibodies to Stat-1, Stat-2, and Stat-3. IFN-α–induced Stat proteins are indicated by arrows.
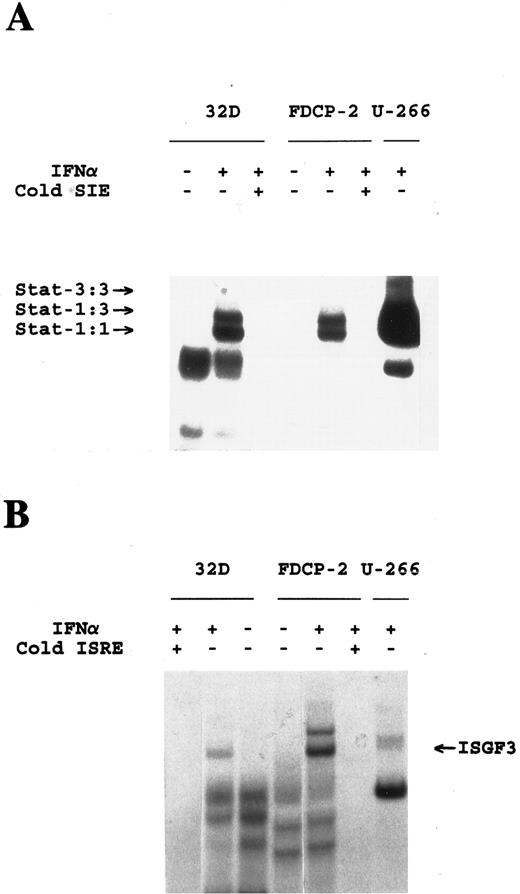
IFN-α induces Stat complexes in the absence of IRS-proteins. Cells were either left untreated or treated with IFN-α as indicated. Cytoplasmic extracts were reacted with 40,000 counts per minute (cpm) of a 32P-end–labeled SIE (A) or ISRE (B) and complexes were resolved by native gel electrophoresis and visualized by autoradiography. Specific complexes were identified by the addition of a 100-fold excess of unlabeled sis-inducible element (SIE) or ISRE to the reaction, as indicated. Composition of Stat complexes was confirmed by supershifting with the appropriate anti-Stat antibodies (data not shown).
IFN-α induces Stat complexes in the absence of IRS-proteins. Cells were either left untreated or treated with IFN-α as indicated. Cytoplasmic extracts were reacted with 40,000 counts per minute (cpm) of a 32P-end–labeled SIE (A) or ISRE (B) and complexes were resolved by native gel electrophoresis and visualized by autoradiography. Specific complexes were identified by the addition of a 100-fold excess of unlabeled sis-inducible element (SIE) or ISRE to the reaction, as indicated. Composition of Stat complexes was confirmed by supershifting with the appropriate anti-Stat antibodies (data not shown).
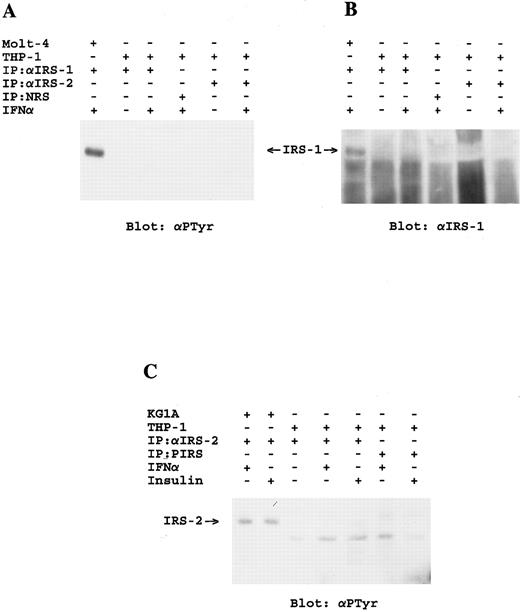
Lack of activation of the IRS-signaling system in THP-1 myelomonocytic cells. (A) Molt-4 or THP-1 cells were incubated for 5 minutes at 37°C in the presence or absence of IFN-α as indicated. Cell lysates were immunoprecipitated with the indicated antibodies and immunoblotted with antiphosphotyrosine. (B) The blot shown in (A) was stripped and reblotted with the αIRS-1CT antibody. (C) KG1A or THP-1 cells were serum starved for 2 hours, and were subsequently incubated for 10 minutes at 37°C in the presence or absence of IFN-α or insulin as indicated. Cell lysates were immunoprecipitated with either an antibody against IRS-2 or preimmune rabbit serum as indicated, analyzed by SDS-PAGE and immunoblotted with antiphosphotyrosine.
Lack of activation of the IRS-signaling system in THP-1 myelomonocytic cells. (A) Molt-4 or THP-1 cells were incubated for 5 minutes at 37°C in the presence or absence of IFN-α as indicated. Cell lysates were immunoprecipitated with the indicated antibodies and immunoblotted with antiphosphotyrosine. (B) The blot shown in (A) was stripped and reblotted with the αIRS-1CT antibody. (C) KG1A or THP-1 cells were serum starved for 2 hours, and were subsequently incubated for 10 minutes at 37°C in the presence or absence of IFN-α or insulin as indicated. Cell lysates were immunoprecipitated with either an antibody against IRS-2 or preimmune rabbit serum as indicated, analyzed by SDS-PAGE and immunoblotted with antiphosphotyrosine.
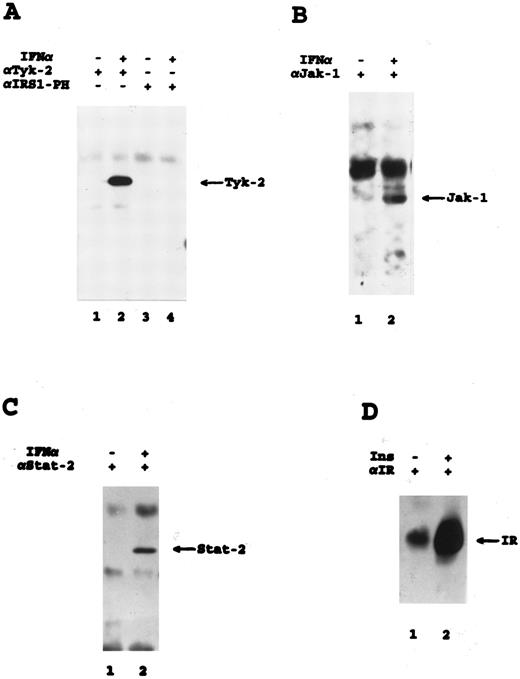
Tyrosine phosphorylation of Jak kinases, Stat-2, and the insulin receptor in THP-1 cells. Antiphosphotyrosine immunoblots are shown. (A) Cells were stimulated with IFN-α for 5 minutes as indicated, and cell lysates were immunoprecipitated with the indicated antibodies. (B) Cells were stimulated with IFN-α for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against Jak-1. (C) Cells were stimulated with IFN-α for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against Stat-2. (D) Cells were stimulated with insulin for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against the insulin receptor.
Tyrosine phosphorylation of Jak kinases, Stat-2, and the insulin receptor in THP-1 cells. Antiphosphotyrosine immunoblots are shown. (A) Cells were stimulated with IFN-α for 5 minutes as indicated, and cell lysates were immunoprecipitated with the indicated antibodies. (B) Cells were stimulated with IFN-α for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against Jak-1. (C) Cells were stimulated with IFN-α for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against Stat-2. (D) Cells were stimulated with insulin for 15 minutes as indicated, and cell lysates were immunoprecipitated with an antibody against the insulin receptor.
Replication of Vesicular Stomatitis Virus in THP-1 Cells Treated With Human IFN-α
| Treatment With IFN-α . | Titer of Virus in PFUs . | Fold Reduction . |
|---|---|---|
| Control | 4.6 × 106 | |
| IFN-α 10 U/mL | 3.1 × 106 | 0.33 |
| IFN-α 50 U/mL | 2.4 × 106 | 0.48 |
| IFN-α 100 U/mL | 1.8 × 106 | 0.61 |
| IFN-α 500 U/mL | 4.0 × 105 | 9.13 |
| IFN-α 1,000 U/mL | 8.0 × 104 | 98.26 |
| Treatment With IFN-α . | Titer of Virus in PFUs . | Fold Reduction . |
|---|---|---|
| Control | 4.6 × 106 | |
| IFN-α 10 U/mL | 3.1 × 106 | 0.33 |
| IFN-α 50 U/mL | 2.4 × 106 | 0.48 |
| IFN-α 100 U/mL | 1.8 × 106 | 0.61 |
| IFN-α 500 U/mL | 4.0 × 105 | 9.13 |
| IFN-α 1,000 U/mL | 8.0 × 104 | 98.26 |
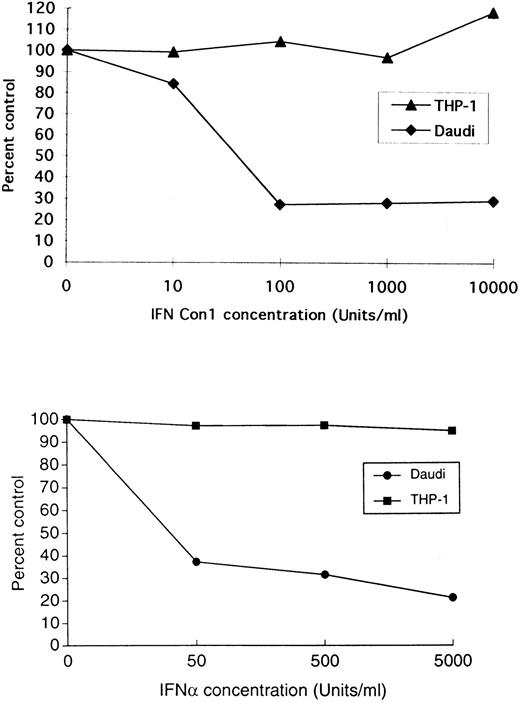
Sensitivity of THP-1 and Daudi cells to the antiproliferative effects of IFNCon1 and IFN-α. Cells were incubated with the indicated concentrations of human IFNCon1 (top panel) or human IFN-α2 (bottom panel), and proliferation was assessed by MTT as indicated in Materials and Methods.
Sensitivity of THP-1 and Daudi cells to the antiproliferative effects of IFNCon1 and IFN-α. Cells were incubated with the indicated concentrations of human IFNCon1 (top panel) or human IFN-α2 (bottom panel), and proliferation was assessed by MTT as indicated in Materials and Methods.
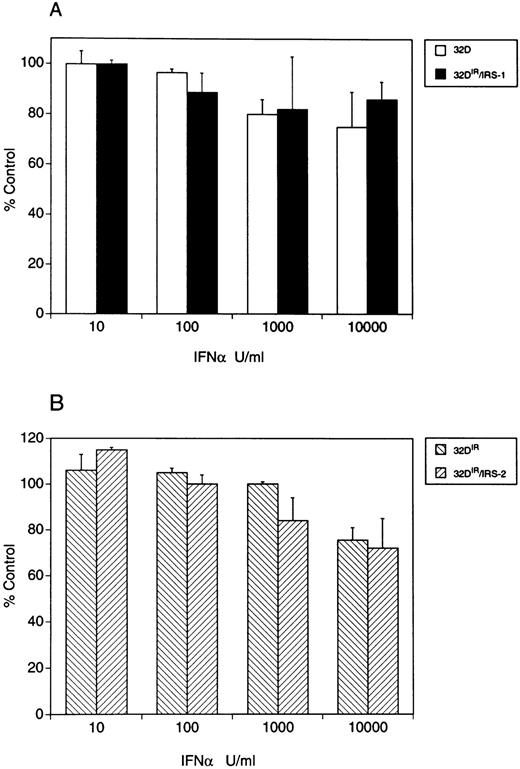
Sensitivity of 32D cells and 32D cells transfected with IRS-1 or IRS-2 cDNAs to the antiproliferative effect of mouse IFNα/β. Cells were incubated with the indicated concentrations of mouse IFNα/β and proliferation was assessed using MTT assays. Each point represents the mean ± SEM of two independent experiments for each cell line.
Sensitivity of 32D cells and 32D cells transfected with IRS-1 or IRS-2 cDNAs to the antiproliferative effect of mouse IFNα/β. Cells were incubated with the indicated concentrations of mouse IFNα/β and proliferation was assessed using MTT assays. Each point represents the mean ± SEM of two independent experiments for each cell line.
MATERIALS AND METHODS
Cells and reagents.The human Molt-4 (acute T-cell lymphocytic leukemia), Daudi (lymphoblastoid), THP-1 (myelomonocytic), and U-266 (multiple myeloma) cell lines were grown in RPMI 1640 (Life Technologies, Inc) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Life Technologies, Inc, Gaithersburg, MD) or 10% (vol/vol) defined calf serum (Hyclone Laboratories, Logan, UT) and antibiotics. The IL-3–dependent mouse myeloid 32D and FDCP-2 cell lines were grown in RPMI-10% FBS with the addition of 5% to 10% WEHI supernatant as a source for IL-3. The 32DIR (32D cells expressing the insulin receptor), 32DIR/IRS-1 and 32DIR/ IRS-2 transfectants have been described elsewhere.22,28 Human recombinant IFN-α2 was provided by Hoffmann Laroche (Nutley, NJ). Human recombinant IFNCon1 (IFN consensus) was provided by Amgen Biologicals (Thousand Oaks, CA). Mouse recombinant IFN-α was provided by Dr D. Gewert (Gemini Research Ltd, Cambridge, UK). Mouse IFN α/β was purchased from Lee and Biomolecular Research Laboratories (San Diego, CA). The antiphosphotyrosine monoclonal antibody (MoAb) (4G10) and the anti-Jak-1 antibody were obtained from Upstate Biotechnology (Lake Placid, NY). A polyclonal antibody raised against Stat-2 (p113, 186-199) has been described elsewhere.30 A polyclonal anti-Tyk-2 antibody has been raised against a synthetic peptide corresponding to the C-terminal 15 aminoacids of Tyk-2.31 A different polyclonal antibody against Tyk-2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies against Vav, Stat-1 and IRS-1, and a MoAb against Stat-3 were also purchased from Santa Cruz Biotechnology. Other polyclonal antibodies against Stat-1 and Stat-2 were provided by C. Schindler (Columbia University College of Physicians and Surgeons, New York, NY). The polyclonal anti-IRS-1PH, anti-IRS-1CT, and anti-IRS-2 antibodies have been previously described.19 28
Preparation of glutathione-S-transferase fusion proteins and binding studies.Preparation of glutathione-S-transferase fusion proteins and binding experiments with cell lysates from IFN-α- or insulin-stimulated cells were performed as previously described.19 32
Preparation of cytoplasmic cell extracts.Cytoplasmic extracts for genomic DNA affinity chromatography (GDAC) and mobility shift assays were prepared by a modification of a previously described procedure.33 Cells were washed twice with ice-cold phosphate-buffered saline containing 1 mmol/L Na3VO4 and 5 mmol/L NaF and once with hypotonic buffer. After incubation for 10 minutes in hypotonic buffer at 108 cells/mL, cells were disrupted by repeated passage through a 25-gauge needle and centrifuged at 12,000g for 20 seconds. The supernatant (cytoplasmic fraction) was supplemented to 60 mmol/L with KCl, clarified by centrifugation at 12,000g for 30 minutes, then made to 12% glycerol, and 0.05% Triton X-100 (Sigma, St Louis, MO). Cytoplasmic fractions yielded approximately 37 μg of protein/106 cells, based on the Bradford method for protein determination (Bio-Rad Labs, Mississaugua, Ontario, Canada). The fractions were aliquoted and stored at −70°C. The hypotonic buffer contains: 12 mmol/L HEPES (pH 7.9), 4 mmol/L Tris (pH 7.9), 0.6 mmol/L EDTA, 10 mmol/L KCl, 5 mmol/L MgCl2 , 1 mmol/L Na3VO4 , 1 mmol/L Na4P2O7 , 1 mmol/L NaF, 0.6 mmol/L dithiothreitol (DTT), 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 10 μg/mL aprotinin, 2 μg/mL leupeptin, and 2 μg/mL pepstatin A. This buffer containing 300 mmol/L KCl and 20% glycerol constitutes high salt buffer.
Oligonucleotides.Double-stranded oligodeoxynucleotides, representing nucleotides −107 to −87 of the human 2′ to 5′ oligoadenylate synthetase (OAS) gene, which contains a functional interferon stimulated response element (ISRE),34 a mutant form of this element, and nucleotides −127 to −109 of the human interferon regulatory factor (IRF)-1 gene, which contains a functional pIRE35 were synthesized. The sequences are, ISRE: CCTTCTGAGGCCACTAGAGCA and pIRE: CTGATTTCCCCGAAATGAC. These oligonucleotides were synthesized with Sal I compatible linkers at the 5′ terminus (TCGAC). Gel-purified oligonucleotides were mixed with their respective components, heated to 65°C for 15 minutes and annealed at room temperature for 18 hours. Double-stranded elements were used directly in competition experiments.
GDAC.GDAC was performed as previously described.36 For the studies described here, 100 μg of cytoplasmic extract from IFN-treated or -untreated cells (4 × 107 FDCP-2/32D cells, treated with 6 × 104 U murine IFN-α for 15 minutes, 2 × 107 U266 cells with IFNCon1 at 5 ng/mL for 15 minutes) were used.
Mobility shift assays.Ten micrograms of cytoplasmic extracts from untreated or IFN-α–treated cells (4 × 107 FDCP-2/32D cells treated with 6 × 104 U murine IFN-α for 15 minutes; 2 × 107 U-266 cells, treated with IFN-α (IFNCon1) at 5 ng/mL for 15 minutes), were analyzed using the electrophoretic mobility shift assay (EMSA), by a modification of the procedure described previously.37 Briefly, extracts were incubated with or without double-stranded oligodeoxynucleotides corresponding to the IRF-1 pIRE, the 2′ to 5′ OAS ISRE or a mutant ISRE, in the presence of 1 μg poly(di:dC). Poly(di:dC), in EMSA buffer for 30 minutes at room temperature (final volume 21 μL). Protein-DNA complexes were resolved on a 4.5% polyacrylamide gel electrophoresis (PAGE) using 0.2 × TBE (Tris, Borate, EDTA) as running buffer. EMSA buffer contains: 12 mmol/L HEPES (pH 7.9), 40 mmol/L KCl, 5 mmol/L MgCl2 , 0.12 mmol/L EDTA (pH 8.0), 0.06 mmol/L EGTA (pH 8.0), 0.5 mmol/L DTT, and 10% glycerol.
Cell proliferation assays.Cells were seeded in flat bottom 96-well plates at a concentration of 1 to 2.5 × 105 cells/mL in the presence or absence of the indicated concentrations of IFNs and were incubated at 37°C for 72 to 96 hours. Cell proliferation was assessed using MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenol tetrazolium bromide] assays, which were performed essentially as previously described.38
Antiviral assays.The protective effect of IFN-α on THP-1 cells from the effects of vesicular stomatitis virus (VSV) was studied using the same methodology as in previous studies.39 The multiplicity of infection of THP-1 cells with VSV was 2 plaque forming units/cell.
RESULTS
Activation of common and dictinct pathways downstream of IRS-1 in response to IFN-α or insulin in U-266 myeloma cells.We undertook experiments to determine whether IRS-1 interacts with Grb-2 during engagement of the Type I IFNR. U-266 cells were treated with IFN-α or insulin, and cell lysates were immunoprecipitated with an anti-Grb-2 polyclonal antibody and then immunoblotted with antiphosphotyrosine. A 170-kD protein, corresponding to tyrosine phosphorylated IRS-1 was clearly detectable in anti-Grb-2 immunoprecipitates from insulin- but not IFN-α–stimulated cells (Fig 1A), suggesting that Grb-2 associates with IRS-1 in vivo during insulin, but not IFN-α stimulation. Reprobing of the same blot with an anti-IRS-1 antibody confirmed that the protein corresponds to IRS-1 (data not shown). We then used a glutathione-S-transferase (GST) fusion protein containing the SH2 domain of Grb-2, to directly determine whether this domain of Grb-2 associates with IRS-1 during IFN-α stimulation. Consistent with the coimmunoprecipitation data, the SH2 domain of Grb-2 bound to tyrosine phosphorylated IRS-1 during insulin-, but not IFN-α stimulation (Fig 1B). On the other hand, in agreement with our previous findings in Daudi cells,19 the carboxy(cSH2)- and amino(nSH2)-terminal SH2 domains of the p85 subunit of the PI 3′-kinase bound to phosphorylated IRS-1 in response to either IFN-α or insulin stimulation (Fig 1C and 1D). Thus, Grb-2 selectively interacts with IRS-1 in response to insulin, but not IFN-α stimulation, suggesting that the Y895VNI motif in IRS-1 that binds the SH2 domain of this protein40 is not phosphorylated during engagement of the Type I IFNR, and represents a specific site for the insulin receptor tyrosine kinase.
The Stat- and IRS-pathways operate distinctively in IFN-α signaling in hematopoietic cells.In subsequent studies we examined whether other proteins involved in IFN-α signaling, in addition to PI 3′-kinase, use IRS-proteins to couple to the Type I IFNR. For these studies we used 32D or 32DIR cells that lack expression of IRS-1 and IRS-2.22 We first determined whether Tyk-2 and Jak-1 are activated by mouse IFNα/β in these cells. Figure 2A and B show that Tyk-2 and Jak-1 are tyrosine phosphorylated/activated during stimulation of these cells. Vav (Fig 2C), Stat-1 (Fig 2D), and Stat-3 (data not shown) were also rapidly phosphorylated on tyrosine, establishing that the SH2-docking function of IRS-1/IRS-2 is not necessary for the interaction of these proteins with upstream Jak kinases.
There is accumulating evidence that, in addition to tyrosine phosphorylation, serine phosphorylation,41 as well as the function of phospholipase A242 regulate Stat-activation. Thus, despite the fact that Stat-proteins were tyrosine phos-phorylated in cells lacking IRS-proteins, it was still possible that the Stat-pathway was not appropriately activated in these cells. To address this issue, we examined IFN-α–inducible Stat-activation in 32D cells in the context of Stat complex formation and DNA-binding activity. Cytoplasmic extracts from IFN-treated 32D cells, or the related FDCP-2 cells that express IRS-2,22 were analyzed using GDAC and the high salt eluate fractions were resolved by sodium dodecyle sulfate (SDS)-PAGE and transferred to nitrocellulose. Anti-Stat immunoblotting revealed that, after IFN treatment, cytoplasmic extracts from both cell lines contained inducible DNA-binding factors that correspond to the Stat proteins, Stat-1, Stat-2, and Stat-3 (Fig 3). To further characterize the IFN-inducible Stat-containing DNA-binding activities, we performed gel mobility shift assays, using the ISRE and pIRE recognition elements. The data show that IFN treatment of both FDCP-2 and 32D cells resulted in the rapid induction of Stat1:1 and Stat1:3 that recognize the pIRE (Fig 4A) and ISGF3, that recognizes the ISRE (Fig 4B). These findings strongly suggest that the Stat-cascade is distinct from the IRS-cascade, and thus these pathways may mediate different IFN-induced biological effects.
Cells defective in IRS-signaling maintain antiviral response to IFN-α.We next studied whether functional IRS-proteins are required for IFN-α–inducible antiviral effects. For this purpose we determined whether IFN-α exhibits a protective effect against the effects of VSV in cells lacking a functional IRS-system. Since 32D cells were relatively insensitive to VSV infection, we used the human myelomonocytic cell line, THP-1, which also lacks expression of a functional IRS-signaling system. In these cells IRS-1 and IRS-2 were not detectable in antiphosphotyrosine or anti-IRS-1 immunoblots of immunoprecipitates from IFN-α- or insulin-stimulated cells (Fig 5A through C, 6A, and data not shown). However, Tyk-2, Jak-1, Stat-2, and the β subunit of the insulin receptor were expressed and tyrosine phosphorylated (Fig 6A through D). Furthermore, the Stat-pathway is intact in these cells, as evidenced by the IFN-α–induced ISGF3 activation and formation of homodimers and heterodimers between Stat-1 and Stat-3 that bind DNA (E.N. Fish and L.C. Platanias, unpublished data, October 1996). THP-1 cells were found to be responsive to the antiviral effect of IFN-α (Table 1), although relatively high doses of IFN-α were required. Thus, a functional IRS-system is not essential for generation of IFN-α–induced antiviral responses, in contrast to the Stat-pathway whose function is required for such effects.45
Activation of more than one signaling pathways is required for the generation of IFN-α–induced antiproliferative responses.We next examined whether cells lacking expression of a functional IRS-system would respond to the antiproliferative effects of Type I IFNs. Figure 7 shows that THP-1 cells are completely refractory to the growth inhibitory effects of IFN-Con1 and IFN-α2. Consistent with previous reports, Daudi cells were sensitive to such effects (Fig 7). As the Jak-Stat pathway is intact in THP-1 cells, these findings raised the possibility that lack of a functional IRS-signaling system may account for such resistance. Similarly, when 32D cells were studied, we also observed that they are resistant to the antiproliferative effects of mouse IFNα/β (Fig 8). Thus, both cell lines that have an intact Stat-pathway but lack expression of a functional IRS-system are resistant to the antiproliferative effect of IFN-α. However, expression of IRS-1 or IRS-2 alone in 32D cells did not reverse such a resistance (Fig 8), suggesting that the functions of IRS-1 or IRS-2 alone are not sufficient to generate antiproliferative signals in these cells.
DISCUSSION
IFNs are potent regulators of hematopoietic cell proliferation and differentiation, but the precise mechanisms that mediate such effects have not been elucidated.2 The IRS-signaling system plays a critical role in signaling by the insulin and IGF-1 receptors.25,43,44,46 IRS-proteins have been previously shown to be essential for the mitogenic effects of insulin/IGF-1 and IL-4 in myeloid hematopoietic cells,22,28 suggesting that their SH2-docking function regulates cellular pathways critical for cell metabolism and growth. Although most of our knowledge on the molecular functions of IRS-proteins has been derived from studies in the insulin-signaling system, there is now accumulating evidence that these proteins participate in signaling for various other ligands. Recent studies have shown involvement of IRS-proteins in Type I IFN,19,20 growth hormone,47 leukemia inhibitory factor,47 IL-2,48 IL-7,48 IL-15,48 IL-9,49 and IL-1350 signal transduction. In addition, there is evidence suggesting that tyrosine phosphorylation of IRS-proteins by receptors that lack intrinsic tyrosine kinase activities is regulated by members of the Jak family of kinases, including Tyk-2,20 as well as Jak-1, Jak-2, and Jak-3.49
The multiplicity of pathways in which IRS-proteins appear to be involved has complicated our understanding on the function of the IRS-system. To clarify the role that the IRS-system plays in the regulation of various cellular functions, it is necessary to characterize the pathways activated downstream of IRS-proteins in response to different cytokines. The activation of the IRS-system by interferons is of particular interest, since in contrast to many other cytokines that engage IRS-proteins, IFNs exhibit antiviral and growth inhibitory activities.
In the present study we sought to determine whether IFN-α–induced IRS-1 phosphorylation leads to association with Grb-2, which, in the case of insulin, provides a link to the Ras signaling cascade. We also sought to determine whether the function of the IRS-system is required for engagement of Stat-proteins and p95vav in IFN signaling, and whether this system participates in the generation of signals that mediate the antiviral and antiproliferative effects of IFNs. Our data show that Grb-2 does not interact with IRS-1 during IFNα stimulation of IFN-α–sensitive U-266 cells, despite the fact that IRS-1 is phosphorylated on tyrosine residues and interacts with the PI-3′ kinase. Apparently, distinct kinase activities are induced by insulin and IFN-α, which likely phosphorylate IRS-1 on different tyrosine residues. Taken together, these results suggest that IRS-1 exhibits signaling specificity, which is determined by the kinase phosphorylating it.
Our studies also show that in cells lacking a functional IRS-system, SH2-containing Stat-proteins and the Vav proto-oncogene are rapidly phosphorylated on tyrosine in an IFN-dependent manner. Furthermore, mature DNA-binding complexes are formed, establishing that Stat-proteins do not require the IRS-system for their activation. This finding clearly documents that, although both the Stat- and IRS-pathways require upstream Jak kinases for their engagement in IFN-α signaling, they operate distinctively. Such a conclusion is further supported by the fact that IFN-α inhibits the replication of VSV in the IRS-defective THP-1 cells. Thus, the function of IRS-proteins is not essential for the generation of signals that mediate the antiviral effects of IFN-α. However, it is still possible that IRS-proteins contribute in part to the induction of such effects, as relatively high doses of IFN-α were required to induce antiviral responses in THP-1 cells.
We also determined whether IRS-defective cells respond to the antiproliferative effects of IFNs. THP-1 cells were refractory to treatment with Type I IFNs, and in a similar manner 32D cells did not respond to treatment with mouse IFNα/β. Interestingly, both of these cell lines exhibit a functional Stat-pathway. Thus, in cells with a defective IRS-system but an intact Stat-pathway, IFNs do not exhibit a growth inhibitory effect. However, expression of either IRS-1 or IRS-2 in 32D cells did not restore sensitivity. Based on these data it appears that IRS-proteins do not mediate the antiproliferative effect of IFN-α in hematopoietic cells. However, we cannot exclude the possibility that the IRS-system may participate in the generation of such responses, but additional elements that are defective in 32D cells are also required. Recent studies have suggested that Stat-1 may be required for the inhibitory effect of IFN-α.51 However, in both IFN-α–refractory hematopoietic cell lines studied here the Stat-pathway is intact, showing that the lack of response in these cells is not due to defective Stat-1 expression or function. Interestingly, a recent report has described the existence of an additional member of the IRS-signaling family, Gab-1.52 This protein is of smaller size than IRS-1 and IRS-2, and in addition to insulin-signaling, is also engaged in signaling by the epidermal growth factor receptor.52 Studies to determine whether Gab-1 is involved in signaling by Type I IFNs are currently under way, and may help to identify the apparently complex network of interactions that mediate the antitumor activities of these cytokines.
ACKNOWLEDGMENT
We thank B. Majchrzak, A. Chamdin, and M.E. Sweet for technical assistance.
Supported by National Institutes of Health (Bethesda, MD) Grants CA73381 (to L.C.P.), DK43808 and DK38712 (to M.F.W.), GM 54709 (to O.R.C.), and by grants from the Hairy Cell Leukemia Foundation (Schaumburg, IL) (to L.C.P.) and Amgen Inc (Thousand Oaks, CA) (to E.N.F.).
Address reprint requests to Leonidas C. Platanias, MD, Section of Hematology-Oncology, The University of Illinois at Chicago, MBRB, MC-734, Room 3150, 900 S Ashland Ave, Chicago, IL 60607-7173.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal