Abstract
The present study was undertaken to assess the predictive value of pretherapeutic determinants of ara-C metabolism and proliferative activity of leukemic blasts for early response to antileukemic therapy in the setting of granulocyte-macrophage colony-stimulating factor (GM-CSF )–based priming before and during TAD-9 induction in 36 consecutive patients with de novo acute myeloid leukemia (AML). Ara-C metabolism was assessed by the activities of deoxycytidine kinase (DCK), deoxycytidine deaminase (DCD), DNA polymerase α (Poly α), and overall polymerase (overall Poly). The fraction of cells in S phase (%S phase) and thymidine kinase (TK) activity were determined as a measure of proliferative activity. Early response to therapy was defined by the percentage of leukemic blasts in the bone marrow 5 to 7 days after completion of TAD-9 with less than 5% signaling an adequate response and greater than 5% indicating an inadequate early reduction, respectively. While neither %S phase, DCK, nor overall Poly activity were predictive for early response, TK and Poly α activities were significantly higher for cases with adequate blast cell clearance. The respective median values were for TK 3.8 versus 1.85 pmol/min/mg protein (P = .012), and for Poly α 1.9 versus 0.69 pmol/min/mg protein (P = .014). An inverse relation was detected for DCD activity which was significantly lower in responding patients with a median of 0.33 nmol/min/mg protein (range, 0.0 to 29.5) as compared to a median of 5.1 nmol/min/mg protein (range, 0.11 to 8.45) in early nonresponders, (P = .009). Taking the respective median values as arbitrary cut-points for high or low enzyme activities, responders and nonresponders could be discriminated prospectively. Hence, 14 of 16 cases (88%) with DCD activities below the median of 1.56 nmol/min/mg protein responded as compared to only 3 of 14 (22%) patients with higher DCD activities (P = .0004). From the 15 patients with TK activity above the overall median of 3.2 pmol/min/mg protein, 11 cases (73%) achieved an adequate blast cell clearance while only 6 of 17 cases (35%) with lower values responded (P = .035). Similarly, 12 of 15 patients (80%) with high Poly α levels (>1.22 pmol/min/mg protein) responded to induction therapy as compared to only 5 of 14 patients (36%) with lower enzyme activities (P = .02). By logistic regression analysis of enzyme activities, DCD activity was found to be the most sensitive parameter to predict an adequate blast cell clearance (P = .032). Activities of DCD and TK were not only associated with initial response but were also found predictive for remission duration. Hence, from 11 patients with low TK levels 8 (73%) relapsed within 1 year, whereas only 2 of 11 (18%) patients with high TK activity experienced a recurrence of their disease (P = .015). Six of 9 (66%) patients with higher than median DCD levels relapsed within 1 year, whereas 10 of 14 patients (71%) with lower DCD levels had a longer remission duration (P = .085). Analysis of DCD gene expression at the mRNA level by a semi-quantitative reverse transcriptase-polymerase chain reaction method showed that a high transcription rate of the DCD gene was associated with high enzyme activities and vice versa. Hence, the observed intraindividual differences in DCD activity are a reflection of differences in gene activity and transcription rate rather than of variants in translation. Although further analyses are needed to elucidate the molecular mechanisms that determine the variation of enzyme activities in individual patients, the present study strongly suggests that pretherapeutic determination of TK and Poly α as well as of DCD allows to predict response to TAD-9 + GM-CSF induction therapy and may provide the means for the development of a risk adapted treatment strategy.
THE SUBSTANTIAL improvements in the treatment of adults with acute myeloid leukemia (AML) that were experienced during the last 15 to 20 years have increased the perspectives for long-term control and final disease eradication substantially and have changed the expectations of therapy from mere palliation to sustained control and, ultimately, cure.1 2 Although this goal is, in fact, achieved by an increasing proportion of patients after intensified induction and postremission therapy, the majority of cases still experience a relapse of their disease to which they ultimately succumb. Besides the development of more effective antileukemic regimens, the early identification of patients with different prognosis and further insights into the biology of the disease still remain major challenges to clinicians and basic scientists.
Efforts of many groups have focused on the evaluation of biologic determinants and their relevance for treatment outcome, such as the proliferative activity of leukemic blasts, their self-renewal capacity, their cytogenetic composition, and their in vitro growth characteristics.3-8 Other approaches are directed toward the analysis of cytostatic drugs with major activity in AML therapy. Besides the anthracyclines, cytosine arabinoside comprises the most effective single agent in the treatment of AML and provides the basis for the majority of currently used combinations.9,10 Because of the cell-cycle–dependent activity of ara-C, extensive investigations have evaluated the relation between blast cell proliferative activity and clinical response to ara-C, but no clear association was found.11-14 Similarly, analyses about the major determinants of ara-C metabolism in leukemic blasts led to different conclusions about their relevance for treatment outcome. While some studies showed a close correlation between the ability of blast cells to retain intracellular ara-CTP15,16 and remission duration, other investigations found a considerable overlap in ara-C metabolite formation among ara-C–responsive and –resistant patients, respectively.17 Inconsistent data also resulted from analyses of deoxycytidine kinase (DCK) activity in AML blasts which was found to be predictive for therapeutic response by several groups.18,19 In contrast, other studies suggested that some still unidentified mechanism of metabolic control rather than the amount of the active enzyme determines the level of ara-C incorporation into blast cell DNA and its final cytotoxicity.20,21 The same controversy also relates to deoxycytidine deaminase (DCD) activity, which was found to be higher in resistant patients by some investigators18,22 while this was not detected by other groups.23 24 These inconsistencies may in part be due to the heterogeneity of patient populations and the small number of cases analyzed. Furthermore, the impurity of the analyzed cell material, variations in methodology, and the application of different clinical protocols using ara-C at various schedules and doses may explain the observed discrepancies.
The interest in the evaluation of cell kinetic determinants and parameters of ara-C metabolism has recently been reemphasized by the introduction of hematopietic growth factors into AML therapy. The administration of growth factors, and granulocyte-macrophage colony-stimulating factor (GM-CSF ) in particular, before and during induction therapy aims at overcoming the cell kinetic resistance of leukemic blasts. This appproach may improve the rational of AML therapy and may lead to therapeutic strategies that are based on the biology of leukemic cells.
The recent cloning of the genes that code for the key enzymes in ara-C metabolism may support these efforts. Cloning of the DCK cDNA has allowed the investigation of DCK deficiency at the molecular level.25 First experiments on ara-C–resistant T-lymphoblast cell lines showed structural alterations of genelike point mutations and deletions as well as decreased mRNA levels.25,26 However, in samples from AML patients structural alterations in the coding region of the DCK gene were detected at a low frequency only and make it unlikely that this mechanism plays a major role in clinical resistance to ara-C.27 Mutations of genes coding for CTP synthetase do not appear to be a mechanism of resistance to ara-C either.28 A cDNA clone for human DCD has also been identified recently,29 but analyses about structural abnormalities or alterations in DCD gene expression in AML blasts have not been reported so far.
The current study was undertaken to further investigate the prognostic relevance of proliferative activity and ara-C metabolism in de novo AML and to improve the basis for the development of a risk-adapted treatment strategy. For this purpose proliferative activity was assessed by thymidine kinase (TK) activity and %S phase, while parameters of ara-C metabolism included the analysis of DCD, DCK, overall Poly and Poly α. In an approach to better understand the regulation of DCD activity at the molecular level, the amount of DCD mRNA was assessed by a semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) method and related to enzyme activity.
PATIENTS, MATERIALS, AND METHODS
Patients and treatment protocol.Consecutive patients with de novo AML who were admitted to the departments of Hematology and Oncolgy of the Universities of Göttingen and Münster between 1992 and 1996 were eligible for the present study. The diagnosis was based on French-American-British (FAB) criteria and complementary cytochemical and immunologic analyses. Furthermore, patients had to have a blast cell infiltration of 50% or more to ensure a reliable assessment of cellular determinants in leukemic cells as described below. Eligible patients underwent the TAD-9 + GM-CSF regimen as first induction cycle.30 TAD-9 comprised 100 mg/m2/d ara-C administered by continuous intravenous (IV) infusion on days 1 and 2 followed by short-term infusions of ara-C 100 mg/m2 every 12 hours on days 3- 8, 6-thioguanine 100 mg/m2 every 12 hours orally on days 3-9, and 60-minute infusions of daunorubicin 60 mg/m2/d on days 3-5. All patients also received GM-CSF at a dose of 250 μg/m2/d starting 24 hours before and continuing during induction chemotherapy until recovery of neutrophils to greater than 1,500/μL. The application of GM-CSF was part of a prospective randomized evaluation of GM-CSF during first-line therapy as carried out by the German AML Cooperative Group.31
Early response to therapy was assessed by a bone marrow (BM) examination 5 to 7 days after the end of TAD-9, ie, between days 14 and 16 after the onset of therapy. A reduction of leukemic blasts below 5% at this point of time was considered as adequate blast cell reduction whereas more than 5% residual blasts indicated an inadequate response.
Patients less than 60 years old subsequently underwent a second induction cycle comprising either TAD-9 again or high-dose ara-C (3.0 g/m2 every 12 hours by IV infusion on days 1-3) and mitoxantrone (10 mg/m2/d by IV infusion on days 3-5). Patients above the age of 60 years were only treated with a second TAD-9 cycle if they revealed persistent blasts at day 16.32
While the analysis of blast cell reduction after the first TAD-9 course served for the early assessment of blast cell sensitivity, the achievement of a complete remission (CR) comprised the clinical endpoint of induction treatment. CR was defined as the absence of leukemia-associated symptoms, a BM of normal cellularity with less than 5% blast cells, and normal peripheral blood (PB) counts with more than 1,500 granulocytes per microliter and more than 100,000 platelets per microliter, and the absence of circulating blasts and extramedullary leukemic manifestations.
After attaining a complete remission all patients received one additional course of TAD-9 for consolidation, which was followed by monthly 5-day maintenance using rotating courses of AD/AT/AC/AT/AD, etc, for 3 years. AD comprised ara-C (100 mg/m2 every 12 hours subcutaneously [s.c.] days 1-5) and daunorubicin (45 mg/m2/d 60-minute IV infusion on days 3 and 4); AT consisted of ara-C (100 mg/m2 every 12 hours s.c. days 1-5) and 6-thioguanine (100 mg/m2 every 12 hours orally on days 1-5); AC comprises ara-C (100 mg/m2 every 12 hours s.c. days 1-5) and cyclophoshamide (1g/m2 IV on day 3).30
Remission duration was defined as the period from fulfillment of CR criteria to relapse. Patients who died in first CR or underwent BM transplantation (BMT) in first CR were censored at the respective time points. Survival was calculated from the first day of chemotherapy to death.
Study conduct.Before its initiation the study was approved by the local ethic committee of the University of Göttingen. It strictly adhered to the updated version of the Helsinki Declaration. All patients gave their informed consent to participate in the current evaluation after having been informed about its purpose and investigational nature.
Cells and cell extract preparation.BM aspirates were obtained from the posterior iliac crest after local anesthesia and collected in heparinized vacutainers. Cells were subjected to Ficoll density gradient (Biochrom, Berlin, Germany) separation. 107 cells were resuspended in 100 μL 50 mmol/L Tris-HCl, pH 7.4, and lyzed by three freeze-thawn cycles. Cellular debris, and unresolved proteins were pelleted by centrifugation at 12,000g for 5 minutes at 4°C. The supernatant was assayed for enzyme activities and protein concentration.
Analysis of cellular DNA content and the percentage of cells in S phase.Cells were dispersed into a 0.5% pepsin-HCl solution (Merck, Heidelberg, Germany), drained through a nylon filter, and fixed in ice-cold 96% ethanol. For DNA analysis by flow cytometry, the samples were centrifuged again at 1,000g for 5 minutes. The pellet was resuspended in 1 to 2 mL of a 0.5% pepsin-HCl solution and stained with ethidium bromide (10 mg/1,000 mL Tris-buffer, pH 7.5) (Serva, Heidelberg, Germany) and mithramycine (25 mg/1,000 mL Tris-buffer, pH 7.5) (Serva) in combination. After 15 to 20 minutes of staining, cellular DNA content was mesured on a PAS II instrument (Partec, Münster, Germany).33
The quantitative proportion of cells in the cell-cycle phases G0/1, S, and G2 + M was determined according to Göhde with an additional correction for cell clumps.34 35
Karyotype analysis.Karyotype analysis was performed on short-term cultures of BM. Cells were incubated for 24 hours at 37°C in RPMI supplemented with 20% fetal calf serum (FCS), 100 U/mL recombinant human (rh) G-CSF (Amgen, Thousand Oaks, CA), rhGM-CSF (Behringwerke, Marburg, Germany), 100 U/mL interleukin-3 (Behringwerke), 1 U/mL erythropoietin (Boehringer, Mannheim, Germany), and 50 ng/mL stem cell factor (Genzyme, Boston, MA). Chromosome preparation and modified GAG-SSC staining was performed as described previously.36,37 The karyotypes were classified according to the International System of Chromosome Nomenclature, ISCN (ISCN 1995).38
Production and purification of the MoAb SJK 237-71 against DNA polymerase α.The hybridoma cell line SJK 237-71 was obtained from the American Type Culture Collection (Rockville, MD). As previously described in detail, SJK 237-71 hybridoma cells secrete monoclonal IgG 1 antibodies which react with DNA polymerase α without exhibiting neutralizing activity.39 Hybridoma cells were cultivated in Minimal Essential Medium (MEM) supplemented with 10% heat inactivated FCS, glutamine (300 μg/mL), and antibiotics (penicillin 60 μg/mL; streptomycin 133 μg/mL) at 37°C in a 5% CO2 atmosphere. MoAbs were isolated from the cell culture supernatants using the commercial Affi-Gel Protein A MAPS II (MoAb purification sytstem) kit (Biorad, Munich, Germany) according to the manufacturer's instruction. The concentration of DNA Polymerase α-MoAb was assessed by enzyme-linked immunosorbent assay (ELISA), using as coating antibody 31500045 (Jackson Immuno Research, Baltimore, MD), as detection antibody peroxidase-conjugated 315035003 (Jackson), o-phenylendiamine dihyrochloride (OPD) (Jackson) as substrate and mouse IgG (Sigma) as internal standard.
DNA polymerase assay.DNase activated calf thymus DNA, which served as a starter of the polymerase assay, was prepared as described by Aposhian and Kornberg.40 The DNA polymerase assay was performed according to Hammond et al,41 with the following modifications. Twenty microliters of cell extract was incubated with 4 mg protein A-Sepharose (Pharmacia, Freiburg, Germany), and 25 μL of purified MAB SJK 237-71 (0.1 mg/mL) in phosphate-buffered saline (PBS) for 2 hours at 4°C. After washing of the immobilized immunocomplexes with 1 mL PBS and 1 mL reaction buffer (5 mmol/L MgCl2 , 1 mL dithiothreitol [DTT], and 50 mmol/L Tris/HCl pH 7.4), a final volume of 100 μL was added containing 30 μg DNase-activated DNA, 10 μmol/L TTP, 10 μmol/L dCTP, 10 μmol/L dATP, 10 μmol/L dGTP, 5 mmol/L MgCl2 , 1 mmol/L DTT, 50 mmol/L Tris-HCl pH 7.4, and 1 μCi dCTP (26 Ci/mmol; Amersham Buchler, Braunschweig, Germany). The reactions were incubated for 15 minutes at 37°C. Incorporation of 3H-dCMP into DNA was determined by using DE81 filter paper (Whatman, Maidstone, UK) as previously described.42 All experiments were performed in triplicate.
Overall polymerase assay.The DNA polymerase assay was performed according to Hammond et al41 with the following modifications: a final volume of 100 μL contained 30 μg DNase-activated DNA, 250 μmol/L TTP, 250 μmol/L dGTP, 250 μmol/L dCTP, 250 μmol/L dATP, 5 mmol/L MgCl2 , 1 mmol/L DTT, 50 mmol/L Tris-HCl pH 7.4, and 1 μCi 3H-dCTP (26 Ci/mmol; Amersham Buchler). The reactions were incubated for 30 minutes at 37°C. Incorporation of 3H-dCMP intoDNA was determined by using DE 81 filter (Whatman) as previously described.42 All experiments were performed in triplicate.
Deoxycytidine deaminase assay.The analysis of DCD activity was performed accoding to Steuart and Burke22 with the following modifications: 90 μL of reaction buffer (final concentrations: 1.9 mmol/L Tris HCl pH 8.0, 60 nmol/L deoxycytidine) and deoxy (5-3H) cytidine (0.006 μCi/mL, 22 Ci/mmol) were added to 10 μL cell extract and incubated at 37°C for 60 minutes. The reaction was terminated by addition of 50 μL 1.2 mol/L trichloracetic acid and the mixture was given on a 0.5 × 7-cm column of Dowex resin (AG 50W-XA), which was subsequently washed with destilled water (2 vol). The product 3H-deoxyuridine eluted with the void volume and radioactivity was determined in an LKB liquid scintillation counter (Turku, Finland).
Thymidine and deoxycytidine kinase assays.For thymidine kinase (TK) and deoxycytidine kinase (DCK) assays,43 100 μL reaction buffer (final concentrations: 100 mmol/L Tris-HCl pH 8.0, 4.5 mmol/L adenosine triphosphate [ATP], 5 mmol/L Mg EDTA, 0.2 mmol/L 3H-TdR [5 Ci/mmol] or 0.1 mmol/L 3H-ara-C [35 Ci/mmol]), respectively, were added to 100 μL cell extract and incubated at 37°C for 1 hour. The reaction was stopped by incubation of the samples at 100°C for 2 minutes. After precipitation of the proteins by centrifugation for 3 minutes at 2,500g and 4°C, 20 μL of the clarified reaction mixture was spotted on DE81 anion exchange filter discs (Whatman), and washed twice with 0.1 mmol/L ammoniumformiat (5 mL) and H2O (5 mL), and once with 96% ethanol. Radioactivity of the phosphorylated nucleosides was counted in an LKB liquid scintillation counter. All experiments were performed in triplicate.
RNA-isolation and RT.For the preparation of cytoplasmic RNA a modified preparation method was adapted from Chomczynski and Cacchi.44 In the RT step 1.5 μg of total RNA was used in an incubation volume of 13 μL. cDNA synthesis was performed using a commercially available kit (Superscript Preamplification System; GIBCO, Eggenstein, Germany). Final concentrations of buffer, deoxynucleotides, oligo-dT primer, and RT were chosen as recommended by the manufacturer. After an incubation time of 40 minutes at 42°C the reaction was stopped by heating to 95°C for 5 minutes.
PCR.The subsequent PCR was performed using an automated thermocycler (Model 480; Perkin-Elmer, Cetus, Emeryville, CA) with 2 μL of the reaction mixture from the RT step, 2.5 U of Taq polymerase (GIBCO), 200 nmol of each specific primer, 10 nmol dNTP, 1.5 mmol/L MgCl2 and 10× buffer: 500 mmol/L KCL, 100 mmol/L Tris-HCl (pH 9.0), 1% Triton X-100, in a total volume of 50 μL. For the DCD PCR the primers 5′ ATGAATTCTTCCTGTGGGGGCTGC3′ for the sense strand and 5′ATGGATCCGGGCGGCGGTGTTCT3′ for the antisense strand (sequence kindly provided by Dr W.M. Bertling, Paul Ehrlich Institute, Langen, Germany) resulted in a 546-bp product. For the β actin PCR a 484-bp product was obtained with the primers 5′ TGACCCAGATCATGTTTGAGA3′ for the sense strand and 5′ACTCCATGCCCAGGAAGGA3′ for the antisense strand. Standard cycle conditions were 40 seconds at 95°C for denaturation of DNA, followed by an annealing reaction for 30 seconds at 60°C and an extension period of 30 seconds at 72°C. The last primer extension and the first denaturation step were extended to 5 minutes. Twenty-five cycles were usually sufficient to detect a signal in all cases of AML. Products were run on a 2% agarose gel (2% wt/vol, Nusieve) and stained with ethidium bromide.
Patient Characteristics
| No. of patients | 36 |
| BM blasts (%) | Range: 50-95 |
| Median: 90 | |
| Age (yr) | Range: 20-72 |
| Median: 48 | |
| Age > 60: 9 | |
| Sex | Male: 16 |
| Female: 20 | |
| FAB type | M1: 5 |
| M2: 9 | |
| M3: 1 | |
| M4: 14 | |
| M5: 4 | |
| M6: 2 | |
| M7: 1 |
| No. of patients | 36 |
| BM blasts (%) | Range: 50-95 |
| Median: 90 | |
| Age (yr) | Range: 20-72 |
| Median: 48 | |
| Age > 60: 9 | |
| Sex | Male: 16 |
| Female: 20 | |
| FAB type | M1: 5 |
| M2: 9 | |
| M3: 1 | |
| M4: 14 | |
| M5: 4 | |
| M6: 2 | |
| M7: 1 |
DNA sequencing.Amplified DNA was purified using the Magic TM PCR Preps Purification System (Promega, Madison, WI) according to the manufacturer's instructions. PCR products were directly sequenced by the Taq cycle sequencing method involving in parallel assay each of the specific primers and the Ready Reaction Dye Deoxy Terminator Cycle Sequencing kit (Applied Biosystems, Weiterstadt, Germany). Sequenced reaction products were analyzed on an automated DNA sequencer (Model 373A; Applied Biosystems).45
Statistical methods.Comparisons between two groups of patients with adequate (<5% blasts) and inadequate (>5% blasts) blast cell clearance after TAD-9 were made by the Wilcoxon test. The Fisher test was used to analyse contingency tables. Logistic regression analyses were performed to evaluate the prognostic impact of the different parameters taking blast cell clearance as main determinant. For all tests, P values <.05 were considered significant. Data were analyzed using the PC-statistic program (Lizenzagentur Lambda, Graz and TopSoft, Version 2.05, Hannover, Germany).
RESULTS
Pretherapeutic parameters of ara-C metabolism and proliferative activity were obtained from 36 consecutive patients with de novo AML who were admitted to the Departments of Hematology/Oncology of the University of Münster or Göttingen and underwent the GM-CSF + TAD-9 induction regimen. Three patients died during the first indution course and 33 patients were eligible for the evaluation of an adequate blast cell clearance. Another five patients were not included in the current evaluation because of a BM blast infiltration less than 50% at initial diagnosis.
Patient characteristis and response to induction therapy.Characteristics of the 36 analyzed consecutive patients that underwent the TAD-9 + GM-CSF regimen are depicted in Table 1. Thirty-three patients completed the first GM-CSF + TAD-9 induction cycle and in 19 cases (58%) an adequate blast cell reduction below 5% blasts at day 14 to 16 was achieved. Fourteen patients (42%) had an inadequate early response. Although patients with an inadequate blast cell reduction after the first TAD-9 + GM-CSF induction course had a tendency toward a lower remission rate after the completion of double induction therapy (78%) as compared to cases with an adequate early response (89%), this difference was not statistially significant (Table 2). Hence, early blast cell reduction was not predictive for the final outcome of induction treatment. However, it represents the first evaluable indication of treatment efficacy and was therefore used to assess the predictive value of the analyzed cellular determinants for the sensitivity of leukemic blasts against the applied chemotherapy.
Response of TAD-9 + GM-CSF Induction Therapy
| Early blast cell clearance | <5% | 19 (58%) |
| >5% | 14 (42%) | |
| Complete remission | <5% | 17 (89%) |
| >5% | 11 (78%) |
| Early blast cell clearance | <5% | 19 (58%) |
| >5% | 14 (42%) | |
| Complete remission | <5% | 17 (89%) |
| >5% | 11 (78%) |
Karyotypes were obtained from 24 patients. Eleven patients had no chromosomal abnormalities, 3 cases had favorable karyotypes [1 case with t(8; 21), 2 cases with inv(16)], 5 patients showed unfavorable cytogenetic aberrations [inv (3), −7, t(9; 11) and complex abnormalities (3 and more)], and 6 patients had cytogenetic findings of unknown prognostic significance [add(2), +4, del(4), (t(7; 11), del(13), del(20)]. Because of the small number of patients in the respective cytogenetic subgroups, no association between karyotype and the subsequently described cellular determinants was found.
Proliferative activity and parameters of ara-C metabolism of leukemic blasts before TAD-9 + GM-CSF induction therapy.Proliferative activity and parameters of ara-C metabolism were analyzed in 36 BM samples and the respective results are summarized in Table 3. The data reveal a substantial interpatient variability for each parameter. Median values for %S phase and TK activity as a measure of proliferative activity were 5.5% (1.8% to 16.2%) and 3.2 pmol/min/mg protein (0.0 to 22.8 pmol/min/mg protein), respectively. The corresponding values for the determinants of ara-C metabolism were: for DCK, 27.5 pmol/min/mg protein (3.8 to 196.0 pmol/min/mg protein); for DCD, 1.56 nmol/min/mg protein (0.0 to 29.5 nmol/min/mg protein); for Poly α, 1.22 pmol/min/mg protein (0.03 to 8.8 pmol/min/mg protein); and for overall Poly, 55.9 pmol/min/mg protein (17.3 to 131.0). The overall median values obtained for the whole group of analyzed patients provided the basis for further discrimination between AML specimens with high or low enzyme activities.
Pretherapeutic Parameters of Proliferative Activity and araC Metabolism
| Parameter . | n . | Median . |
|---|---|---|
| TK (pmol/min/mg protein) | 34 | 3.2 |
| S phase (%) | 26 | 5.5 |
| DCK (pmol/min/mg protein) | 35 | 27.5 |
| DCD (nmol/min/mg protein) | 35 | 1.56 |
| Overall Poly (pmol/min/mg protein) | 19 | 55.9 |
| Poly α (pmol/min/mg protein) | 32 | 1.22 |
| Parameter . | n . | Median . |
|---|---|---|
| TK (pmol/min/mg protein) | 34 | 3.2 |
| S phase (%) | 26 | 5.5 |
| DCK (pmol/min/mg protein) | 35 | 27.5 |
| DCD (nmol/min/mg protein) | 35 | 1.56 |
| Overall Poly (pmol/min/mg protein) | 19 | 55.9 |
| Poly α (pmol/min/mg protein) | 32 | 1.22 |
There was no relation between %S phase and TK activity nor between the different parameters of ara-C metabolism. Neither age nor karyotype showed any influence on enzyme activities or %S phase.
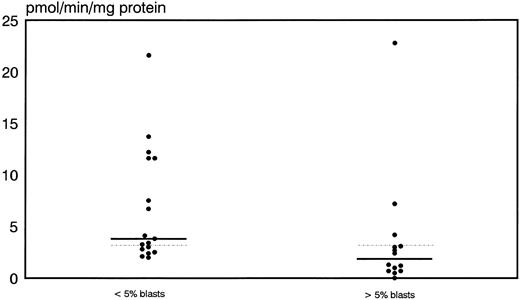
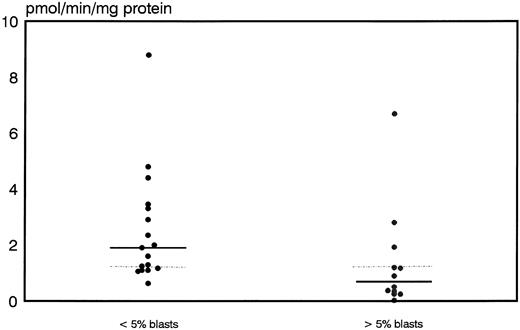
Prognostic significance of proliferative activity and determinants of ara-C metabolism for early clinical response to TAD-9 + GM-CSF induction therapy.Proliferative activity and parameters of ara-C metabolism were related to early clinical response as determined by the reduction of blast cells at day 14 to 16. High TK activity but not high %S phase was associated with an adequate blast cell clearance. Hence, patients with an adequate blast cell clearance had a substantially higher median TK activity of 3.8 pmol/min/mg protein (range, 2.0 to 21.6) compared with nonresponders revealing a median TK activity of only 1.85 pmol/min/mg protein (0.0 to 22.8) (P = .012) (Fig 1). From the 15 patients with TK activity above the overall median of 3.2 pmol/min/mg protein, 11 cases (73%) achieved an adequate blast cell clearance while only 6 of 17 cases (35%) with lower values responded (P = .035). Similarly, high Poly α activity of leukemic blasts was predictive for early response. In responding cases the median value of Poly α activity was 1.9 pmol/min/mg protein (0.63 to 8.8) and thus significantly higher than for patients with inadequate blast cell reduction: 0.69 pmol/min/mg protein (0.03 to 6.7) (P = .014) (Fig 2). Correspondingly, 12 of 15 patients (80%) with higher than median activity of Poly α (1.22 pmol/min/mg protein) had an adequate early response to induction therapy as compared to only 5 of 14 patients (36%) with lower enzyme activities (P = .02). Values for DCK activity varied substantially among individual cases and no relation to treatment response could be detected.
Distribution of thymidine kinase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
Distribution of thymidine kinase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
Distribution of polymerase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
Distribution of polymerase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
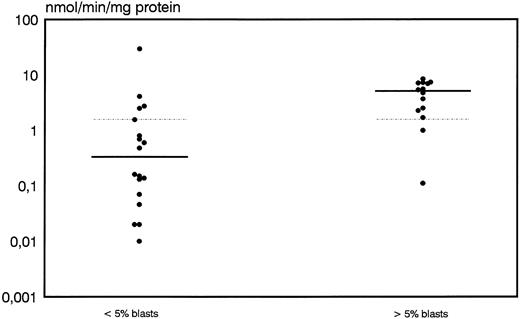
A high discrimination between responders and nonresponders was also shown for DCD activity. Median values and range for DCD activity were 0.33 nmol/min/mg protein (0.0 to 29.5) for patients with an adequate blast cell reduction whereas nonresponders showed a significantly higher median of 5.1 nmol/min/mg protein and a range of 0.11 to 8.45 (P = .009) (Fig 3). From 16 patients with DCD activity below the overall median of 1.56 nmol/min/mg protein, 14 (88%) cases achieved an adequate response whereas only 3 of 16 (22%) patients with higher than median values obtained an adequate blast cell clearance (P = .0004).
Distribution of deoxycytidine deaminase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
Distribution of deoxycytidine deaminase activity in leukemic blasts for early responders (<5% blasts) and nonresponders (<5% blasts) to induction therapy. Median values of the respective groups and the overall median are indicated by the solid and the dotted lines, respectively.
By logistic regression analysis of enzyme activities, DCD activity was found to be the most sensitive parameter to predict an adequate blast cell clearance (P = .032). Logistic regression analysis with blast cell clearance as main parameter showed a significane level of P = .348 for Poly α, .248 for TK, and .59 for DCK activity.
Activities of TK and DCD were not only associated with initial response to TAD-9 + GM-CSF but were also found of relevance for remission duration. From the 28 patients who achieved a CR, pretherapeutic results of TK and DCD activity were evaluable in 22 (TK) and 23 (DCD) cases, respectively. From the 11 patients with TK levels below the median, 8 (73%) relapsed within 1 year while only 2 of 11 patients (18%) with high TK activity experienced a recurrence of their disease during that time (P = .015). Correspondingly, 6 of 9 patients (66%) with higher than median DCD levels relapsed within 1 year whereas 10 of 14 patients (71%) with lower DCD levels had a longer than 1 year CR duration (P = .085).
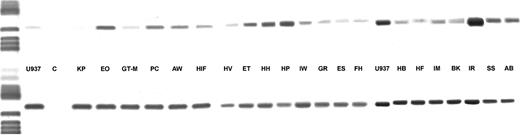
DCD gene expression and its relation with DCD activity.Total RNA was extracted from leukemic blasts of 21 patients with AML and from the cell line U937 and was tested for possible RNA degradation by amplification of a 484-bp segment from β-actin cDNA. Sterile water was used as negative, RNA from U937 as positive control. As shown in Fig 4 a 546-bp PCR product was detected in all AML cases. Specificity of this amplification product for DCD gene expression was verified by direct DNA sequencing. For quantitation of initial DCD mRNA the relative signal intensity of the ethidium bromide stained bands was determined as compared with the signal intensity of the β-actin amplification product. Values for the relative optical densities (OD) provided the basis for discriminating between AML specimens with high or low DCD gene expression (Table 4). On this basis 8 of 9 patients with a high transcription rate also showed higher than median DCD enzyme activity, whereas in blast cells from all 10 patients with low DCD mRNA levels a low enzyme activity was detected. These data indicate that DCD activity is regulated by the transcription rate of the DCD gene itself rather than by subsequent steps of translation and activation, respectively.
DCD gene expression in AML blasts. In the upper row the amplification products for the DCD gene are shown, in the lower row the respective β-actin PCR products are depicted.
DCD gene expression in AML blasts. In the upper row the amplification products for the DCD gene are shown, in the lower row the respective β-actin PCR products are depicted.
Relation of DCD Gene Expression With DCD Enzyme Activity
| Patient . | DCD Gene Expression . | DCD Activity . |
|---|---|---|
| . | . | (nmol/min/mg protein) . |
| G.R. | Low | 0.02 |
| H.B. | Low | 0.02 |
| B.K. | Low | 0.13 |
| K.P. | Low | 0.14 |
| I.W. | Low | 0.16 |
| H.F. | Low | 0.6 |
| G.T.-M. | Low | 0.64 |
| H.V. | Low | 0.8 |
| E.S. | Low | 0.8 |
| H.F. | Low | 1.0 |
| HI.F. | Median | 0.28 |
| I.M. | Median | 7.0 |
| A.B. | High | 0.21 |
| H.P. | High | 1.64 |
| E.O. | High | 1.7 |
| P.C. | High | 2.2 |
| E.T. | High | 2.3 |
| I.R. | High | 2.4 |
| H.H. | High | 2.7 |
| S.S. | High | 6.6 |
| A.W. | High | 29.6 |
| Patient . | DCD Gene Expression . | DCD Activity . |
|---|---|---|
| . | . | (nmol/min/mg protein) . |
| G.R. | Low | 0.02 |
| H.B. | Low | 0.02 |
| B.K. | Low | 0.13 |
| K.P. | Low | 0.14 |
| I.W. | Low | 0.16 |
| H.F. | Low | 0.6 |
| G.T.-M. | Low | 0.64 |
| H.V. | Low | 0.8 |
| E.S. | Low | 0.8 |
| H.F. | Low | 1.0 |
| HI.F. | Median | 0.28 |
| I.M. | Median | 7.0 |
| A.B. | High | 0.21 |
| H.P. | High | 1.64 |
| E.O. | High | 1.7 |
| P.C. | High | 2.2 |
| E.T. | High | 2.3 |
| I.R. | High | 2.4 |
| H.H. | High | 2.7 |
| S.S. | High | 6.6 |
| A.W. | High | 29.6 |
DISCUSSION
The evaluation of pretherapeutic determinants with prognostic relevance for induction therapy and/or remission duration in AML has been a target of numerous investigations. Except for cytogenetics, results have been too inconsistent, however, to be used for pretreatment stratification. Interest in the field of pretherapeutic cell kinetics has been renewed recently when hematopoietic growth factors were introduced into AML therapy with the aim to stimulate leukemic cell growth and to render leukemic blasts more sensitive to subsequently applied chemotherapy.
The current study was performed to evaluate the relevance of pretherapeutic proliferative activity and parameters of ara-C metabolism for the early response to induction therapy under the conditions of a GM-CSF–based priming regimen comprising the TAD-9 combination. The obtained data indicate that a high proliferative activity of leukemic blasts as represented by high TK and Poly α activity is associated with a favorable early response but that the activity of DCD is the most valuable prognostic determinant. Hence, 14 of 16 (88%) patients with lower than median DCD values experienced an adequate early blast cell reduction as compared to only 3 of 16 (22%) cases with higher values. Although TK and Poly α activity were also useful for the discrimination between early responders and nonresponders, logistic regression analysis identified DCD as the most powerful prognostic determinant. The finding that DCD activity is a very sensitive parameter to predict early response to induction therapy is not contradictory to previous reports by Preisler et al.15 16 These studies indicate a correlation beween the ability of AML blasts to retain intracellular ara-CTP, but outcome of remission induction therapy was not related with ara-CTP retention. The investigators explain these observations with differences between the conditions of exposure to ara-C during induction and consolidation therapy. In the former situation, ara-C was administered by continous infusion while during consolidation therapy ara-C was given subcutaneously, resulting in short durations of therapeutically effective plasma ara-C levels. Under the latter conditions of exposure ara-CTP retention might be expected to be significant. In the present study induction therapy comprised short-term infusions of ara-C every 12 hours on days 3-8, so that under these conditions of exposure to ara-C the ability of cells to retain ara-CTP might well account for differences in early response to therapy.
The clinical relevance of a high DCD activity as a major cause for ara-C resistance has been emphasized by several investigators already. Hence, Steuart and Burke22 found a high DCD activity in the leukemic cells of patients who did not respond to conventional dose ara-C therapy. Similarly, Colly et al19 reported about a group of 21 patients with resistant AML who had either a very low DCK activity or substantially elevated DCD levels. Kreis et al46 took a different approach and investigated the ratio of araU/ara-C in the plasma of patients with AML. Although two groups of ‘deaminators’ could be discriminated, the respective differences were not statistically significant.
These findings raise the question about the mechanisms underlying the differences in DCD activity between individual patients. First results about the regulation of DCD activity are described in this study and indicate that DCD activity correlates with the amount of mRNA in AML blasts. This association suggests that differences in DCD activity obviously emerge from differences in transcription and stimulate the search for the respective regulatory events.
In contrast to DCD, which inactivates ara-C by deamination to its noncytotoxic metabolite araU, DCK, which regulates the phosphorylation of ara-C and the formation of ara-CTP as the main cytotoxic component, was not found predictive for early treatment response. This finding is in contrast to cell culture and animal systems in which a low level of DCK activity comprises the most prevalent mechanism of ara-C resistance.47-49 In the clinical setting, however, few studies only have assessed the relevance of DCK deficiency and have shown inconclusive results.18,19,23 24
The lacking relevance of DCK activity for response to induction therapy is supported by the recently reported finding that, in contrast to in vitro cell systems, structural alterations of the coding region for the DCK gene occur at a low frequency in leukemic blasts from AML patients and thus do not constitute a major mechanism of resistance to ara-C under in vivo conditions.27
Impaired incorporation of ara-CTP into DNA because of a low activity or altered sensitivity of DNA polymerase has also been discussed as a mechanism of cellular resistance to ara-C.17,50 To our knowledge, the present investigation is the first to show that low Poly α activity of leukemic blasts in vivo is associated with an inadequate response to induction therapy. These results confirm previous data from our group that were obtained in a separate group of patients demonstrating that Poly α activity of leukemic blasts in vitro is highly predictive for clinical response to TAD-9 induction therapy.13
The results of the current study reemphasize the relevance of proliferative activity as an important prognostic factor for the response to induction therapy in the context of GM-CSF–based priming. Besides Poly α, TK activity but not %S phase proved as predictive determinant. Because duration of S phase may vary substantially between individual patients,51 the activity of the ongoing DNA synthesis process as expressed by TK rather than by %S phase cells appears more relevant to assess the susceptibility of the leukemic blasts to cell-cycle–specific agents such as ara-C. However, the current study did not discriminate between classical drug resistence and biological resistance, as suggested by Preisler et al.52 The relevance of cell-cycle parameters may be more pronounced for ara-C–sensitive blasts as for cases with primary resistance to the drug.
However, compared with evaluations of non–growth factor supported trials, the predictive value of parameters of proliferative activity appears less striking. Hence, the analysis of determinants of proliferative activity and of ara-C metabolism during TAD-9 therapy without GM-CSF priming showed that TK and Poly α activity were highly predictive for early response. However, the in vitro analyses demonstrated an inverse correlation between proliferative activity and sensitivity to GM-CSF.13 The data suggest that the predictive value of proliferation-associated determinants may be influenced by the coadministration of hematopoietic growth factors during induction chemotherapy rendering growth factor independent parameters of ara-C metabolism and DCD in particular more important.
To our knowledge, the current study is the first one to evaluate determinants of proliferative activity and ara-C metabolism for their impact on early response to induction therapy in the setting of a growth factor priming strategy. Further data are certainly needed to substantiate these findings. The current evaluation shedds further light on the complex interactions between proliferation and drug metabolism in leukemic cells and adds to understanding the determinants of response or failure to induction therapy, respectively. Hence, these results are a step further toward improving the rational of AML therapy and toward the development of risk-adapted treatment strategies.
Supported by a grant from the Dr Mildred-Scheel Stiftung für Krebsforschung, Germany (W 131/94/Hi7).
Address reprint requests to Wolfgang Hiddemann, MD, PhD, Department of Hematology and Oncology, Georg-August-University, Robert Koch Str. 40, D-37075 Göttingen, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal