To the Editor:
Platelet glycoprotein (GP) IIb-IIIa (integrin αIIbβ3) is essential for normal hemostasis and plays a central role in platelet aggregation by binding to its adhesive ligands such as fibrinogen and von Willebrand factor. The GPIIb-IIIa is the most abundant member of the integrin family found on the platelet surface, with 80,000 molecules/platelet.1 Mature GPIIIa protein is a single chain polypeptide composed of 762 amino acids.2 GPIIIa contains 56 cysteine residues in highly conserved locations within the extracellular domain of the molecule, all of which are normally disulfide-linked to form 28 nonconsecutive disulfide bridges. Two large loops extending from amino acids Cys5-Cys435 and Cys406-Cys655 have been proposed.3 Mature GPIIb has 1,008 amino acids containing 9 disulfide bonds formed by 18 cysteines.4 The overall role of these cysteine residues in functional expression of GPIIb-IIIa is not fully understood.
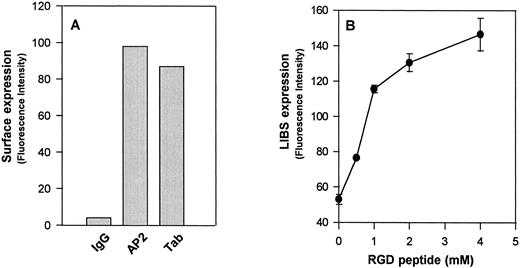
Flow cytometric analysis of surface-expressed GPIIb-IIIaCys655Tyr on CHO cells. (A) Expression of GPIIb-IIIaCys655Tyr on the surface of transfected CHO cells as measured by monoclonal antibodies directed against GPIIb (Tab) or the intact complex (AP2). Cells were incubated with the indicated antibody or normal mouse IgG (IgG) at a concentration 20 μg/mL at room temperature for 60 minutes. After washing, the cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragments of goat-antimouse IgG for 30 minutes and analyzed by flow cytometry. (B) Binding of the LIBS antibody D3 to GPIIb-IIIa transfected CHO cells. Transfected CHO cells were incubated with LIBS antibody, D3 (20 μg/mL), in the presence of different concentrations of RGDW peptides. After washing, the surface-bound antibody was analyzed by the addition of FITC-labeled goat antimouse IgG, followed by flow cytometry. Note that functional expression of the D3 epitopes on these transfected CHO cells is in an RGD dose-dependent manner. Similar results were obtained from wild-type GPIIb-IIIa transfected CHO cells (not shown).
Flow cytometric analysis of surface-expressed GPIIb-IIIaCys655Tyr on CHO cells. (A) Expression of GPIIb-IIIaCys655Tyr on the surface of transfected CHO cells as measured by monoclonal antibodies directed against GPIIb (Tab) or the intact complex (AP2). Cells were incubated with the indicated antibody or normal mouse IgG (IgG) at a concentration 20 μg/mL at room temperature for 60 minutes. After washing, the cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragments of goat-antimouse IgG for 30 minutes and analyzed by flow cytometry. (B) Binding of the LIBS antibody D3 to GPIIb-IIIa transfected CHO cells. Transfected CHO cells were incubated with LIBS antibody, D3 (20 μg/mL), in the presence of different concentrations of RGDW peptides. After washing, the surface-bound antibody was analyzed by the addition of FITC-labeled goat antimouse IgG, followed by flow cytometry. Note that functional expression of the D3 epitopes on these transfected CHO cells is in an RGD dose-dependent manner. Similar results were obtained from wild-type GPIIb-IIIa transfected CHO cells (not shown).
We generated a stable cell line using wild-type GPIIb and a GPIIIa cDNA containing an A2062G mutation. This substitution results in an amino acid change from Cys → Tyr at residue 655 of GPIIIa, a residue that is thought to participate in the formation of a long-range disulfide bond with Cys406 .3 When this mutant GPIIIa cDNA was transfected into CHO cells with GPIIb, it was capable of forming a complex with GPIIb (GPIIb-IIIaCys655Tyr) on the cell surface, as shown by the binding of the complex-specific antibody AP2 (Fig 1A). This integrin complex could bind to RGD-containing peptides and undergo conformational changes, as shown by increased binding of the ligand-induced binding site (LIBS) antibody, D3 (Fig 1B). D3 recognizes a conformation of the integrin complex that is induced by ligand binding.5 A cell adhesion assay showed that this mutant GPIIb-IIIa complex behaved like wild-type GPIIb-IIIa to mediate cell adhesion to immobilized fibrinogen (not shown). These data suggest that the mutation at the highly conserved cysteine residue 655 of GPIIIa subunit has no effect on integrin function.
The position and number of the 56 cysteines in GPIIIa are absolutely conserved within β-subunits of integrin family, ranging from Drosophila to humans.6,7 Considering this fact, one might have expected the cysteine mutant of GPIIIa to be dysfunctional. Surprisingly, this mutant form of the GPIIb-IIIa complex functions normally in terms of its ability to express on the cell surface and to bind ligand. A cysteine-addition mutation in GPIIIa (Arg636Cys) has been shown to be associated with Sra platelet alloantigenic epitope.8 This mutation, conferring 57 cysteine residues rather than 56, upon the GPIIIa does not seem to affect biosynthesis of the GPIIIa subunit. Although it is not yet clear whether this unpaired cysteine mutation affects the function of GPIIb-IIIa, Sra-positive individuals appear to have normal hemostatic function. The finding that removal of cysteine 655 or addition of a cysteine at position 636 of GPIIIa has no apparent effects on the synthesis, surface expression, or function of GPIIb-IIIa indicates that not all the highly conserved cysteine residues in β3 integrin are critical for the function of the GPIIb-IIIa complex. In contrast, a recently described Cys374Tyr mutation of GPIIIa in a Glanzmann thrombasthenic patient led to a defect in surface expression of GPIIb-IIIa on the patient's platelets.9 Thus, it will be of future interest to evaluate the role of the highly conserved cysteine residues in stabilization or direction of the proper folding of GPIIIa and subsequent complex formation and function.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal