To the Editor:
To provide samples for neonatal mass screening, a few drops of blood are routinely collected from the heel of the newborn infant and allowed to dry on a thick filter paper called the Guthrie card.1 Guthrie cards represent a valuable and comprehensive genetic repository because genomic DNA can be isolated from blood samples processed on these filter papers for use in polymerase chain reaction (PCR). Besides the extraction of DNA from card blood, amplification from small pieces of blood-stained paper directly placed into the PCR reaction mixture has been reported.2,3
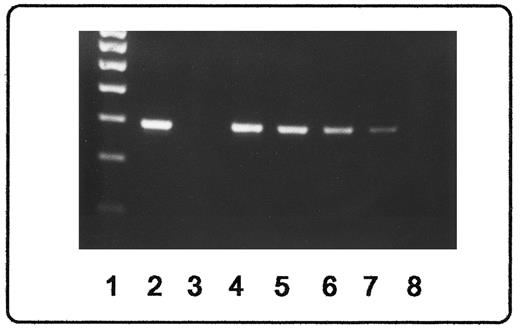
(A) Analysis of bcr-abl rearrangement from dried blood specimen in patients with CML. Lanes 1 and 2, patient no. 1 post-BMT (d +28 and d +49, respectively). Note that a faint bcr-abl signal is visible in lane 1 but not in lane 2. Lane 3, patient no. 2 under treatment with interferon-α2; sample was processed after 7 months of storage. Lane 4, patient no. 3 at the time of diagnosis exhibiting a b3a2 rearrangement. Lane 5, patient no. 4 without amplificable bcr-abl rearrangement on the time of diagnosis; note, however, that the abl-specific mRNA was amplified (see [B]). Lane 6, negative control (water). Lane 7, positive control (cell line K562). Lane 8, 100-nt length marker. (B) Seminested RT-PCR amplification using primers for abl exon 1a, exon 1b, and exon 2, showing that intact abl-gene mRNA is present in all extracted specimen, including those cases (patients no. 1 and 4; compare with lane 2 and lane 5 in [A]) in which no bcr-abl rearrangement could be detected. Lanes 6 through 8 are the same as in (A).
(A) Analysis of bcr-abl rearrangement from dried blood specimen in patients with CML. Lanes 1 and 2, patient no. 1 post-BMT (d +28 and d +49, respectively). Note that a faint bcr-abl signal is visible in lane 1 but not in lane 2. Lane 3, patient no. 2 under treatment with interferon-α2; sample was processed after 7 months of storage. Lane 4, patient no. 3 at the time of diagnosis exhibiting a b3a2 rearrangement. Lane 5, patient no. 4 without amplificable bcr-abl rearrangement on the time of diagnosis; note, however, that the abl-specific mRNA was amplified (see [B]). Lane 6, negative control (water). Lane 7, positive control (cell line K562). Lane 8, 100-nt length marker. (B) Seminested RT-PCR amplification using primers for abl exon 1a, exon 1b, and exon 2, showing that intact abl-gene mRNA is present in all extracted specimen, including those cases (patients no. 1 and 4; compare with lane 2 and lane 5 in [A]) in which no bcr-abl rearrangement could be detected. Lanes 6 through 8 are the same as in (A).
In contrast to DNA, processing of RNA requires precautions as the macromolecule is notoriously susceptible to rapid degradation by endogenous ribonuclease (RNase) activity. Recent reports showed that undegraded mRNA can be obtained in sufficient amounts from dried blood stains on filter paper without special pretreatment.4,5 The unexpected stability of RNA when recovered from dried blood may be due to the storage condition in which the RNA appears to be protected from RNases once the nucleated cells are dried. Consequently, the ability to extract RNA from dried blood on filter paper was used to diagnose infectious diseases and to unravel genetic mutations.3-6
We report here on the extraction of RNA from filter paper in pediatric patients with chronic myelogenous leukemia (CML). The characteristic chromosome translocation that results in the formation of a Philadelphia (Ph+) chromosome [t(9; 22)(q34; q11)] is the hallmark of CML and juxtaposes the abl gene on chromosome 9 to the bcr gene on chromosome 22. As a consequence of this fusion, a unique 8.5-kb bcr-abl hybrid mRNA transcript arises.7 After reverse transcription into cDNA, this bcr-abl–rearranged mRNA can be detected by the PCR (RT-PCR). Recently, an approach has been reported to dissolve bcr-abl mRNA directly on the glass slide from blood smears kept stored either stained with May-Grünwald-Giemsa solution or unstained for up to 6 months.8 We investigated whether also filter paper might be an even more suitable storage and transportation medium than glass slides for the extraction of bcr-abl–rearranged mRNA.
Specimens spotted on Guthrie cards were mailed as regular letter from all over Germany by physicians participating in the ongoing pediatric CML-paed trial for treatment of childhood CML.9 Twenty-four samples from 7 patients before bone marrow transplantation (BMT) were analyzed as follows. Dried blood spots equivalent to 100 μL of blood were punched out of the filter paper and suspended in RNA extraction buffer from a commercially available kit (RNeasy-Blood-Mini Kit; Qiagen, Ltd, Hilden, Germany). After incubation for 1 hour at room temperature, the pooled suspensions together with the paper chips were added on microcolumns included in the kit and processed as described by the manufacturer. This extraction resulted in a yield of 0.3 to 0.5 μg total RNA. To assure the quality of the RNA, a fragment of the abl gene was successfully amplified by RT-PCR in all cases investigated (Fig 1B). For that purpose, two 5′ abl primers (for exons 1a and 1b, respectively) and one 3′ abl primer (for exon 2) were used, considering that alternative splicing of the normal abl mRNA results in the presence of two distinct mRNAs.10 Nested RT-PCR for bcr-abl amplification was performed with primers located on abl exon a2 and bcr exons e11 and e12, respectively, as described.7 10 Sensitivity of the assay was determined by diluting cells from the CML cell line K562 into blood from healthy donors, which then was spotted on filter paper and stored for 1 month before mRNA extraction was performed. Down to 1 cell in 10,000 cells could be detected by this method (Fig 2).
Sensitivity for detection of bcr-abl–rearranged mRNA. Products from RT-PCR amplification were visualized by ethidium staining after agarose gel electrophoresis. Lane 1, 100-nt length marker; lane 2, positive control (cell line K562); lane 3, negative control (healthy donor); lanes 4 through 8, serial dilution of K562 cells (1:101, 1:102, 1:103, 1:104, and 1:105, respectively) into cells from a healthy donor. Down to 1 K562 cell diluted in 104 donor cells is easily detectable.
Sensitivity for detection of bcr-abl–rearranged mRNA. Products from RT-PCR amplification were visualized by ethidium staining after agarose gel electrophoresis. Lane 1, 100-nt length marker; lane 2, positive control (cell line K562); lane 3, negative control (healthy donor); lanes 4 through 8, serial dilution of K562 cells (1:101, 1:102, 1:103, 1:104, and 1:105, respectively) into cells from a healthy donor. Down to 1 K562 cell diluted in 104 donor cells is easily detectable.
A major breakpoint (M-bcr) bcr-abl fusion transcript could be shown in 6 of 7 patients before BMT regardless of the leukocyte count (hematologic remission v partial remission) or therapy (hydroxyurea and/or interferon-α2). Of these 6 patients with M-bcr transcripts 4 showed a b2a2 rearrangement, 1 showed a b3a2 rearrangement (for details, see Roth et al10 ), and 1 exhibited both rearrangements (Fig 1A). In the seventh patient, the abl gene had been amplified successfully; however, neither a M-bcr nor a minor bcr transcript (m-bcr, ela2 rearrangement; for details, see Roth et al10 ) of the bcr-abl gene could be demonstrated. Although diagnosis of CML was confirmed by conventional cytogenetics exhibiting the Ph+ chromosome, the negative RT-PCR results were repeated on freshly collected blood. Thus, an unusual bcr-abl rearrangement or a lacking mRNA transcript cannot be excluded in this case.
Analysis from filter papers that had been kept stored at room temperature for more than 1 year was possible. Matching results were obtained when compared with samples collected later on during the course of the disease that had been stored only for a few days before mRNA extraction.
During follow-up examinations of 3 patients after BMT, a total of 13 peripheral blood specimens spotted on filter paper were collected over a period of 2 years. Only 2 specimens, collected from either 1 patient at 1 month and 9 months after BMT, respectively, still exhibited the M-bcr rearrangement.
We conclude that mRNA extraction for diagnosis of the bcr-abl rearrangement in CML from dried blood spotted on filter paper is feasible. Filter papers enclosed in transparent storage sleeves may easily be filed with the patient's records and thus allow comfortable access to DNA and RNA specimens collected at the time of diagnosis or during regular follow-up examinations for prolonged periods of time. In conclusion, simple spilling blood on filter paper may (1) facilitate the cooperation between laboratories and hospitals separated by long distances, (2) save transportation costs, and (3) contribute to standardize diagnostic procedures on a molecular level in CML and related diseases.

![Fig. 1. (A) Analysis of bcr-abl rearrangement from dried blood specimen in patients with CML. Lanes 1 and 2, patient no. 1 post-BMT (d +28 and d +49, respectively). Note that a faint bcr-abl signal is visible in lane 1 but not in lane 2. Lane 3, patient no. 2 under treatment with interferon-α2; sample was processed after 7 months of storage. Lane 4, patient no. 3 at the time of diagnosis exhibiting a b3a2 rearrangement. Lane 5, patient no. 4 without amplificable bcr-abl rearrangement on the time of diagnosis; note, however, that the abl-specific mRNA was amplified (see [B]). Lane 6, negative control (water). Lane 7, positive control (cell line K562). Lane 8, 100-nt length marker. (B) Seminested RT-PCR amplification using primers for abl exon 1a, exon 1b, and exon 2, showing that intact abl-gene mRNA is present in all extracted specimen, including those cases (patients no. 1 and 4; compare with lane 2 and lane 5 in [A]) in which no bcr-abl rearrangement could be detected. Lanes 6 through 8 are the same as in (A).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1713/3/m_bl_0054f1.jpeg?Expires=1769122670&Signature=vDKq0HB2eSl7gKMdTRiZjB-4SOP-EBHXgdkfbSMo9fq0qak4uCzCNq7RSp0JWE45wQeEJp82GsCb~IR-sFbOgtlN-R1TcQv9IbqNMVtdDZSjeqKTYB~0SKlSd5duyWwwRguIOBnmxjyBVSD~H2FYqk4Q71xVmIE8dl0PH6hJWp8o8vvj-lVPujeHuYtajmzN3oXHqvfkKueinggpyl76USkM9osIrg8mL2L6NIFqE-QmNsInr5nghfeiPbrLzZnBqD1PtIK3LM-H~OQC75QL8Fa2conn9kMm5NJVW784v5Flgqyepp0qybQCzT4GN2jI9ubcCVq8LjipQx8iom-E~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal