Abstract
N-acetylmuramyl-L-alanine amidase (NAMLAA) specifically degrades peptidoglycan, which is a major component of bacterial cell walls with strong inflammatory properties. For instance, peptidoglycan is capable of stimulating peripheral blood cells to release pro-inflammatory cytokines and is capable of inducing chronic arthritis in an animal model. In a previous study we found that degradation of peptidoglycan by purified NAMLAA reduced its inflammatory effects. To determine where NAMLAA is located in tissues, monoclonal antibodies against purified NAMLAA were produced for use in immunohistochemistry, immunoelectron microscopy, flow cytometric analysis, and Western blotting. The immunohistochemical studies showed NAMLAA-positive cells in human spleen, liver, arthritic synovial tissues, and lymph nodes. In flow cytometric studies of blood and bone marrow, neutrophilic and eosinophilic granulocytes proved to be positive. Monocytes were negative, although they do contain lysozyme, the other important peptidoglycan-degrading enzyme. However, mature macrophages obtained by bronchoalveolar lavage and subsequent selection based on auto-fluorescence did possess NAMLAA. In immunocytochemical staining of blood smears, thrombocytes were also positive for NAMLAA. Western blot analysis and immunoelectron microscopy of neutrophils and eosinophils showed that NAMLAA is located in azurophilic granules of neutrophils and in secretory vesicles and crystalloid-containing granules of eosinophils. Flow cytometric analysis of blood and bone marrow from different French-American-British–classified acute myeloid leukemia (AML) patients showed that AML-M2 myeloblasts were the first in the granulocyte maturation lineage that were positive for NAMLAA. The more immature AML, such as AML-M0 and AML-M1, did not express NAMLAA. CD15- and CD13-negative megakaryoblasts, corresponding to AML-M7, were also positive for NAMLAA. The expression pattern of NAMLAA in the myeloid lineage suggests that the monoclonal antibody AAA4, recognizing NAMLAA, is useful for discrimination between AML in the monocyte lineage and in the granulocyte lineage.
IN HUMAN SERA N-acetylmuramyl-L-alanine amidase (NAMLAA) activity was reported for the first time by Ladešić et al.1 This relatively unknown human enzyme degrades peptidoglycan, a major component of most bacterial cell walls, by hydrolysis of the lactyl bond that connects N-acetylmuramic acid in the glycan strands with L-alanine of the peptide side chain.2 In a previous study we described the purification of NAMLAA from human plasma, the preparation of monoclonal antibodies (MoAbs) against it, and the properties of this enzyme.3
Peptidoglycan is present in the cell walls of almost all bacteria and is the major constituent of the cell walls of Gram-positive bacteria. The inflammatory properties of peptidoglycan in vivo include complement activation,4 arthritis induction,5 and activation of monocytes and macrophages resulting in the release of inflammatory cytokines.6 7
Following the lysis of bacteria during an infection, cell wall fragments containing peptidoglycan are rapidly cleared through the action of phagocytic cells and their hydrolytic enzymes.8 Lysozyme is thought to be the most important enzyme for the inactivation of peptidoglycan, because it degrades the sugar backbone of peptidoglycan by hydrolyzing the bond between N-acetylglucosamine and N-acetylmuramic acid. However, in previous studies we and others showed that cell wall fragments from Gram-positive bacteria still possessed most of their inflammatory properties after degradation by hen egg-white lysozyme,9,10 but lost most of their cytokine and arthritis-inducing capacity after degradation by NAMLAA.11 This indicates that the presence of lysozyme alone is not sufficient for complete inactivation of the inflammatory properties of peptidoglycan.
The presence of a peptidoglycan-degrading enzyme other than lysozyme in phagocytic cells is therefore required for the complete processing of peptidoglycan. Apart from lysozyme, N-acetylglucosaminidase was reported to be present in granulocytes and was shown to hydrolyze the N-acetylglucosamine group from the nonreducing end of the glycan chains,12 although its primary function is the degradation of oligosaccharides containing β-glucosaminide linkages, present on many glycoproteins.13 Whether the degradation by N-acetylglucosaminidase could have an effect on the inflammatory properties of peptidoglycan has not been investigated yet. However, it is reasonable to assume that this enzyme has very little effect on these properties because of its limited enzymatic activity. Therefore, the presence of NAMLAA in phagocytic cells might well complement the hydrolytic actions of lysozyme.
In the present study, MoAbs raised against human NAMLAA were used for immunohistochemistry, electron microscopy, flow cytometric analysis, and Western blotting to determine the localization of NAMLAA in human tissues. Blood and bone marrow (BM) cells from healthy donors and from acute myeloid leukemic patients were analyzed to determine in which stages of myeloid differentiation NAMLAA is expressed, and to investigate whether this marker can be used for the diagnosis of acute leukemias.
MATERIALS AND METHODS
Preparation of MoAb AAA4 against NAMLAA.Purified NAMLAA was used for preparing mouse MoAbs as described previously.3 The IgG1 MoAb AAA4 showed the highest affinity for NAMLAA and was used for conjugation with FITC (AAA4-FITC) by standard procedures.14 In a previous study, AAA4 proved to be highly specific for NAMLAA and was used for the purification of NAMLAA in one step from human plasma.3
Bronchoalveolar lavage.Bronchoalveolar lavage samples (generously supplied by the Department of Pulmonary Medicine, University Hospital Rotterdam, The Netherlands) were obtained as follows. After informed consent, bronchoalveolar lavage was performed on individuals during anesthesia for surgery. The lavage was performed with a flexible bronchoscope placed in wedge position in the right middle lobe. Four aliquots of 50 mL of saline were subsequently instilled and aspirated. The obtained fluid was collected in siliconized bottles.
Immunohistochemical Double Labeling With AAA4 on Human Spleen
| Code . | Specificity . | Concentration Used . | Double Labeling . | Reference/Source . |
|---|---|---|---|---|
| RV202 | Vimentin | 1:10 | − | [21] |
| 5B5 | Fibroblasts | 1:20 | − | DAKO |
| HLE-1 | CD45, leukocytes | 1:100 | + | Becton Dickinson |
| HLA-DR | MHC-II | 1:250 | − | Becton Dickinson |
| Leu4 | CD3, T cells | 1:100 | − | Becton Dickinson |
| B4 | CD19, B cells | 1:100 | − | Coulter Clone |
| Mac | Macrophages | 1:500 | − | DAKO |
| L25 | Dendritic cells | 1:500 | − | [22] |
| LeuM3 | CD14, monocytes | 1:100 | − | Becton Dickinson |
| CD24 | Granulocytes | 1:100 | + | Ortho Diagnostics, Raritan, NJ |
| VIM-D5 | CD15, neutrophilic granulocytes | 1:100 | + | W. Knapp, Vienna, Austria |
| Code . | Specificity . | Concentration Used . | Double Labeling . | Reference/Source . |
|---|---|---|---|---|
| RV202 | Vimentin | 1:10 | − | [21] |
| 5B5 | Fibroblasts | 1:20 | − | DAKO |
| HLE-1 | CD45, leukocytes | 1:100 | + | Becton Dickinson |
| HLA-DR | MHC-II | 1:250 | − | Becton Dickinson |
| Leu4 | CD3, T cells | 1:100 | − | Becton Dickinson |
| B4 | CD19, B cells | 1:100 | − | Coulter Clone |
| Mac | Macrophages | 1:500 | − | DAKO |
| L25 | Dendritic cells | 1:500 | − | [22] |
| LeuM3 | CD14, monocytes | 1:100 | − | Becton Dickinson |
| CD24 | Granulocytes | 1:100 | + | Ortho Diagnostics, Raritan, NJ |
| VIM-D5 | CD15, neutrophilic granulocytes | 1:100 | + | W. Knapp, Vienna, Austria |
The cells in the lavage fluid were kept at 4°C, washed twice in phosphate-buffered saline (PBS) containing 0.5% (wt/vol) bovine serum albumin (BSA) and 0.45% (wt/vol) glucose. The cells were then filtered through a 100-μm and a 30-μm gauze and subsequently sorted on a FACS-Vantage (Becton Dickinson, Erembodegem, Belgium) with a laser operating at 488 nm. The highly autofluorescent (530 nm) population was used to prepare cytospins and was shown to contain 100% alveolar macrophages, positive for acid phosphatase and CD68.23 The cells were stained with AAA4 as described under Immunocytochemistry (see below).
Immunocytochemistry.For the preparation of smears, blood and small BM samples were obtained after informed consent from a healthy donor by the Department of Hematology, Erasmus University Rotterdam, The Netherlands. The smears were fixed and permeabilized by incubation for 10 minutes in acetone. The smears were then rinsed in PBS containing 0.2% BSA and incubated for 1 hour at room temperature with 70 μL of AAA4 (diluted 40 μg/mL in PBS-BSA) or, for control staining, with an irrelevant MoAb of the same isotype and concentration. Subsequently, the smears were rinsed in PBS-BSA and incubated for 30 minutes at room temperature with rabbit–antimouse immunoglobulin (Z259; DAKO, Glostrup, Denmark) diluted 1:20 in PBS-BSA with 1% normal human serum. After rinsing in PBS-BSA, a 1:40 dilution of mouse–anti-alkaline phosphatase in a complex with alkaline phosphatase (APAAP, D651; DAKO) was applied for 30 minutes. To develop the stain, the smears were incubated for 30 minutes at room temperature with 0.012% (wt/vol) naphthol-ASMX phosphate, 0.025% (wt/vol) fast blue BB base, and 0.025% (wt/vol) levamisol (all from Sigma, St Louis, MO), which stains positive cells blue. Granulocytes were easily identified by the negatively stained nucleus, rendering a counter stain unnecessary. Finally, the smears were mounted in Kaiser's glycerol gelatin (Merck, Darmstadt, Germany).
Immunohistochemistry.For the immunohistochemical studies, spleen tissue was obtained from the Department of Pathology immediately after surgery (SSDZ, Delft, The Netherlands). The spleen was removed for technical reasons from a patient with gastric adenocarcinoma. Liver biopsy specimens obtained from the Department of Pathology, Erasmus University Rotterdam, were taken from an organ being prepared for transplantation. Lymph nodes were obtained from a patient undergoing surgery for tonsillitis. Synovial tissues were obtained at the time of reconstructive surgery from the knees and hips of patients with definite or classical rheumatoid arthritis according to the American Rheumatism Association criteria.
For immunohistochemical staining with the MoAb AAA4, tissue cryostat sections were acetone-fixed as described previously.15 Only the spleen cryostat sections were fixed with pararosaniline. The staining procedure was the same as described above for immunocytochemistry.
Immunohistochemical double staining.For double staining with two MoAbs, the tissue cryostat sections were fixed as described above. Endogenous peroxidase activity was blocked by a 10-minute incubation in 0.5% H2O2 in PBS. The sections were washed in PBS/BSA and subsequently incubated with MoAb AAA4 for 1 hour at room temperature. As the second step, APAAP was used as described above. After incubation and washing in PBS/BSA, the sections were incubated with the second MoAb for 1 hour at room temperature. The antibodies used are listed in Table 1. The staining was detected with a peroxidase-conjugated rabbit–antimouse-Ig (P161, DAKO) in a 1:250 dilution in PBS/BSA with 1% normal human serum. After a 30-minute incubation and washing in PBS/BSA, the sections were incubated with the alkaline phosphatase substrate for 45 minutes in the dark, followed by rinsing in PBS/BSA and 0.2 mol/L sodium acetate buffer, pH 4.6. The peroxidase substrate 3-amino-9-ethylcarbazole was applied for 30 minutes. Finally, the sections were rinsed in PBS/BSA and distilled water and mounted in Kaiser's glycerol gelatin.
Flow cytometric analysis.Blood and BM was sampled for diagnostic purposes from AML patients and were French-American-British (FAB) classified.16,17 As a control, peripheral blood (PB) and BM, obtained for diagnostic purposes, from a healthy donor were used. The cells were stained for intracellular antigens according to Syrjalä et al.18 In short, 100 μL of heparinized blood was incubated with the relevant conjugated antibodies for 10 minutes at room temperature. The antibodies used for flow cytometric analysis were: CD3-PerCP, CD4-FITC, CD8-PE (Becton Dickinson Immunocytometry systems, San Jose, CA); CD10-PE, CD14-PE (Coulter, San Fransisco, CA); CD15-PE (CLB-CD15-PE; Immuno Quality Products, Groningen, The Netherlands); and CD19-TC (Sanbio, Uden, The Netherlands). The cells were then lysed and fixed by adding 2 mL of FACS Lysing Solution (Becton Dickinson) and incubating for 10 minutes at room temperature. The cells were centrifuged and washed twice with PBS + 0.5% BSA and incubated with AAA4-FITC for 10 minutes. Again the cells were washed twice with PBS-BSA and were finally resuspended in 200 μL of FACS Flow (Becton Dickinson). The analyses were performed with a FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA).
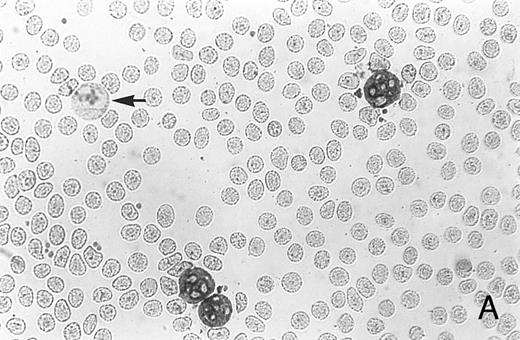
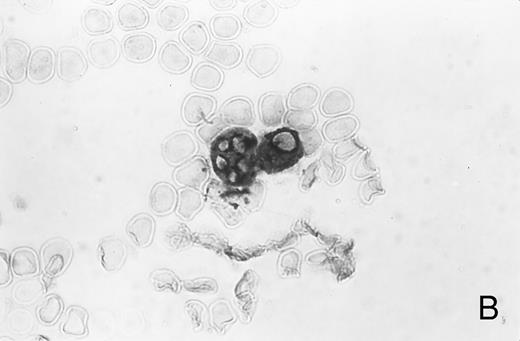
Immunocytochemistry of blood and BM. (A) Blood smear from a healthy donor stained with MoAb AAA4 shows NAMLAA-positive granulocytes and an NAMLAA-negative monocyte (arrow). (B) BM smear after staining with AAA4 shows an NAMLAA-positive promyelocyte and a mature granulocyte, both NAMLAA-positive.
Immunocytochemistry of blood and BM. (A) Blood smear from a healthy donor stained with MoAb AAA4 shows NAMLAA-positive granulocytes and an NAMLAA-negative monocyte (arrow). (B) BM smear after staining with AAA4 shows an NAMLAA-positive promyelocyte and a mature granulocyte, both NAMLAA-positive.
Bronchoalveolar macrophages, sorted for high auto-fluorescence, were stained for NAMLAA. Twenty percent of the macrophages stained positive.
Bronchoalveolar macrophages, sorted for high auto-fluorescence, were stained for NAMLAA. Twenty percent of the macrophages stained positive.
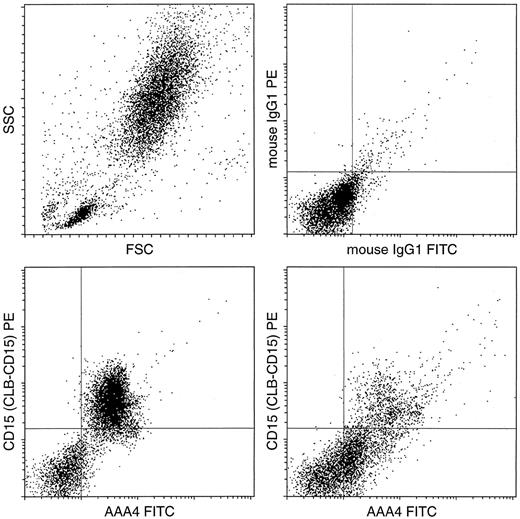
Flow cytometric analysis of BM from a healthy donor compared with an FAB-classified AML-M3 patient. Double labeling was performed with AAA4-FITC (NAMLAA specific) and CD15-PE. The scatter morphology of the BM sample and the control stainings with IgG-FITC and IgG-PE are given in the two upper plots. Double-labeling of BM cells with the antibody AAA4-FITC and the granulocyte marker CD15-PE shows that in the normal situation (lower left) the (immature) granulocytes are CD15 and NAMLAA double positive, whereas the AML cells are only positive for NAMLAA.
Flow cytometric analysis of BM from a healthy donor compared with an FAB-classified AML-M3 patient. Double labeling was performed with AAA4-FITC (NAMLAA specific) and CD15-PE. The scatter morphology of the BM sample and the control stainings with IgG-FITC and IgG-PE are given in the two upper plots. Double-labeling of BM cells with the antibody AAA4-FITC and the granulocyte marker CD15-PE shows that in the normal situation (lower left) the (immature) granulocytes are CD15 and NAMLAA double positive, whereas the AML cells are only positive for NAMLAA.
Expression of NAMLAA in FAB Classified AML Patients
| FAB . | Patient No. . | NAMLAA . |
|---|---|---|
| AML-M0/M1 | 270296 | Negative |
| 120796 | Negative | |
| AML-M1/M2 | 141195 | Negative |
| 020496 | Negative | |
| AML-M2 | 071195 | Negative |
| 201195 | Negative | |
| 300196 | Positive | |
| 310596 | Negative | |
| 060896 | Positive | |
| AML-M3 | 220196 | Positive |
| 090296 | Positive | |
| AML-M4eo | 290196 | Negative |
| AML-M4 | 240796 | Negative |
| AML-M5 | 150296 | Negative |
| 151096 | Negative | |
| AML-M5a | 120796 | Negative |
| AML-M5b | 260496 | Negative |
| AML-M7 | 191296 | Positive |
| 230996 | Positive | |
| CML | 301195 | W. positive |
| 030196 | W. positive |
| FAB . | Patient No. . | NAMLAA . |
|---|---|---|
| AML-M0/M1 | 270296 | Negative |
| 120796 | Negative | |
| AML-M1/M2 | 141195 | Negative |
| 020496 | Negative | |
| AML-M2 | 071195 | Negative |
| 201195 | Negative | |
| 300196 | Positive | |
| 310596 | Negative | |
| 060896 | Positive | |
| AML-M3 | 220196 | Positive |
| 090296 | Positive | |
| AML-M4eo | 290196 | Negative |
| AML-M4 | 240796 | Negative |
| AML-M5 | 150296 | Negative |
| 151096 | Negative | |
| AML-M5a | 120796 | Negative |
| AML-M5b | 260496 | Negative |
| AML-M7 | 191296 | Positive |
| 230996 | Positive | |
| CML | 301195 | W. positive |
| 030196 | W. positive |
Abbreviation: FAB, French-American-British; W., weak.
Cryo ultramicrotomy and immunolabeling.Leukocytes from PB were fixed with a mixture of 0.5% (vol/vol) glutaraldehyde and 4% (wt/vol) paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.2) for 1 hour and were pelleted in 10% (wt/vol) gelatin in PBS. Ultrathin frozen sections were incubated at room temperature with AAA4 (60 μg/mL), followed by incubation with rabbit–antimouse-IgG (1/40) and 10-nm gold-conjugated goat–antirabbit-IgG (1/40). In double-labeling experiments, the cryosections were incubated first with AAA4, followed by incubation with rabbit–antimouse IgG and 5 nm gold-conjugated goat–antirabbit-IgG and then, to prevent any interference between the different antibody gold complexes on the sections,19 for 10 minutes with 1% glutaraldehyde. Subsequently, the sections were incubated with rabbit–antihuman myeloperoxidase (MPO; 1/1,500) or rabbit–antihuman lactoferrin (LF, 1/200) and antirabbit-IgG linked to 10 nm gold (MPO and LF were from Cappel Laboratories, Cocharanville, PA; antirabbit gold conjugates were from Amersham Nederland, 's-Hertogenbosch, The Netherlands). As a control, the primary antibody was replaced by a nonrelevant murine or rabbit antibody. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined with a Philips CM10 electron microscope (Eindhoven, The Netherlands).
Preparation of neutrophil fractions and Western blot analysis.Neutrophil fractions were isolated essentially as described by Bolscher et al,20 except that a sucrose gradient of 15/40/52/60% (wt/vol) was used to recover the plasma membrane, specific granule, and azurophilic granule fractions.
The granulocyte fractions were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples of 20 μL were boiled for 3 minutes with 5 μL of loading buffer (60 mmol/L Tris⋅HCl pH 6.8; 23% glycerol; 3% SDS; 0.06% bromophenol blue; 10% 2-mercaptoethanol). Ten microliters of these samples was analyzed on 10% SDS-PAGE (Mini Protein; BioRad, Richmond, CA). Samples were transferred to nitrocellulose in 25 mmol/L Tris, 190 mmol/L glycine, and 20% methanol transfer buffer. Aspecific binding sites were blocked by incubating the nitrocellulose sheets in low-fat milk for 30 minutes and subsequently washing three times with 0.5% Tween-20 in PBS. The nitrocellulose sheets were then incubated for 1 hour at room temperature with MoAbs diluted 500 times in PBS-Tween. After three washes, goat–antimouse-IgG conjugated to alkaline phosphatase (DAKO) was added in a 1,000-times dilution, and the blots were incubated for 1 hour at room temperature. The blots were washed three times with PBS-Tween and then three times with PBS. For visualization of antibody-antigen complexes, the alkaline-phosphatase substrate nitroblue tetrazolium/5-bromo-4-chloro indoxyl phosphate (NBT/BCIP) was used as described.21
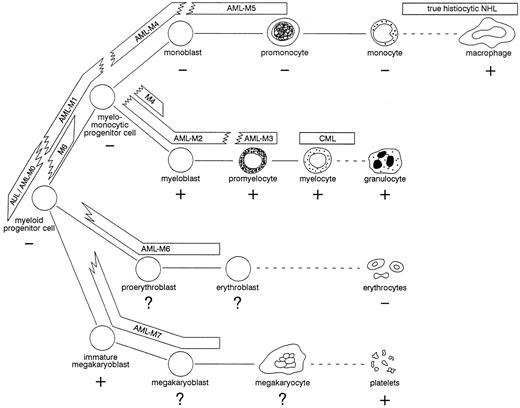
Hypothetical scheme of myeloid differentiation. The expression of NAMLAA is indicated for each differentiation stage with + and −. The bars represent the various types of leukemias and non-Hodgkin's lymphomas and indicate where these malignancies can be located according to their maturation arrest. To indicate the heterogeneous phenotype found in most AML patients, the bars fade into each other. CML, chronic myeloid leukemia; NHL, non-Hodgkin's lymphoma.
Hypothetical scheme of myeloid differentiation. The expression of NAMLAA is indicated for each differentiation stage with + and −. The bars represent the various types of leukemias and non-Hodgkin's lymphomas and indicate where these malignancies can be located according to their maturation arrest. To indicate the heterogeneous phenotype found in most AML patients, the bars fade into each other. CML, chronic myeloid leukemia; NHL, non-Hodgkin's lymphoma.
MPO activity in the fractions was used to measure the presence of proteins from the azurophilic granules in the specific, membrane, and cytosol fractions. Serial dilutions (25 μL) of the fractions in peroxidase buffer (18.25 g/L Na2HPO4 , 10.5 g/L citric acid pH 5.0) were prepared in titertec flatbottom plates and subsequently incubated with 75 μL peroxidase substrate solution (peroxidase buffer + 0.012% H2O2 [vol/vol]) final concentration + 400 mg/L orthophenylene diamine; Sigma). After 10 minutes at room temperature, the reaction was stopped with 50 μL 4 mol/L H2SO4 . The absorbance was measured at 492 nm in a Titertec Multiscan (Flow Lab, Irvine, Scotland).
RESULTS
Localization of NAMLAA in human tissues.NAMLAA-positive cells were found in human spleen, liver, lymph nodes, and synovial tissues of arthritis patients and healthy controls. Double staining of spleen tissue for NAMLAA and a variety of markers for fibroblasts, HLA class-II, T cells, B cells, monocytes, macrophages, dendritic cells, and granulocytes (Table 1) showed double staining of granulocytes only (data not shown).
Immunocytochemical studies of blood and BM showed that granulocytes, but not monocytes, stained positive for NAMLAA (Fig 1A). Figure 1B shows that band-form granulocytes from BM were also positively stained for NAMLAA. Although monocytes and spleen macrophages did not express NAMLAA, a 20% subpopulation of highly fluorescent bronchoalveolar macrophages was positive for NAMLAA (Fig 2), indicating that differentiation of monocytes to macrophages induces NAMLAA expression.
The observation that granulocytes stain positive, and that monocytes and lymphocytes were negative, was confirmed by flow cytometric analysis of PB and BM from a healthy donor. Double-labeling for NAMLAA and CD15 of BM cells from a healthy donor compared with BM cells from an FAB-classified AML-M3 patient showed that the granulocytes were double positive for NAMLAA and CD15 whereas the promyelocytes are only positive for NAMLAA (Fig 3). In double-labeling experiments, neither the B-cell marker CD10 or CD19 nor the T-cell marker CD3, CD4, or CD8 stained NAMLAA-positive cells. This indicates that in blood and BM NAMLAA expression is restricted to the myeloid lineage.
Expression of NAMLAA in various acute myeloid leukemias (AMLs).Blood and BM from FAB-classified AML patients were screened for NAMLAA-positive cells (Table 2). M0 (myeloid precursor), M1 (myelo-monocytic precursor), and M4 (monoblast) leukemic cells were negative, whereas M3 (promyelocyte) leukemic cells were positive. Chronic myeloid leukemia (CML) (myelocyte) and M7 (megakaryoblast) cells were also positive. In the myeloblast stage (M2), leukemic cells from two patients were NAMLAApositive, while those of three other patients were negative, suggesting that expression of NAMLAA starts at this stage of granulocyte maturation (Fig 4).
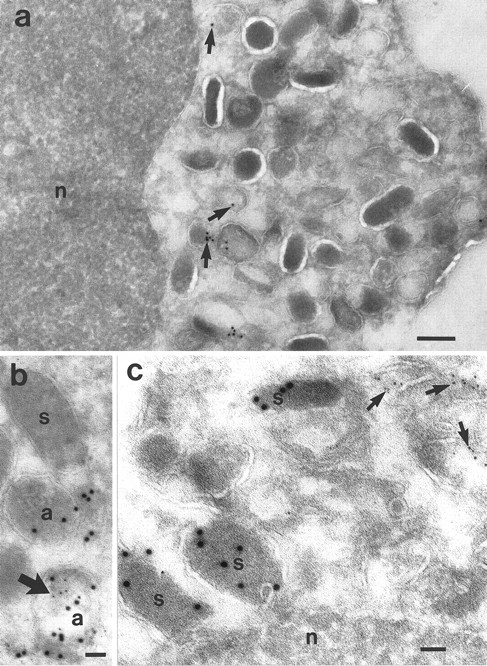
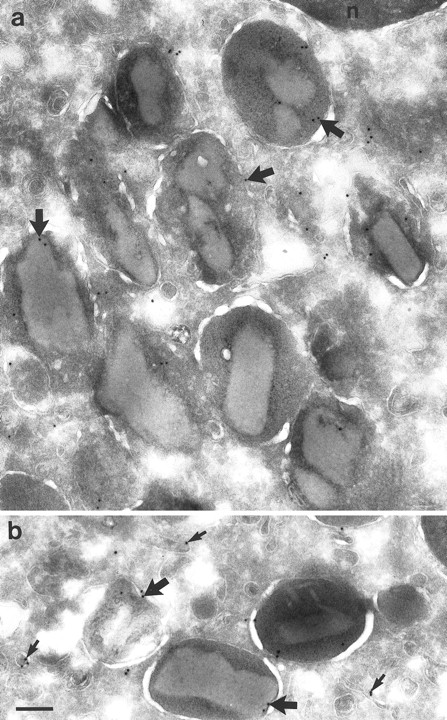
Localization of NAMLAA in granulocytes.Immunoelectron microscopic studies were performed on ultrathin cryosections of neutrophils and eosinophils. The sections were incubated with the NAMLAA-specific MoAb AAA4 and were subsequently stained with gold-labeled antibodies. The gold label was found in vesicles and granules of neutrophils (Fig 5a). No background staining was found on the nucleus. The granules were subsequently characterized in double-labeling experiments. Myeloid peroxidase, a marker of azurophilic granules, colocalized with NAMLAA in some but not all azurophilic granules (Fig 5b). We have counted a total of 195 granules and 26.2% were positive for NAMLAA and MPO, 25.6% were positive for MPO alone, 2.0% were positive for NAMLAA alone, and 46.2% of the granules were negative. No colocalization was found with lactoferrin (Fig 5c), a marker for specific granules. We have counted 208 granules: 17.2% were positive for NAMLAA, 61.0% were positive for lactoferrin, and 21.2% were negative. NAMLAA was more abundant in the crystalloid-containing granules and vesicles of eosinophils (Fig 6). Monocytes present in the same sections were negative for NAMLAA.
Cryosections of neutrophils from PB. (a) Localization of NAMLAA after incubation with the NAMLAA-specific MoAb AAA4. The gold label is shown in vesicles and granules (arrows). No background is shown on the nucleus (n). (b) Micrograph showing double labeling for NAMLAA (5 nm gold) and MPO, a marker for azurophilic granules (10 nm gold). Both labels were seen in some, but not in all, MPO-positive azurophilic granules. (c) Localization of lactoferrin (10 nm gold) and NAMLAA (5 nm gold) showed that the lactoferrin-positive specific granules (s) were not labeled with AAA4, whereas other granules/vesicles were labeled with AAA4 (arrows). Bars: (a), 200 nm; (b), 50 nm; (c), 100 nm.
Cryosections of neutrophils from PB. (a) Localization of NAMLAA after incubation with the NAMLAA-specific MoAb AAA4. The gold label is shown in vesicles and granules (arrows). No background is shown on the nucleus (n). (b) Micrograph showing double labeling for NAMLAA (5 nm gold) and MPO, a marker for azurophilic granules (10 nm gold). Both labels were seen in some, but not in all, MPO-positive azurophilic granules. (c) Localization of lactoferrin (10 nm gold) and NAMLAA (5 nm gold) showed that the lactoferrin-positive specific granules (s) were not labeled with AAA4, whereas other granules/vesicles were labeled with AAA4 (arrows). Bars: (a), 200 nm; (b), 50 nm; (c), 100 nm.
Cryosections of eosinophils from PB incubated with MoAb AAA4. (a) Area of an eosinophil showing labeling for NAMLAA on the crystalloid-containing granules (arrows). (b) Area of another eosinophil showing labeling on the same granules (thick arrows) and on the membrane of small vesicles (small arrows). Bar = 200 nm.
Cryosections of eosinophils from PB incubated with MoAb AAA4. (a) Area of an eosinophil showing labeling for NAMLAA on the crystalloid-containing granules (arrows). (b) Area of another eosinophil showing labeling on the same granules (thick arrows) and on the membrane of small vesicles (small arrows). Bar = 200 nm.
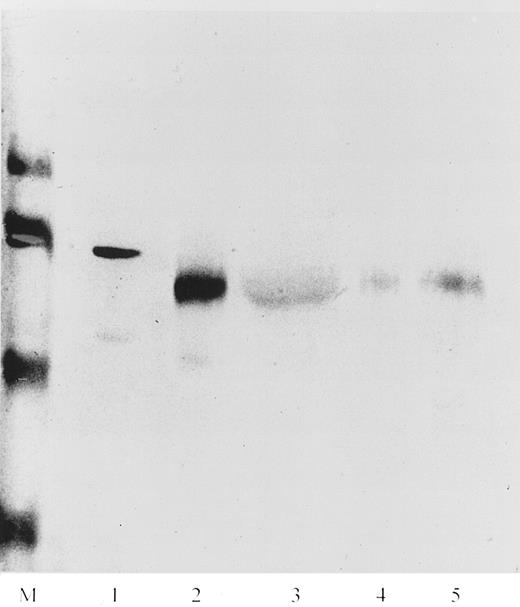
To confirm the results obtained by immunoelectron microscopy the localization of NAMLAA in neutrophilic granulocytes was investigated also by Western blotting. The neutrophilic granulocytes were fractionated into a semi-purified membrane fraction, a cytosolic fraction without membranes, a fraction containing mainly specific granules and a fraction containing mainly azurophilic granules. The presence of proteins from the azurophilic granules in the other neutrophil fractions was based on the content of MPO activity. The membrane fraction contained about 10% of the total specific activity whereas the cytosol and specific granules fractions contained about 5% MPO activity. The fractions were then analyzed by Western blot. As shown in Fig 7, the membrane fraction (lane 5) and the azurophilic granule–containing fraction (lane 2) showed a band with a molecular mass of approximately 65 kD. The glycosylated form of NAMLAA, purified from human plasma, had a molecular mass of 70 kD (lane 1). The cytosol fraction and the specific granule-containing fraction were stained very faintly.
Western blot of neutrophil cell fractions. Lane M corresponds to prestained molecular weight markers of 116, 80, 49, and 32 kD. Lane 1 corresponds to purified NAMLAA from human plasma. Lanes 2, 3, 4, and 5 correspond to the azurophilic granule–containing fraction, the cytosol fraction, the specific granule–containing fraction, and the purified plasma membrane fraction, respectively. Only the azurophilic granule–containing fraction and the membrane fraction were recognized by MoAb AAA4, producing a band with a lower molecular mass than the NAMLAA purified from plasma in lane 1.
Western blot of neutrophil cell fractions. Lane M corresponds to prestained molecular weight markers of 116, 80, 49, and 32 kD. Lane 1 corresponds to purified NAMLAA from human plasma. Lanes 2, 3, 4, and 5 correspond to the azurophilic granule–containing fraction, the cytosol fraction, the specific granule–containing fraction, and the purified plasma membrane fraction, respectively. Only the azurophilic granule–containing fraction and the membrane fraction were recognized by MoAb AAA4, producing a band with a lower molecular mass than the NAMLAA purified from plasma in lane 1.
DISCUSSION
The two most important enzymes capable of degrading peptidoglycan in humans are lysozyme and NAMLAA. Lysozyme is present in specific and azurophilic granules of neutrophils24,25 and in the granules of monocytes,26 but its presence was not detectable in eosinophilic granulocytes.27 28 NAMLAA is expressed differently. It is present in the azurophilic granules of neutrophils and in the crystalloid-containing granules of eosinophilic granulocytes. In a previous study, in which we investigated the inflammatory properties of peptidoglycan products before and after NAMLAA degradation, we found that the cooperative action of lysozyme and NAMLAA is essential for optimal degradation of peptidoglycan. Therefore, the difference in localization between NAMLAA and lysozyme is remarkable.
Azurophilic granules store a number of digestive enzymes, which are essential for the inactivation of invading microorganisms. Using some of these enzymes as a marker, Egesten et al29 were able to classify heterogeneous forms of azurophilic granules in promyelocytes. By using antibodies against MPO, bactericidal permeability increasing protein, cathepsin G, elastase, and proteinase 3, they classified azurophilic granules into nucleated azurophils, large spherical azurophils, and small azurophils. In Fig 5b it is shown that NAMLAA did not always colocalize with MPO, which suggests that NAMLAA is also heterogeneously expressed between the different forms of azurophilic granules.
In previous studies it was shown that NAMLAA is a glycoprotein with a molecular mass of 70 kD, but its mass is 60 kD after deglycosylation.3 Using two-dimensional gel electrophoresis, we detected multiple isoforms of NAMLAA with the main spots between pH 7.5 and 7.8. This proves that NAMLAA is a cationic protein, as are most of the proteins in the azurophilic granules.29 The neutrophil fractions subjected to Western blot analysis showed a broad protein band with a molecular mass between the fully glycosylated 70-kD band and the deglycosylated 60-kD band. The molecular mass and the broadness of the band indicate that the enzyme may have been partly degraded during the isolation procedure. The presence of the band in the azurophil fraction but not in the fraction containing the specific granules confirms the results obtained by immunoelectron microscopy. The fact that the membrane fraction also contained the same band is probably caused by contamination of the membrane fraction with components of the azurophil fraction.
The NAMLAA-positive cells in the tissues tested were granulocytes migrated into the tissues and granulocytes present in the blood vessels of the tissues. Surprisingly, the NAMLAA-containing cells in the liver were not Kupffer cells, which are known to be involved in the clearing of bacterial debris from the circulation. Why these cells do not express NAMLAA is unknown, but it is consistent with the findings of Daldorff et al30 and Lichtman et al,31 who showed in a rat model that bacterial cell walls can persist in the liver.
The expression of NAMLAA during the maturation of the myeloid lineage shows that NAMLAA expression starts early in granulocyte maturation and late in monocyte/macrophage maturation. This difference is probably related to the effector function of these cell types. Monocytes are actually precursor cells of macrophages and may not need NAMLAA for their effector function in this stage, whereas granulocytes and macrophages are mature phagocytizing cells. The expression pattern of NAMLAA during myeloid differentiation shows that NAMLAA is also expressed in the megakaryocyte lineage, corresponding with the finding that thrombocytes in blood were also stained positive for NAMLAA.
In conclusion, the results presented in this report show the presence of a novel enzyme, capable of degrading bacterial cell walls, in azurophylic granules of neutrophilic granulocytes, in the crystalloid-containing granules of eosinophilic granulocytes, in thrombocytes, and in a sub-population of activated alveolar macrophages. The expression pattern of NAMLAA in the myeloid lineage suggests that AAA4 is useful for discrimination between AML in the monocyte lineage and in the granulocyte lineage. Therefore, AAA4 might contribute to the diagnosis and classification of acute leukemias. The cloning of the gene coding for NAMLAA is in progress.
ACKNOWLEDGMENT
We thank Tar van Os for photographic assistance, Annemieke ten Bokum for careful reading of the manuscript, and Harm de Wit for supplying the sorted BAL macrophages.
Address reprint requests to M.A. Hoijer, PhD, Department of Immunology, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal