Abstract
Primary effusion (body cavity–based) lymphoma (PEL) is a recently recognized subtype of malignant lymphoma that exhibits distinctive clinical and biological features, most notably its usual infection with the Kaposi's sarcoma–associated herpesvirus (KSHV). The vast majority of cases also contain Epstein-Barr virus (EBV). This dual viral infection is the first example of a consistent dual herpesviral infection in a human neoplasm and provides a unique model to study viral interactions. We analyzed the pattern of EBV latent gene expression to determine the pathogenic role of this agent in PELs. We examined five PELs coinfected with EBV and KSHV by reverse transcription-polymerase chain reaction (RT-PCR), in situ hybridization, and immunohistochemistry. EBER1 mRNA, a consistent marker of viral latency, was positive in all PEL cases, although at lower levels than in the non-PEL controls due to EBER1 expression by only a variable subset of lymphoma cells. Qp-initiated mRNA, encoding only EBNA1 and characteristic of latencies I and II, was positive in all PEL cases. Wp- and Cp-initiated mRNAs, encoding all EBNAs and characteristic of latency III, were negative in all cases. LMP1 mRNA, expressed in latencies II and III, was present in three cases of PEL, although at very low levels that were not detectable at the protein level by immunohistochemistry. Low levels of LMP2A mRNA were detected in all cases. BZLF1, an early-intermediate lytic phase marker, was weakly positive in four cases, suggesting a productive viral infection in a very small proportion of cells, which was confirmed by ZEBRA antigen expression. Therefore, PELs exhibit a restricted latency pattern, with expression of EBNA1 in all cases, and low LMP1 and LMP2A levels.
A SUBSET OF high-grade non-Hodgkin's lymphomas (NHLs) growing mainly or exclusively in the body cavities as lymphomatous effusions has been reported in acquired immune deficiency syndrome (AIDS) patients.1-4 Subsequently, these lymphomas, called body cavity–based lymphomas (BCBL) or primary effusion lymphomas (PEL), were found to contain a new member of the herpesvirus family, the Kaposi's sarcoma–associated herpesvirus (KSHV),5 also designated human herpesvirus-8 (HHV-8).6 The close and specific association of KSHV with PELs has since been confirmed by other investigators.7-10 The unique clinical and biological features of PELs include: (1) an epidemiology similar to that of Kaposi's sarcoma (KS), occurring mostly in male homosexual AIDS patients and rarely in elderly HIV-negative individuals5,11; (2) a morphology that bridges large cell immunoblastic and anaplastic large cell lymphomas; (3) an indeterminate immunophenotype with a B-cell genotype; (4) a lack of c-myc oncogene alterations12; and (5) the presence of Epstein-Barr virus (EBV) in addition to KSHV in the vast majority of cases.12
EBV can establish a latent infection in B lymphocytes, giving rise to three patterns based on the selective expression of nine latent genes.13 The most restricted pattern of latency corresponds to latency I (Lat I). This pattern is found characteristically in Burkitt's lymphoma14 and in circulating mononuclear cells of healthy EBV-infected individuals.15 The most complete pattern of expression of latent viral genes represents latency III (Lat III), which can be detected in lymphoblastoid cell lines (LCL)16 and in lymphoproliferative disorders occurring in immunosuppressed individuals in the setting of transplantation17 and AIDS.18 A third type of latency, latency II (Lat II), has been recognized in undifferentiated nasopharyngeal carcinoma19 and in EBV-positive cases of Hodgkin's disease.20 Understanding the pattern of latent gene expression is important for evaluating the role of EBV in these diseases because the degree of activity of the latent virus correlates with its transforming properties. For example, in Lat I (restricted latent gene expression) the virus mainly expresses EBNA1, a gene required for episomal replication, allowing for maintenance of its genome. In contrast, in Lat III (full pattern of latent gene expression), the virus expresses nine genes, including the transforming EBNA2 and LMP1, and thus can be the main driving force for cell proliferation.
The EBV latency pattern of PELs has not been previously characterized, and the role of EBV in the development of this subset of NHLs is unknown. The dual viral infection with EBV and KSHV, the first example of a dual herpesviral infection in a human neoplasm, provides a unique model to study viral interactions. We analyzed the type of EBV latent gene expression in five PELs to better understand these interactions and the role of both viruses in the pathobiology of PEL.
MATERIALS AND METHODS
Pathological samples.A group of five malignant lymphomas containing KSHV and EBV sequences meeting the suggested clinical, morphological, immunophenotypic, and molecular genetic criteria for PEL,5,12,21 were selected for inclusion in this study. Four of the five PELs are HIV-related. Cases 1 and 2 represent the BC1 and BC2 cell lines established from two PELs.22 Case 3 is a previously reported malignant lymphomatous effusion occurring in an HIV-negative elderly man11 (case 18). Cases 4 and 5 represent adult AIDS patients with malignant lymphomatous effusions not reported previously. A LCL called UH-1, which was established by infecting normal human cord blood lymphocytes with EBV obtained from B95-8 cells, was used as a positive control for Lat III. This LCL is not tightly latent, and thus also serves as a positive control for productive infection. As an additional control we used RNA extracted from an AIDS-related large-cell immunoblastic lymphoma (IBL), which had very similar clinical, morphologic, immunophenotypic, and molecular features to the PELs, with the exception that it was negative for KSHV and that it presented and remained as a solid tumor mass. Because of these similarities, this case was reported together with two cases of PEL before the identification of KSHV (patient no. 2).1 A myeloid cell line (HL60) lacking EBV was used as negative control.
Heparin-treated samples of the effusions were collected under sterile conditions during the course of standard diagnostic procedures. Mononuclear cells were isolated and cryopreserved as described.5
Nucleic acid extraction.Genomic DNA was extracted from cryopreserved mononuclear cell suspensions by a salting-out procedure as previously described.23
Total RNA was isolated from the cell lines and lymphoma cells using the TRI REAGENT nucleic acids extraction kit (Molecular Research Center Inc, Cincinnati, OH) according to the manufacturer's instructions.
Southern blot hybridization analysis.Five-microgram aliquots of genomic DNA were digested with the appropriate restriction endonucleases according to the manufacturer's instructions (Boehringer Mannheim, Indianapolis, IN), electrophoresed in 0.8% agarose gels, denatured by alkali, neutralized, and transferred to nitrocellulose filters according to Southern.24 The filters were then hybridized as previously described25 to probes labeled by 32P with the Random Primed DNA labeling system (Boehringer Mannheim, Mannheim, Germany). Autoradiography was performed at −70°C for 16 to 48 hours. The presence of KSHV sequences was determined by hybridizing BamHI-digested DNA to the KS330Bam probe.26 The presence of a clonal EBV infection was shown by hybridization of BamHI-digested DNAs to a probe specific to EBV genomic termini.27
Oligonucleotide primers and probes.The primer-probe combinations used in this study have been previously published by Lin et al28 (for EBNA2, EBNA3c, and EBER1), and Tierney et al15 (for Cp, Wp, Qp initiated EBNAs, LMP1, LMP2A, and BZLF1). A primer set specific for β-actin cDNA (Stratagene, La Jolla, CA) was used as a quantitative control for the RT-PCR. The sequence of the β-actin internal oligonucleotide probe was selected from its cDNA sequence.
Polymerase chain reaction (PCR) amplification and hybridization.PCR analysis was used to determine the type of the EBV strain with primer sets for EBNA2 and EBNA3c region of the EBV. The conditions used for amplification and hybridization were as previously described.29
Reverse transcription (RT)-PCR amplification and hybridization.RNA samples were initially treated by 10 U RNase free DNase I (Boehringer Mannheim, Germany) in 20 mmol/L TrisHCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2 reaction buffer for 15 minutes at room temperature. The DNase was heat-inactivated after addition of 25 pmol/L of ethylenediaminetetraacetic acid (EDTA). RT was performed with 1 μg RNA and 0.5 ng random hexamers using the Superscript reverse transcriptase system (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer's instructions. DNase-treated RNA was also amplified without the RT reaction to rule out DNA contamination of the RNA samples. The synthesized first strand cDNAs were subjected to PCR with an initial denaturing phase of 94°C for 3 minutes, and then subjected to 30 cycles of amplification in DNA thermal cycler (Perkin-Elmer, Norwalk, CT) as follows: denaturation, 94°C for 1 minute; primer annealing, 45°C (Wp, Cp, Qp), 48°C (LMP2A), 50°C (LMP1, BZLF1), or 60°C (β-actin) for 2 minutes; and extension, 72°C for 3 minutes. PCR was performed with 0.2 pmol/L of each primer, 20 mmol/L TrisHCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2 , 200 μmol/L, and 0.5 U of the Taq DNA polymerase. Serial dilutions of the cDNA preparations were amplified with the β-actin primers to ensure a similar amount of cDNA in all the samples. Amplified products were visualized on an ethidium-bromide–stained 2% agarose gel after electrophoresis, then transferred to a nitrocellulose membrane and hybridized with 32P end-labeled internal oligonucleotide probe. The filters were then rinsed once and washed at room temperature for 15 minutes and for 10 minutes at 55°C (U, LMP1, BZLF1), 58°C (LMP2A), 61°C (W2), or 57°C (β-actin) as previously described.29 The blots were exposed at −80°C with an intensifying screen for 1 to 2 hours or overnight for LMP1.
Immunocytochemistry.Immunocytochemical analysis for EBV-specific proteins was performed with monoclonal antibodies against LMP1 (CS 1 to 4), EBNA2 (pE2), and ZEBRA (BZ.11) purchased from DAKO (Carpenteria, CA) in cases 1, 2, 3, and 4. Expression of these three antigens was evaluated on acetone fixed cytospin preparations. In addition, expression of EBNA2 and ZEBRA was analyzed on paraffin sections from formalin fixed agarose-embedded cell blocks after antigen retrieval for 10 minutes in 10 mmol/L sodium citrate buffer, pH 6.0, using a Black and Decker (model HS800) steamer. Antibodies were applied at a 1:200 dilution (anti-LMP1) or 1:100 dilution (anti-EBNA2 and anti-ZEBRA) and incubated overnight at 4°C. For demonstration of LMP1 immunoreactivity, a biotinylated horse anti-mouse antibody and Vectastain Elite ABC peroxidase kit (Vector, Burlingame, CA) were used.30 Horseradish peroxidase was developed with 3,3′-diaminobenzidine (Sigma Chemical Co, St Louis, MO) as previously described.31 The immuno-alkaline phosphatase (APAAP) technique was applied to show binding of anti-EBNA2 and ZEBRA antibodies. Rabbit anti-mouse antibody and mouse APAAP (DAKO) were used as second and third steps respectively. Alkaline phosphatase reaction was revealed by new fuchsin substrate.32 Slides were counterstained with hematoxilin. Hodgkin's disease tissue, UH-1 LCL, a posttransplantation lymphoproliferative disorder and 12-O-tetradecanoylphorbol-13-acetate (TPA)–stimulated BC-2 cells were used as controls.
In situ hybridization.EBV-encoded RNA (EBER) expression was detected by in situ hybridization using the Epstein-Barr virus Probe system (Vector) according to the manufacturer's protocol.
RESULTS
KSHV infection.The presence of KSHV DNA sequences was determined by Southern blot hybridization of BamHI digested DNA with the KS330Bam probe. All cases showed the characteristic strong band at the expected position (330 bp; Fig 1).
Southern blot analyses for EBV terminal repeat and KSHV. DNA samples were digested with the BamHI restriction enzyme and hybridized to the BamHI NJ-het probe (upper panel) and to the KS330Bam probe (lower panel). UH1, an established LCL, was used as a positive control for EBV and as a negative control for KSHV. The number above each lane corresponds to the PEL case.
Southern blot analyses for EBV terminal repeat and KSHV. DNA samples were digested with the BamHI restriction enzyme and hybridized to the BamHI NJ-het probe (upper panel) and to the KS330Bam probe (lower panel). UH1, an established LCL, was used as a positive control for EBV and as a negative control for KSHV. The number above each lane corresponds to the PEL case.
EBV clonality and type.The clonality of EBV infection was investigated by Southern blot hybridization of BamHI digested DNAs to a probe specific for the EBV genomic termini.27 The detection of a single band in each of the five PELs indicates that each lymphoma arose from the clonal expansion of a single EBV-infected cell (Fig 1). PCR for the EBNA2 and EBNA3C regions28 showed that case 1 contained EBV2; and cases 2, 3, 4, and 5 contained EBV1.
In situ hybridization for EBER.Because the EBERs are the most abundant EBV latent transcripts and are also common to all forms of latency, we performed in situ hybridization using a cDNA probe cocktail specific for EBER1 and EBER2. While EBER was expressed in practically all tumor cells of our positive controls, we identified EBER positivity in a variable percentage of PEL tumor cells, which ranged from 20% to 60% (not shown).
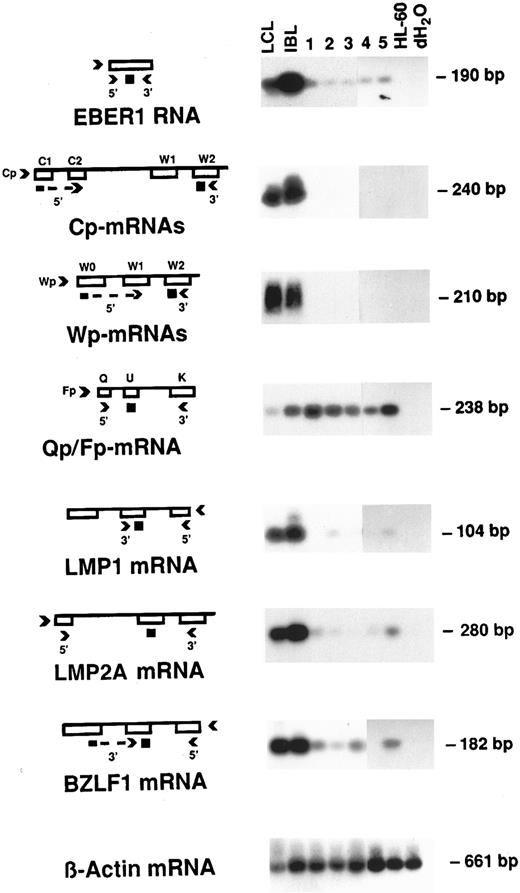
RT-PCR analysis of EBV latency.The type of EBV latency was investigated using the RT-PCR method. EBER1 RNA expression was detected it in all five PEL cases, although the signal was relatively weak, as compared with the LCL and IBL positive controls (Fig 2). This is consistent with the lack of EBER expression in a fraction of the tumor cells.
RT-PCR for EBV latent transcripts. RNA samples were subjected to RT reaction, then cDNA was amplified with the appropriate primers for each latent gene region. The PCR products were hybridized to a 32P-labeled internal oligonucleotide probe. LCL was used as positive control; IBL was used as an additional control because of its similarities to PELs. HL60 is a promyelocytic cell line used as negative control; H2O represents reactions carried out in the absence of cDNA. The number above each lane corresponds to the PEL case. Diagrams on the left illustrate the corresponding transcripts, with arrows indicating primers and small black squares representing the probes used. β-Actin was used as a control for equal amounts of mRNA used for the RT-PCR reaction.
RT-PCR for EBV latent transcripts. RNA samples were subjected to RT reaction, then cDNA was amplified with the appropriate primers for each latent gene region. The PCR products were hybridized to a 32P-labeled internal oligonucleotide probe. LCL was used as positive control; IBL was used as an additional control because of its similarities to PELs. HL60 is a promyelocytic cell line used as negative control; H2O represents reactions carried out in the absence of cDNA. The number above each lane corresponds to the PEL case. Diagrams on the left illustrate the corresponding transcripts, with arrows indicating primers and small black squares representing the probes used. β-Actin was used as a control for equal amounts of mRNA used for the RT-PCR reaction.
The C1 /C2 /W1 /W2 splice structure is typical of Cp-initiated EBNA mRNAs and is characteristic of Lat III. The expected 240 bp was shown in the Lat III LCL control and the case of IBL, but not in any of the five PELs. The W0 /W1 /W2 splice structure is typical of Wp-initiated EBNA mRNAs and is also characteristic of Lat III. The expected 210 bp band was shown in the Lat III LCL control and the IBL specimen, but not in any of the five PELs (Fig 2).
The BamHI Q/U/K splice structure is expressed from the Q promoter of the EBNA1 gene in Lat I Burkitt's lymphomas,33-35 as well as from the F promoter in LCL cells when they switch from Lat III to lytic cycle.33 36 Q/U/K transcripts were clearly detected in all PELs, yielding the expected 165-bp product (Fig 2). These are probably due to Qp initiation, although we have not characterized these transcripts. The LCL and IBL controls also showed a signal with the Q/K primer pair, which was fainter in the LCL control. The transcripts in these controls are most likely caused by Fp-initiated mRNA expression because both of these controls have significant lytic viral replication as seen by expression of BZLF1 mRNA (discussed later).
LMP1, a protein present in Lat II and Lat III, is one of the major transforming factors of EBV. RT-PCR analysis of LMP1 mRNA yielded the expected 104-bp product in cases 2, 3, and 5, although the level of expression was much lower than in the LCL and IBL controls and detection of the positive signal required long (overnight) autoradiographic exposure. No LMP1 mRNA could be detected in cases 1 and 4 (Fig 2).
Because LMP2A is expressed more consistently and at higher levels than LMP1 in LatII nasopharyngeal carcinomas,19,37 and is also expressed in Hodgkin's disease,38 expression of this gene was evaluated. We found LMP2A mRNA to be present in all five cases of PEL, although the levels appeared to be lower than in the LCL and IBL controls (Fig 2).
BZLF1 mRNA is an intermediate-early transcript indicative of the switch from latency to lytic cycle. It was present in the LCL and IBL controls and, more weakly, in four cases of PEL (cases 1, 2, 3, and 5) as shown by the presence of the 182-bp product. Case 4 appeared to be tightly latent, being negative for BZLF1 (Fig 2).
β-actin mRNA, a “housekeeping gene,” was used as a control for the amount of mRNA used in all of the previous RT-PCR reactions. A uniformly intense signal was obtained in all the cases as well as in the controls, showing the equal amounts of RNA used for each sample (Fig 2).
Immunocytochemistry.To confirm the results obtained by RT-PCR, we performed immunocytochemistry for LMP1, EBNA2, and ZEBRA antigens (not shown). Whereas the tumor cells in the relevant positive controls showed strong staining for LMP1 and EBNA2, no staining above background levels was identified in the PEL cases. Expression of ZEBRA was identified in rare cells in all the cases of PEL, comprising less than 1% of the entire cell population. This result is consistent with lytic viral replication in a very small proportion of PEL tumor cells.
DISCUSSION
PELs are a recently defined group of non-Hodgkin's lymphomas that appear to represent a distinct biological entity, characterized by a unique set of clinico-pathological as well as molecular features. One of these features is the presence of both the EBV and KSHV genomes in the vast majority of cases examined. Although we do not have definitive evidence that all the PEL cells carry the EBV genome, we have found the KSHV genome to be present in all the lymphoma cells by in situ hybridization (Nador et al, manuscript submitted). Therefore, all the cells containing EBV, and thus being evaluated in this study, have both viral genomes within them. To the best of our knowledge, this is the first example of a dual herpesviral infection consistently associated with a specific human neoplasm and thereby provides a unique model to study viral interactions. To begin to understand these interactions and the role of these viruses in the pathobiology of KSHV-associated lymphomas, we analyzed the type of EBV latent gene expression in five PELs.
RT-PCR analysis for EBERs detected EBER1 RNA in all five PELs, although the signal was weak as compared with the LCL control. These low levels of EBER were shown by in situ hybridization to be caused by the lack of expression in a variable, but frequently significant, proportion of the tumor cell population. This result is consistent with a previous study in which three EBV-positive PELs were reported to contain EBER hybridization signals in 25% to 75% of the neoplastic cells.39 While diminished EBER expression is a feature of cells entering the EBV lytic cycle, this does not appear to be the reason for the low EBER expression in PEL cells because less than 1% of cells in all cases express ZEBRA antigen, as seen by immunohistochemistry. Furthermore, this pattern of in situ hybridization for EBER is reminiscent of that described for nasopharyngeal carcinomas, in which considerable variation in hybridization intensity among the malignant cells has been reported.40 Thus, this lack of expression of EBER from a subset of tumor cells is not unprecedented, although its biological significance remains to be determined.
A relevant finding that emerged from this study is that PELs express EBNA1 mRNA using the Q/U/K splice structure, and thus probably through Qp, as in Lat I Burkitt's lymphomas.13,33 PELs do not use the BamHI Wp or BamHI Cp, which generate EBNA1, 2, 3A, 3B, 3C, and LP in Lat III. EBNA1 is necessary for episomal persistence in latent infection; one of its domains reacts with ori P and a second domain interacts nonspecifically with chromosomal proteins to ensure partitioning of EBV episomes to progeny nuclei.41 EBNA1 has also been recently shown to induce B cell lymphomas when expressed in B cells as a transgene in mice.42 Thus, expression of EBNA1 may contribute to transformation in the PEL cells.
Because Lat I and II share the presence of Q/U/K transcripts and differ only with respect to LMP transcription, we evaluated expression of LMP1 by RT-PCR as well as by immunoperoxidase staining. While the PEL cases analyzed were largely negative for LMP1 expression, we did detect low levels of LMP1 mRNA after long autoradiographic exposures in three cases. We were not able to detect LMP1 at the protein level by immunocytochemistry in any of the cases included in this study. However, it appears that some cases of PEL do express LMP1, as shown by our detection of LMP1 immunoreactivity in approximately 30% of the neoplastic cells of one case of PEL which was not included in this study because of the lack of frozen tissue for RNA extraction (unpublished observation, January 1997). Furthermore, LMP1 antigen expression has been reported in two of three cases of EBV-positive PEL analyzed, in which 5% and 35% of the neoplastic cells were found to be positive.39 Thus, it appears the LMP1 expression is frequently low but can be highly variable in PEL. In contrast, low levels of LMP2A expression were identified in all five cases of PEL. The pattern of LMP1 and LMP2A expression was thus similar to that of nasopharyngeal carcinomas.19 It has been suggested that the LMP expression levels in Lat II should be equivalent to those seen in LCLs.13 Therefore, a sharp distinction between Lat I and Lat II in the presence of borderline detectability of LMP is difficult.
It is interesting to note that PELs do not use the Wp/Cp promoters, which are active in other lymphomas that are associated with immunodeficiency, such as many AIDS-related NHLs18,43 and posttransplantation lymphoproliferative disorders.44 45 This difference is particularly surprising because the morphology of PELs is similar to that of immunoblastic lymphomas, which, when EBV-positive, have a Lat III pattern of gene expression. Thus, the pathobiology of PELs differs from that of EBV-positive immunoblastic lymphomas and posttransplantation lymphoproliferative disorders, not only by the presence of KSHV, but also by the different pattern of EBV gene expression.
In summary, PELs exhibit a restricted pattern of EBV latency. The sole expression of EBNA1 in the absence of significant expression of the major EBV growth transforming factors, such as Wp/Cp-initiated EBNAs and LMP1, suggests that EBV is not singly responsible for transformation. In this setting, it is probable that KSHV plays a major role in the pathogenesis of PELs. Furthermore, a secondary role for EBV in PELs has been suggested by the fact that a few cases containing KSHV but lacking EBV have been identified. It remains to be determined, however, whether in such cases KSHV is sufficient or whether additional genetic mechanisms are involved in the transformation process. The studies presented here provide a foundation for understanding the role of KSHV and EBV in PELs, as well as the interactions which are likely to take place between these two viruses in this unique and naturally occurring herpesviral coinfection.
ACKNOWLEDGMENT
The authors thank Liang Li Ying for technical assistance in immunocytochemistry.
Supported in part by National Institutes of Health Grant No. EY06337 to D.M.K. and No. CA68939 to E.C.
Address reprint requests to Ethel Cesarman, MD, PhD, The New York Hospital-Cornell Medical Center, Department of Pathology, 525 E 68th St, New York, NY 10021.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal