Abstract
After allogeneic bone marrow transplantation (BMT), the beneficial graft-versus-leukemia (GVL) effect but also the life-threatening graft-versus-host disease (GVHD) are mediated by T cells of the grafted marrow. The identification of leukemia cell–reactive T cells and their ligands are, therefore, crucial for the development of new anti leukemia strategies. Here we describe a leukemia-reactive allo-HLA class II restricted CD4+ T-cell clone, 6.2, isolated from a healthy individual after stimulation with allogeneic leukemic cells. Clone 6.2 recognizes leukemic cells from several AML patients without showing reactivity to unfractioned peripheral blood mononuclear cells, monocytes, B cells, T-cell blasts, and proximal tubulus epithelial cells. Interestingly, clone 6.2 also recognizes BM cells derived from healthy individuals and inhibits the colony formation of myeloid and erythroid cell lineages. In the BM, clone 6.2 recognizes only CD34+ early precursor cells but not CD34−, more differentiated cells. Thus, the target antigen of clone 6.2 is developmentally regulated and expressed only by leukemic cells and CD34+ early progenitor cells in the hematopoietic system. We suggest that targeting the T-cell immune response to leukemia-associated, developmentally regulated antigens of the hematopoietic system can provide a basis for the separation of GVL from GVHD, and may lead to new therapeutic approaches for residual and relapsed leukemia.
ALLOGENEIC bone marrow transplantation (BMT) is the therapy of choice for the treatment of several hematologic malignancies, such as leukemia.1 Today the occurrence of graft-versus-host disease (GVHD) remains a major complication of the BMT.2-5 In the current view, both GVHD and graft-versus-leukemia (GVL) effects of the BMT are mediated by donor derived mature T cells. Depletion of T cells from the BM effectively prevents GVHD, but results in a high rate of leukemia relapses.6-8 Furthermore, patients with relapsed chronic myeloid leukemia can be effectively treated by administration of donor lymphocytes.9 Several clinical trials show a direct association between GVL and GVHD and suggest that the GVL effect may be the reflection of the antihost reactivity against leukemic cells and is, therefore, not separable from GVHD.4,10,11 On the other hand, recent analyses of a large number of patients in the International Bone Marrow Transplantation Register point out that GVL can be observed independent of serious GVHD.12 Also, results from experimental murine models suggest that GVL and GVHD can be mediated by separate as well as by identical T-cell populations.13-16 These data have been also supported by some recent studies in humans where T-cell lines and clones could be isolated, and these preferentially recognized leukemic cells.17-22 These in vitro studies, together with the clinical data, suggest that leukemia-associated antigens must exist. Nevertheless, in most cases the isolated T cells appeared not solely leukemia specific or difficult to maintain in vitro.18-21
In our search for T cells that are reactive with leukemia-associated antigens, we have investigated the in vitro T-cell response of a healthy individual against the leukemic cells of an HLA class II mismatched unrelated AML patient with AML-M1 classification. We have isolated several T-cell clones, of which the majority was directed against subtle HLA class II differences between the responder and the stimulator. One CD4+ proliferative and cytotoxic T-cell clone recognized only leukemic cells from several AML patients in an allo-HLA-DR restricted fashion. This T-cell clone also recognized HLA class II matched BM cells. Neither CD34−, more differentiated precursors in the BM nor mononuclear cells in the peripheral blood (PB) are recognized by T-cell clone 6.2, suggesting that its target antigen is expressed only on leukemic cells and on early hematopoietic progenitor cells.
MATERIALS AND METHODS
Isolation of PB Mononuclear Cells (PBMCs), Leukemic Cells, and BM Cells
PBMCs or BM cells from healthy donors and acute myeloid leukemia (AML) patients were isolated by Ficoll-hypaque (Pharmacia, Uppsala, Sweden) density centrifugation. Cell samples from AML patients that contained greater than 95% morphologically recognizable leukemic cells were further assigned as “leukemic cells.” Leukemic cells, PBMCs, and BM cells were cryopreserved in 10% dimethyl sulfoxide and stored in liquid nitrogen until use.
Isolation of Monocytes, Untransformed B Cells, and BM Cell Fractions
Patients' PBMCs or BM cells from healthy individuals were labeled with the indicated fluorescein-conjugated antibodies (Becton Dickinson). Monoclonal antibody (MoAb)-labeled cells were positively sorted using a fluorescein-activated cell sorter (FACS).
Epstein-Barr Virus–Transformed B Lymphoblastoid Cell Lines (EBV-BLCL)
PBMCs were incubated with EBV during 1.5 hours at 37°C. After washing, the cells were cultured in RPMI + 20% fetal calf serum (FCS) in the presence of 30 Gy irradiated feeder cells consisting of PBMCs from six random donors. The EBV-transformed B cells were further expanded in RPMI + 10% FCS.
Phytohemagglutinin (PHA) Blasts
PBMCs were cultured in the presence of 0.1 μg/mL PHA for 3 days. Activated T-cell blasts (PHA blasts) were further expanded for three days using recombinant interleukin-2 (rIL-2) (20 U/mL) containing culture medium.
Generation of Leukemia-Reactive T-Cell Lines and Clones
Stimulator cells.Leukemic cells of an AML patient with AML-M1 subclassification were used as stimulator cells. The HLA typing of the leukemic cells was: HLA-A3,-B7,-B62,-Cw7,DR13(DRB1*1302),-DR15(DRB1*1501), -DR52(Dw26)(-DRB3*0301), -DQw6(DQB1*0602, *0604), -DPB1*0301,*0601.
Responder cells.PBMCs of a healthy individual were used as responder cells. The HLA typing of the responder cells was: HLA-A 3 , - B 7 , - B 6 2 , - C w 7 , - D R 1 3 ( D R B 1 * 1 3 0 1 ), - D R 1 5 ( D R B 1 * 1 5 0 1 ) , D R 5 2 ( D w 2 5 ) ( - D R B 3 * 0 2 0 2 ), - D Q w 6 ( D Q B 1 * 0 6 0 2 , * 0 6 0 3 / 0 7), --DPB1*0401,*0402.
Before the induction of primary T-cell cultures, stimulator leukemic cells were cultured for 72 hours with a cocktail of cytokines consisting of 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; kindly provided by Dr Osanto, Leiden, The Netherlands), 1,000 U/mL IL-4 (Genzyme, Leuven, Belgium), and 150 U/mL tumor necrosis factor-α (TNF-α; Genzyme), in RPMI supplemented with 10% FCS, and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL). A total of 107 cytokine-treated, irradiated (30 Gy) leukemic cells were cocultured with 107 responder cells in 5 mL of culture medium (RPMI supplemented with 15% human serum [HS] and antibiotics), at 37°C, and 5% CO2 . On day 6 20 U/mL of rIL-2 was added in the culture. On day 8 the T-cell line was restimulated with irradiated leukemic cells. On day 15 the T-cell line was cloned by limiting dilution at 0.3 cells/well in 96-well round-bottom microtiter plates in the presence of a feeder cell mixture containing irradiated PBMCs (30 Gy) from six random donors (1 × 106 cells/mL), irradiated leukemic cells (30 Gy) (2.5 × 105 c/mL), 20 U/mL rIL-2, and 1% Leucoagglutinin-A (Sigma, St Louis, MO). rIL-2, 20 U/mL, was added to the cultures every 72 hours. The T-cell clones were expanded by weekly restimulations with the above-described feeder cell-cytokine mixture and tested for leukemia-specific cytotoxic and proliferative activities.
T-cell proliferation assays.One to 2 × 104 responder T cells were cocultured with irradiated stimulator cells (2 to 10 × 104 c/well) in 96-well flat-bottom microtiter plates during 88 hours. Sixteen hours before harvesting the cultures were labeled with 0.5 μCi of 3H-thymidine. The 3H-thymidine incorporation was determined by liquid scintillation counting. The results are expressed as the mean of duplicate or triplicate cultures. The SEM of the results never exceeded 15%.
Cytotoxicity assays.51Cr-labeled target cells (3,000/well) were incubated with serial dilutions of effector T cells in 96-well round-bottomed microtiter plates (Costar 3799; Cambridge, MA). After 4 hours of incubation at 37°C, cell-free supernatants were obtained for gamma counting. The percent specific lysis was calculated as follows: % Specific Lysis = (Experimental Release − Spontaneous Release)/(Maximal Release − Spontaneous Release) × 100%. Spontaneous release and the maximal release are the chromium release of target cells in culture medium alone and in culture medium containing 1% Triton-X 100 (Fluka, Buchs, Switzerland), respectively.
To use as target cells, proximal tubulus epithelium cells (PTEC) were trypsinated and seeded at 3,000 c/well in 96-well flat-bottom microtiter plates. After allowing adherence, TNF-α (150 U/mL) and interferon-γ (IFN -γ) (200 U/mL) were added to the wells to induce the HLA class II expression. PTEC were cultured in the presence of cytokines for 72 hours. Twelve hours before the assay 51Cr (3 μCi/well) was added to the wells. After washing, 51Cr labeled, adherent PTEC were used as target cells in cytotoxicity assays.
Hematopoietic precursor cell (HPC) growth inhibition assays.HPC growth inhibition assays were performed as described previously.23 Briefly, 1.25 × 105 bone marrow mononuclear cells (BMMNC) was mixed with effector T cells at different T:BM cell ratios in 0.2 mL of HPC culture medium (Iscove's modified Dulbecco's medium [IMDM] supplemented with 30% plasma, 0.5 % bovine serum albumin [BSA], 0.47 g/L transferrin, 5 × 10−5 mol/L mercaptoethanol, and 10% culture supernatant of T cells). The cells were then either immediately resuspended to a final volume of 1.4 mL with semisolid HPC medium supplemented with 10 ng/mL rGM-CSF, 50 ng/mL rIL-3 (both from Sandoz, Basel, Switzerland), 2 IU r-erythropoietin (Cilag AG Int, Zug, Switzerland), and 1.3% methyl cellulose, or briefly centrifuged (1,000g, 15 seconds) to establish BM–T-cell contact and incubated for 4 hours at 37°C in 5% CO2 before transferring to the semisolid medium. One milliliter of the semisolid suspension was plated in 30-mm plastic dishes and incubated at 37°C in 5% CO2 . After 14 days the number of erythrocyte burst-forming units (BFU-E), colony-forming unit-granulocytes (CFU-G), and CFU-monocytes (CFU-M), defined as typical cell aggregates of more than 20 cells, were scored under an inverted microscope. The percentage HPC growth inhibition is calculated as follows: % HPC Growth Inhibition = (1 − No. of Colonies in the Presence of Effector Cells/No. of Colonies Without Effector Cells) × 100%.
RESULTS
In Vitro Generation of Leukemia Reactive T-Cell Lines and Clones
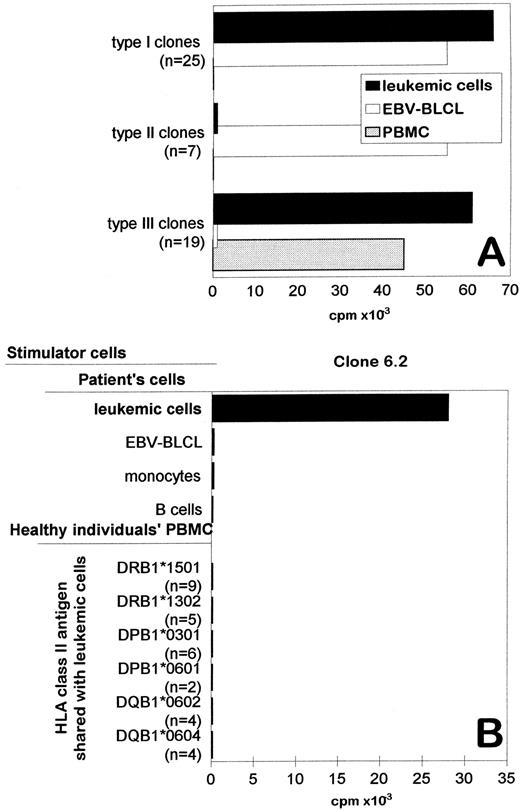
The PBMCs of a healthy individual were used to generate in vitro T-cell responses against leukemic cells of an unrelated AML patient. The responder individual and the patient were serologically HLA identical, but showed subtle differences in HLA-DR, -DP, and -DQ genotypings (see Materials and Methods). Before using as stimulator cells, the leukemic cells were cultured with GM-CSF, IL-4, and TNF-α during 72 hours to increase their antigen-presenting capacity. The generated T-cell line showed proliferative and cytotoxic activity against leukemic cells at day 8 (data not shown). Several T-cell clones derived from this T-cell line were tested against leukemic cells, patient's EBV-BLCL, and HLA-typed PBMCs from healthy individuals. Based on the reaction patterns, four types of T-cell clones could be distinguished (Fig 1): (1) The type I T-cell clones (n = 25) were reactive against both leukemic cells and EBV-BLCL. Thus, these clones were not able to discriminate leukemic from nonleukemic cells. (2) The type II T-cell clones (n = 7) were reactive only to EBV-BLCL, suggesting that EBV-associated antigens were recognized. (3) The type III T-cell clones (n = 19) were reactive against leukemic cells but did not show reactivity against patient's EBV-BLCL, suggesting that their target antigen(s) were present on leukemic cells but not on nonleukemic EBV-BLCL. However, when these T-cell clones were tested against a panel of PBMCs, which expressed the mismatched HLA class II antigens of the patient, all T-cell clones recognized one of more PBMCs. Thus, type III T-cell clones were not directed against leukemia-associated antigens, but most probably to alloantigens that were expressed by PBMCs but not by EBV-BLCL.
Specific recognition of original stimulator leukemic cells by the CD4+ T-cell clone 6.2. (A) The proliferative activity of different types of T-cell clones against patient's leukemic cells (AML cells), patient's EBV-BLCL, and HLA matched PBMCs. For each type of clone, the reactivity of one representative clone is shown. The number of clones displaying similar reactivity patterns is indicated in the brackets. (B) The proliferative activity of T-cell clone 6.2 against leukemic cells, patient's monocytes, patient's transformed or untransformed B cells, and unrelated PBMC that share HLA class II antigens with the AML patient. CD14+ monocytes and CD19/20+ B cells were isolated from PBMC of the patient by FACS sorting. Similar results were obtained in at least three independent experiments.
Specific recognition of original stimulator leukemic cells by the CD4+ T-cell clone 6.2. (A) The proliferative activity of different types of T-cell clones against patient's leukemic cells (AML cells), patient's EBV-BLCL, and HLA matched PBMCs. For each type of clone, the reactivity of one representative clone is shown. The number of clones displaying similar reactivity patterns is indicated in the brackets. (B) The proliferative activity of T-cell clone 6.2 against leukemic cells, patient's monocytes, patient's transformed or untransformed B cells, and unrelated PBMC that share HLA class II antigens with the AML patient. CD14+ monocytes and CD19/20+ B cells were isolated from PBMC of the patient by FACS sorting. Similar results were obtained in at least three independent experiments.
Specific Recognition of Patient's Leukemic Cells by the CD4+ T-Cell Clone 6.2
One remaining CD4+ T-cell clone, designated as 6.2, recognized leukemic cells but did not show reactivity against EBV-BLCL, CD19/20+ untransformed B cells, or CD14+ monocytes that were derived from the PBMCs of the patient (Fig 1B). Furthermore, clone 6.2 did not show reactivity against a panel of PBMCs that shared HLA-class II antigens with leukemic cells (Fig 1B). These results indicated that T-cell clone 6.2 was not directed against antigens expressed by PBMCs, monocytes, or B cells but reacted to an antigen that is expressed only by leukemic cells.
T-Cell Clone 6.2 Is Restricted Via the Allo HLA-DRw26 Molecule
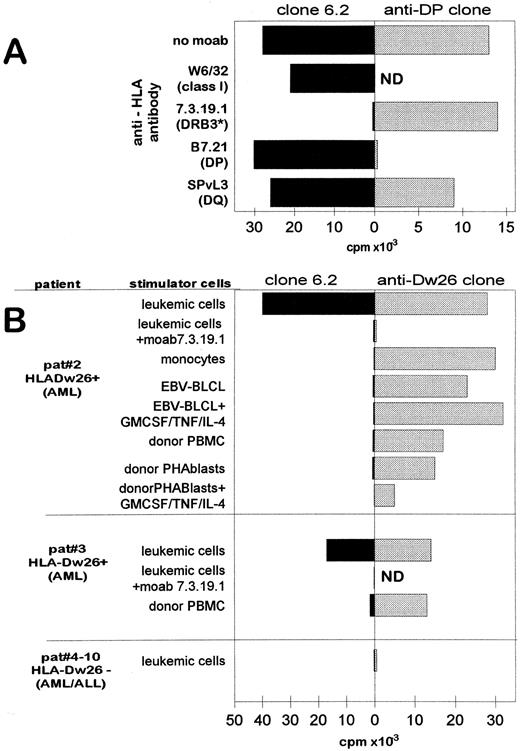
As shown in Fig 2A, the proliferative activity of the clone 6.2 against leukemic cells was completely blocked by MoAb 7.3.19.1 that is directed to HLA-DRB3 locus (HLA-DR52).24 Antibodies directed to HLA class I, HLA-DP, or HLA-DQ25 26 were not inhibitory. The MoAb 7.3.19.1 did not inhibit the recognition of the leukemic cells by a control HLA-DP–reactive T-cell clone (Fig 2A, right panel) illustrating its specific inhibitory effect on clone 6.2. Because all cells that are positive for HLA-DRB1*1302 express the DR52 subtype HLA-Dw26 (DRB3*0301), the antigen recognition by clone 6.2 is restricted by HLA-Dw26. Note that the responder cells are also HLA-DR52–positive but are genotypically typed as HLA-DRB3*0202 (see Materials and Methods). Thus, the T-cell clone 6.2 recognizes its leukemia-associated target antigen in the context of a mismatched, allo HLA-Dw26 (DRB3*0301) molecule.
T-cell clone 6.2 is restricted by HLA-DRB3 locus product HLA-Dw26 and specifically recognizes HLA-Dw26–positive leukemic cells. (A) Effect of HLA specific MoAbs on the recognition of leukemic cells by T-cell clone 6.2 (left panel) and a control DP reactive T-cell clone (right panel). The target specificities of the MoAbs are indicated in brackets. MoAbs were added in the proliferation assays at 1:100 dilution. ND, not tested. (B) Proliferative activity of T-cell clone 6.2 (left panel) and the HLA-Dw26 specific alloreactive T-cell clone (right panel) against different leukemic and nonleukemic cells. Patients no. 2 and 3 are HLA-Dw26–positive AML-M1 patients; patients no.4 to 10 are HLA-Dw26–negative AML or ALL patients (3 AML-M1, 1 AML-M3, 1 AML-M5, 1 ALL patient). MoAb 17.3.19.1 is added in the assay. CD14+ monocytes from patient no. 2 were obtained from PBMC by FACS sorting. EBV-BLCL and PHA blasts were also tested after culturing with GM-CSF (800 U/mL) + TNF-α (50 U/mL) + IL-4 (500 U/mL) for 72 hours. The thymidine uptake of T-cell clone 6.2 alone and the stimulator cells alone did not exceed 500 cpm.
T-cell clone 6.2 is restricted by HLA-DRB3 locus product HLA-Dw26 and specifically recognizes HLA-Dw26–positive leukemic cells. (A) Effect of HLA specific MoAbs on the recognition of leukemic cells by T-cell clone 6.2 (left panel) and a control DP reactive T-cell clone (right panel). The target specificities of the MoAbs are indicated in brackets. MoAbs were added in the proliferation assays at 1:100 dilution. ND, not tested. (B) Proliferative activity of T-cell clone 6.2 (left panel) and the HLA-Dw26 specific alloreactive T-cell clone (right panel) against different leukemic and nonleukemic cells. Patients no. 2 and 3 are HLA-Dw26–positive AML-M1 patients; patients no.4 to 10 are HLA-Dw26–negative AML or ALL patients (3 AML-M1, 1 AML-M3, 1 AML-M5, 1 ALL patient). MoAb 17.3.19.1 is added in the assay. CD14+ monocytes from patient no. 2 were obtained from PBMC by FACS sorting. EBV-BLCL and PHA blasts were also tested after culturing with GM-CSF (800 U/mL) + TNF-α (50 U/mL) + IL-4 (500 U/mL) for 72 hours. The thymidine uptake of T-cell clone 6.2 alone and the stimulator cells alone did not exceed 500 cpm.
T-Cell Clone 6.2 Proliferates Against Other HLA-Dw26–Positive AML Cells Without Showing Reactivity to Nonleukemic Cells
The T-cell clone 6.2 was subsequently tested against a panel of leukemic and nonleukemic cells derived from different leukemia patients (Fig 2B, left panel). A CD4+ alloreactive T-cell clone specific for the HLA-Dw26 molecule was used as control (Fig 2B, right panel). Beside its reactivity against the original stimulator leukemic cells of the AML-M1 patient (no. 1) (Figs 1B and 2A), clone 6.2 recognized two other leukemic cells from AML patients (nos. 2 and 3) (Fig 2B, left panel). These leukemic cells were also recognized by the control HLA-Dw26 reactive T-cell clone (Fig 2B, right panel), confirming the expression of HLA-Dw26 on the cell surface. Note that the proliferative activity of both clone 6.2 and HLA-Dw26 specific alloreactive T-cell clone against the leukemic cells of patient no. 2 were blocked by antibody 7.3.19.1, illustrating the HLA-Dw26 dependency of the recognition. Leukemic cells from six HLA-Dw26–negative AML or acute lymphoblastoid leukemia (ALL) patients were not recognized either by clone 6.2 or by the control HLA-Dw26–specific T-cell clone (Fig 2B).
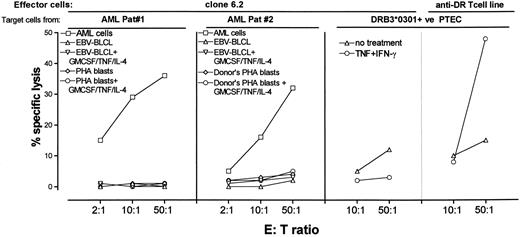
Specific cytotoxic activity of T-cell clone 6.2 against leukemic cells. Patients no. 1 and 2 are leukemia patients with AML-M1 designation. EBV-BLCL and PHA blasts were also tested after culturing with GM-CSF (800 U/mL) + TNF-α (50 U/mL) + IL-4 (500 U/mL) for 72 hours. Adherent PTEC were prepared as targets as indicated in Materials and Methods. Percent specific release of target cells was determined in 4-hour standard Cr release assays.
Specific cytotoxic activity of T-cell clone 6.2 against leukemic cells. Patients no. 1 and 2 are leukemia patients with AML-M1 designation. EBV-BLCL and PHA blasts were also tested after culturing with GM-CSF (800 U/mL) + TNF-α (50 U/mL) + IL-4 (500 U/mL) for 72 hours. Adherent PTEC were prepared as targets as indicated in Materials and Methods. Percent specific release of target cells was determined in 4-hour standard Cr release assays.
The leukemic cell–specific reactivity of clone 6.2 is further demonstrated by absence of proliferation against different nonleukemic cell types obtained from patients no. 2 and 3: Figure 2B shows that clone 6.2 did not recognize the EBV-BLCL and monocytes derived from patient no. 2; clone 6.2 also failed to recognize PHA blasts or PBMCs derived from the HLA-identical BM donors of patients no. 2 and 3. The EBV-BLCL and PHA blasts that were treated with GM-CSF/TNF-α/IL-4 cocktail were also not stimulatory, indicating that the target antigen of clone 6.2 was not induced on BLCL or T cells by these cytokines. All tested HLA-Dw26–positive nonleukemic cells, including those that were treated with cytokines, were recognized by the control Dw26-reactive T-cell clone (Fig 2B, right panel), showing that all assayed nonleukemic cells expressed the restriction molecule and were capable of stimulating T cells (Fig 2B, right panel).
T-Cell Clone 6.2 Is Cytotoxic to Leukemic But Not to Nonleukemic Cells
T-cell clone 6.2 not only possessed proliferative activity against leukemic cells but also efficiently lysed the leukemic cells (Fig 3). Similar to its proliferative activity, the cytotoxic activity of clone 6.2 was confined to leukemic cells. Nonleukemic cells such as PHA blasts or EBV-BLCL derived from the patients no. 1 and 2 or from their HLA-identical BM donors were not lysed (Fig 3). BLCL or PHA blasts that were treated with the GM-CSF/TNF/IL-4 cocktail were also not lysed (Fig 3).
To assess whether nonhematopoietic cells can express the target antigen of clone 6.2, an HLA-DRB3*0301–positive PTEC line was tested as target cell (Fig 3). Clone 6.2 and a control alloreactive T-cell line that is directed to HLA-DRB1*1302 and HLA-DRB3*0301 allo-determinants were used as effector cells. Because PTECs do not constitutively express HLA class II, they were cultured with 200 U/mL IFN-γ + 150 U/mL TNF-α during 72 hours to induce HLA class II expression. The control alloreactive T-cell line showed a nonsignificant lysis against untreated PTEC which lacked HLA-class II. The induction of HLA class II expression by IFN-γ and TNF-α led to the recognition of the PTEC by the control alloreactive T-cell line only, suggesting that the PTEC did not expressed the target antigen of clone 6.2.
T-Cell Clone 6.2 Recognizes CD34+ Hematopoietic Progenitor Cells and Inhibits the Growth of Erythroid, Monocytic, and Granulocytic Cell Lineages
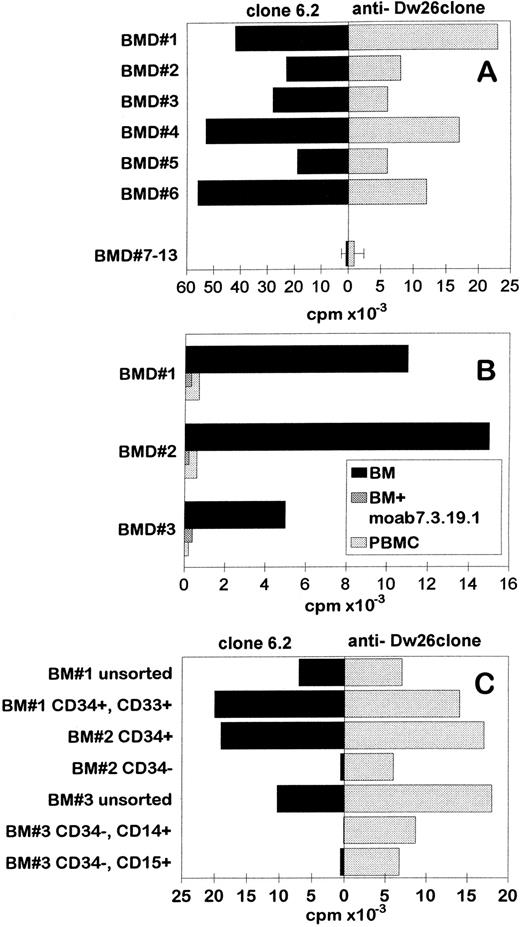
T-cell clone 6.2 was tested against a panel of BM cells derived from healthy individuals (Fig 4A, left panel). Likewise, the control HLA-Dw26–specific alloreactive T-cell clone, clone 6.2, recognized all HLA-Dw26–positive BM cells but did not recognize HLA-Dw26–negative BM cells (Fig 4A). The proliferation of clone 6.2 against BM cells was inhibited by the antibody 7.3.19.1, confirming its HLA-DR–restricted reactivity (Fig 4B). As expected, the PBMC derived from the HLA-Dw26–positive BM donors were not recognized (Fig 4B). These results suggested that the clone 6.2 might recognize a developmentally regulated antigen that is expressed only by early progenitor cells present in the BM. To address this assumption, several FACS sorted BM cell populations were tested for their capacity to stimulate T-cell clone 6.2 (Fig 4C). T-cell clone 6.2 recognized the unsorted BM cells, CD34+ early progenitor cells, CD34+, CD33+ early myeloid cell precursors, but not CD34− cells, including CD34−, CD14+ monocytic lineage and CD34−, CD15+ granulocytic cell lineage. These results indicated that the target antigen recognized by the antileukemic T-cell clone 6.2 was expressed by CD34+ BM early progenitor cells but not by more differentiated cells, including PBMCs. Note that the control HLA-Dw26–specific alloreactive T-cell clone recognized all cell subsets, indicating that the unresponsiveness of clone 6.2 to CD34− cells was not caused by a lack of stimulatory capacity (Fig 4C, right panel).
Specific recognition of HLA-Dw26–positive BM early progenitor cells by T-cell clone 6.2. (A) HLA-Dw26–positive or –negative BM cells from healthy individuals were used to stimulate clone 6.2 (left panel) and the control HLA-Dw26–specific alloreactive T-cell clone (right panel) in 88-hour standard proliferation experiments. (B) MoAb 7.3.19.1 is added in the assay. (C) Different subsets of BM cells were obtained by FACS sorting after labeling with appropriate antibodies, irradiated, and used in proliferation experiments as stimulator cells at 5 × 104 cells/well.
Specific recognition of HLA-Dw26–positive BM early progenitor cells by T-cell clone 6.2. (A) HLA-Dw26–positive or –negative BM cells from healthy individuals were used to stimulate clone 6.2 (left panel) and the control HLA-Dw26–specific alloreactive T-cell clone (right panel) in 88-hour standard proliferation experiments. (B) MoAb 7.3.19.1 is added in the assay. (C) Different subsets of BM cells were obtained by FACS sorting after labeling with appropriate antibodies, irradiated, and used in proliferation experiments as stimulator cells at 5 × 104 cells/well.
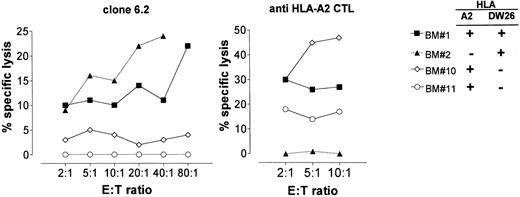
Because T-cell clone 6.2 displays cytotoxic activity, we addressed the question of whether it could also lyse BM cells and inhibit the outgrowth of different hematopoietic cell lineages. Therefore, we used BM cells as target cells in cytotoxicity assays (Fig 5) and in parallel we performed HPC growth inhibition assays (Fig 6). A CD8+ HLA-A2–specific CTL clone was used as control.
Cytotoxic activity of clone 6.2 to BM cells. Unfractionated BM cells were tested as target cells for clone 6.2 (left) and for an HLA-A2–specific, CD8+ alloreactive CTL (right) in 4-hour standard CML assays.
Cytotoxic activity of clone 6.2 to BM cells. Unfractionated BM cells were tested as target cells for clone 6.2 (left) and for an HLA-A2–specific, CD8+ alloreactive CTL (right) in 4-hour standard CML assays.
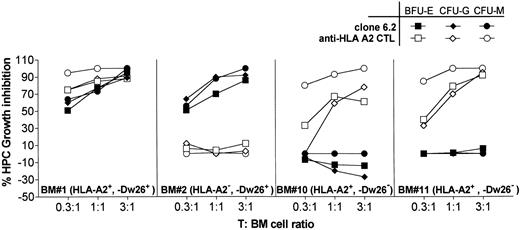
HPC growth inhibition by T-cell clone 6.2. T-cell clone 6.2 is preincubated with BM cells at indicated T-cell:BM cell ratios for 4 hours at 37°C. The cells were then transferred into semisolid HPC growth medium and cultured in cell culture dishes to allow HPC growth. The colony formation in semisolid medium is determined by light-microscopy after 10 days. Percent inhibition of the HPC growth was determined as follows: % Inhibition = (1 − No. of Colonies in the Presence of T-Cell Clone/No. of Colonies in the Absence of T-Cell Clone ) × 100%.
HPC growth inhibition by T-cell clone 6.2. T-cell clone 6.2 is preincubated with BM cells at indicated T-cell:BM cell ratios for 4 hours at 37°C. The cells were then transferred into semisolid HPC growth medium and cultured in cell culture dishes to allow HPC growth. The colony formation in semisolid medium is determined by light-microscopy after 10 days. Percent inhibition of the HPC growth was determined as follows: % Inhibition = (1 − No. of Colonies in the Presence of T-Cell Clone/No. of Colonies in the Absence of T-Cell Clone ) × 100%.
T-cell clone 6.2 showed specific cytolysis against two HLA-Dw26–positive, unfractionated BM cells. As expected, HLA-Dw26–negative BM cells were not lysed (Fig 5, left panel). The moderate levels of lysis observed against these unfractionated BM cells was not surprising because clone 6.2 was expected to recognize only CD34+ cells in the BM. The control HLA-A2–specific CD8+ alloreactive T-cell clone significantly lysed the BM cells that were HLA-A2–positive (Fig 5, right panel).
In HPC growth inhibition assays (Fig 6), clone 6.2 strongly inhibited the outgrowth of BFU-E, CFU-G, and CFU-M from two HLA-Dw26–positive BM (BM no. 1 and BM no. 2) at T:BM ratios as low as 0.3:1. HLA-Dw26–negative BM (BM no. 10, BM no. 11) were not inhibited, showing that the HPC inhibition was specific and dependent on antigen presentation by BM cells. Likewise, the control HLA-A2–specific CTL inhibited the HPC growth from only HLA-A2–positive BMs no. 1, 10, and 11. Both clone 6.2 and alloreactive T-cell clone inhibited the number of the growing colonies, and no difference in the size of the colonies was observed, suggesting that the further outgrowth of the colonies was not inhibited. Furthermore, neither clone 6.2 nor the CD8+ alloreactive clone inhibited the HPC growth when the T cells and the BM cells were only mixed immediately before plating in the semisolid culture medium (data not shown), indicating that the inhibition of HPC growth was dependent to cell-cell contact. These results were similar to those obtained by other CD8+ CTL directed to alloantigens or to minor histocompatibility antigens.23
Taken together, the strong HPC growth inhibition by clone 6.2 may reflect its cytotoxic activity against CD34+ early precursor cells. However, other mechanisms such as induction of apoptosis via fas-fasL interactions cannot be excluded.
DISCUSSION
After an allogeneic BMT mature T cells present in the graft inoculum appear to play a major role in the elimination of residual leukemic cells.6-9 The understanding of the nature of the T-cell response directed to leukemic cells and the identification of leukemia-associated T-cell antigens is the basis of new therapeutic methods against leukemia. This issue was the main focus of the present study.
Over the past few years it became clear that the proper activation of naive T-cell precursors in primary in vitro cultures requires efficient antigen presentation supported by strong costimulatory signals delivered by the antigen presenting cell (APC).27 In this respect, leukemic cells may not function as proper APC since they usually lack or weakly express costimulatory molecules such as B7.1 and B7.2. This may be the reason why in some studies antileukemic T-cell lines and clones were reported to be functionally or physically unstable in culture. Therefore, we decided to culture leukemic cells with GM-CSF, IL-4, and TNF-α. These cytokines, which are known to generate adequate APC from monocytes,28-30 also increased the antigen-presenting capacity of acute myeloid leukemia cells (manuscript in preparation).
Using this strategy, we investigated primary T-cell responses induced against allogeneic leukemic cells. Beside T-cell clones that were reactive to leukemic cells and patient's EBV-BLCL, we have isolated other alloreactive T-cell clones that recognized leukemic cells, HLA-matched, allogeneic PBMCs, but not patient's EBV-BLCL (Fig 1A, type III clones). These latter T-cell clones are probably directed to allo-HLA determinants, which are apparently absent on EBV-BLCL.
More interestingly, we have isolated a T-cell clone, designated as “6.2,” recognizing an antigen present on various leukemic cells but absent on nonleukemic cells isolated from the PB, and on the kidney-derived PTEC. This CD4+ T-cell clone appeared to recognize its target antigen not in a self-restricted but in an allo HLA-DR–restricted fashion. In this respect our results are in agreement with previous studies of Sosman et al,17,18 who also have shown that allogeneic T cells can display specific antileukemic activity in vitro. Similar to the T cells described by Sosman et al, the antileukemic T-cell clone 6.2 described here is CD4+, class II restricted, and not only proliferates but also displays cytotoxic activity against leukemic cells. Thus, it is plausible that GVL effect after BMT can be mediated also by antileukemic CD4+ T cells. Some recent studies indeed suggest that CD4+ T cells can mediate GVL effects without the apparent induction of GVHD.31 Moreover, in a recent clinical trial, the GVL effect of the buffy coat transfusion was preserved by the depletion of CD8+ cells, whereas the GVHD was largely prevented after this procedure.32
So far little is known about the nature of the leukemia-associated antigens that trigger T-cell immune responses. Several investigators consider leukemia-specific fusion proteins, such as the characteristic BCR-ABL fusion protein in chronic myeloid leukemia (CML), as potential candidates for targeting the T-cell immune response specifically to leukemic cells.33 Although T-cell responses can be generated against the BCR-ABL fusion peptides, it has not been convincingly shown that BCR-ABL fusion product is naturally expressed in the context of HLA molecules. In acute leukemias a variety of tumor-specific chromosome abnormalities occur. Although the fusion products of these chromosomal translocations can be also considered as target antigens for the development of leukemia-specific therapies, not a large percentage of patients can be cured by such strategies because of the interindividual variation in the chromosomal abnormalities. However, several of these divers chromosomal translocations led to the activation of common transcription factors that are important in differentiation.34 As yet it is not known whether these developmentally regulated antigens could trigger T-cell immune responses and serve as leukemia-associated T-cell antigens. Our current results suggest that possibly such developmentally regulated antigens can be recognized by T cells: The HLA-DR restricted T-cell clone 6.2 described here specifically recognizes leukemic cells from several AML patients and reacts to CD34+ BM cells without showing reactivity against CD34− BM cells or against nonleukemic cells derived from PBMC. The target antigen of clone 6.2 is also not present on a nonhematopoietic system–derived cell line PTEC. Although we were not able to test other somatic tissue cells, and thus it still remains possible that an other cell type can be recognized, so far clone 6.2 shows a very limited target cell specificity in the hematopoietic system. The most likely explanation of this specific target cell specificity is that clone 6.2 recognizes a developmentally regulated antigen of the hematopoietic system, which is involved in the differentiation of early progenitor cells. This antigen is also expressed on leukemic cells that are arrested in an early stage of the differentiation. Because the T-cell clone effectively inhibits the outgrowth of hematopoietic precursor cells and lyses the leukemic cells, the use of this clone for the treatment of leukemia after BMT may result in elimination of residual leukemic cells in the PB and their precursors in the BM without the risk of GVHD. The main criticism against such a therapeutic approach is that the T-cell clone 6.2 will also recognize grafted BM and presumably will not allow the outgrowth of several cell lineages. This risk can be avoided by transplantation of an HLA-Dw26–negative, thus a one-allele HLA-DR mismatched BM graft, because the T-cell clone 6.2 will recognize its target antigen only in the context of HLA-Dw26. It is obvious that in such a transplantation setting the BM graft should be T-cell depleted to avoid the risks of serious GVHD and the rejection of the CTL clone. Alternatively, furnishing the T-cell clone 6.2 with a suicide gene, such as Hsv-tk gene, may allow the control of the T-cell clone in vivo before BMT.
In conclusion, our results suggest that targeting the T-cell immune response to the developmentally regulated antigens of the hematopoietic system may be possible, and lead to new approaches in the leukemia treatment.
ACKNOWLEDGMENT
We thank Drs F. Koning and T. Ottenhoff for critical discussions.
Supported by grants from the Dutch Cancer Foundation (Koningin Wilhelmina Fonds) and the J.A. Cohen Institute for Radiopathology and Radiation Protection (IRS).
Address reprint requests to Tuna Mutis, MD, PhD, Department of Immunohematology and Blood Bank, Leiden University Hospital, Bldg1, E3-Q, Box 9600, 2300 RC Leiden, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal