Abstract
The translocation t(12; 21)(p13; q22) is difficult to detect by classic cytogenetics. However, using fluorescence in situ hybridization (FISH) and by screening for the TEL/AML1 rearrangement by the polymerase chain reaction (PCR), it has been demonstrated to be the most frequent known structural chromosomal abnormality in childhood acute lymphoblastic leukemia (ALL). It is closely correlated with a B-cell precursor (BCP) phenotype and is considered a favorable prognostic factor. However, little is known about the incidence of the translocation in relapsed patients and the duration of complete remission (CR) in children expressing the TEL/AML1 fusion gene. We therefore examined 49 bone marrow samples from children with ALL at first or second relapse that were consecutively mailed to our laboratory to test for the presence of t(12; 21) using reverse transcriptase (RT)-PCR. The TEL/AML1 rearrangement could be identified in nine of 44 (20%) of the patients, a result similar to the reported incidence at diagnosis. Most of the TEL/AML1–positive children showed no adverse clinical features at diagnosis (eg, white blood cell [WBC] count <100 × 109/L or age <10 years), and regarding these data, there were no differences versus children who were negative for the fusion gene. However, the period of remission was about 1 year longer in children expressing TEL/AML1 (P = .046), and the majority of relapses in this group appeared late (<2 years after diagnosis). Our findings therefore reinforce the urgent need for further prospective studies with a long follow-up period to determine the true prognostic significance of t(12; 21) and to avoid premature changes of treatment strategies.
THE TRANSLOCATION t(12; 21)(p13; q22), which is difficult to detect by classic cytogenetics (<0.05%), was originally considered a rare abnormality. With the use of additional techniques such as fluorescence in situ hybridization (FISH)1 and reverse transcriptase–polymerase chain reaction (RT-PCR) that identifies the molecular genetic equivalent, the TEL/AML1 rearrangement, more information has become available. The t(12; 21) has been shown to be the most frequent structural chromosomal aberration of childhood acute lymphoblastic leukemia (ALL),2-13 and is rare in adults (1.5%).2,5,7,14,15 This translocation typically occurs in patients between 1 and 10 years of age with a low white blood cell (WBC) count (∼25.0 × 109/L), a B-cell precursor (BCP) phenotype, and nonhyperdiploidy (>50). The reported frequency is between 12% and 36%, with an average of about 25%. Most investigators correlate the appearance of the TEL/AML1 rearrangement with a favorable prognosis, ie, 89% to 100% event-free survival (EFS).2,3 10
If the presence of the TEL/AML1 rearrangement per se is a good prognostic factor, one might expect a very low incidence of this abnormality at relapse. To our knowledge, no investigation has yet been published exclusively describing children at relapse. There are only two reports8 16 mentioning the t(12; 21) being included among a small number of relapse patients from a larger series of initial leukemia. All of these studies were retrospectively performed and may be biased by selection.
To gain further knowledge about the prognostic meaning of t(12; 21) in childhood ALL, we screened for the TEL/AML1 rearrangement using RT-PCR in bone marrow samples from children with relapse that were consecutively referred to our laboratory, and compared the clinical features of TEL/AML1-positive and -negative children at relapse and at diagnosis.
SUBJECTS AND METHODS
From May to November 1996, 634 bone marrow samples were referred to our laboratory. Forty-nine were from children with relapsed ALL mailed from 26 pediatric centers in Germany. Relapse was diagnosed by criteria described previously.17 Whereas most of the children were initially treated in one of the German multicenter trials (Berlin-Frankfurt-Münster or Cooperative ALL), four of them had received leukemia therapy in Turkey, Kazakhstan, Singapore, or Dubai, respectively.
In this period, 204 ALL patients at diagnosis were successfully analyzed by RT-PCR for the TEL/AML1 rearrangement in a prospective study. The gene fusion was detected in 36, for an incidence of 18% in all cases and 21% in children with BCP-ALL at diagnosis.
RT-PCR. Total RNA was extracted by a single-step method.18 cDNA synthesis using random hexamer primers (Boehringer Mannheim, Germany) was performed in a total volume of 20 μL using a standard protocol.19 Amplification of the chimeric TEL/AML1 gene fragment was performed in a model 9600 thermocycler (Perkin Elmer, Langen, Germany) using a nested PCR protocol. After an initial melting step (1.5 minutes at 94°C), 35 amplification cycles of 15 seconds at 94°C, 45 seconds at 64°C, and 45 seconds at 72°C were performed in a 20-μL final volume containing 1.6 pmol of each outer primer during the first round of PCR. One microliter of the first-round product was subjected to the second round of PCR. This differed from the first round by the number of cycles (which was reduced to 25), the amount of internal primers (8 pmol), and the annealing temperature (60°C). PCR products were analyzed on a 1% agarose gel and visualized by ethidium bromide staining.
To check the integrity of the isolated RNA and the correctness of cDNA synthesis, the ubiquitously expressed ABL gene was amplified in a separate PCR. PCR primer sequences are shown in Table 1.
PCR Primers for Amplification of the Chimeric TEL/AML1 Fragment
| Primer . | Sequence 5′-3′ . | Orientation . |
|---|---|---|
| TEL | ||
| External | AGCCCCATCATGCACCCTCTGATCC | Sense |
| Internal | GCAGAATTCCACTCCGTGGATTTCAAACAGTCC | Sense |
| AML1 | ||
| External | GTGGTCGGCCAGCACCTCCACC | Antisense |
| Internal | AACGCCTCGCTCATCTTGCCTGGGCTC | Antisense |
| Primer . | Sequence 5′-3′ . | Orientation . |
|---|---|---|
| TEL | ||
| External | AGCCCCATCATGCACCCTCTGATCC | Sense |
| Internal | GCAGAATTCCACTCCGTGGATTTCAAACAGTCC | Sense |
| AML1 | ||
| External | GTGGTCGGCCAGCACCTCCACC | Antisense |
| Internal | AACGCCTCGCTCATCTTGCCTGGGCTC | Antisense |
Moreover, at diagnosis, all ALL patients were routinely screened for expression of a BCR/ABL mRNA as reported elsewhere.20
FISH. For FISH analysis, whole-chromosome paints of chromosomes 12 (wcp12) and 21 (wcp21) were used (Appligene Oncor, Illkirch, France). Hybridization was performed according to the manufacturer's protocol.
Statistics. For statistical evaluation of the association between the clinical data and the appearance of the TEL/AML1 rearrangement, the Mann-Whitney test was used.21
RESULTS
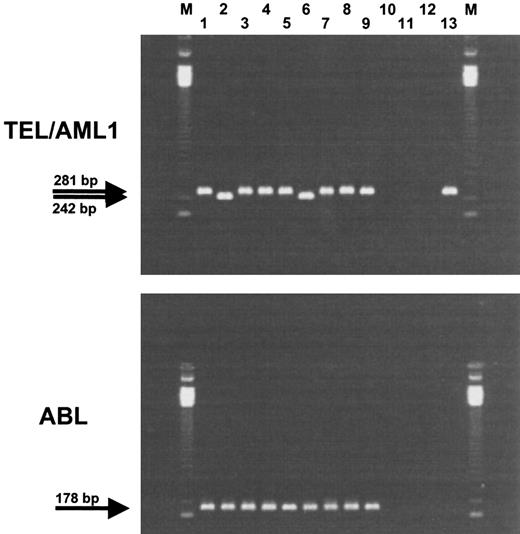
During a period of 7 months (May to November 1996), 49 bone marrow samples from children with relapsed ALL were consecutively mailed to our laboratory for molecular and cytogenetic analysis of chromosomal aberrations. RT-PCR for BCR/ABL and TEL/AML1 rearrangement was performed for all of them, but failed in three patients because of RNA degradation. Nine of 46 children were positive for the TEL/AML1 rearrangement (19.6%; Fig 1). This was confirmed by FISH with whole-chromosome paints for chromosomes 12 and 21 in seven of nine cases (Table 2). Two patients who were diagnosed and treated abroad were excluded from further evaluation because no data on the initial leukemia were available. However, another patient who had been diagnosed and treated in Turkey as AML was included (patient no. 96256).
RT-PCR detection of the TEL/AML1 fusion transcript. Upper panel, TEL/AML1 fusion transcript. Two different sizes of TEL/AML1 products are detectable (242 and 281 bp). To check integrity of the RNA, a 178-bp fragment of ABL was amplified from each patient (bottom panel). Lane M, 123-bp marker; lanes 1 to 9, patients no. 87086, 88615, 89100, 90568, 93437, 94112, 94565, 96526, 96569; lanes 10 to 12, water control for cDNA synthesis, first round of PCR, and second round of PCR; lane 13, positive control, REH cell line.
RT-PCR detection of the TEL/AML1 fusion transcript. Upper panel, TEL/AML1 fusion transcript. Two different sizes of TEL/AML1 products are detectable (242 and 281 bp). To check integrity of the RNA, a 178-bp fragment of ABL was amplified from each patient (bottom panel). Lane M, 123-bp marker; lanes 1 to 9, patients no. 87086, 88615, 89100, 90568, 93437, 94112, 94565, 96526, 96569; lanes 10 to 12, water control for cDNA synthesis, first round of PCR, and second round of PCR; lane 13, positive control, REH cell line.
Clinical Data for TEL/AML 1-Positive Patients
| Patient No. . | Sex . | Initial Leukemia . | First Relapse . | Second Relapse . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Age (yr) . | Immunophenotype . | WBC . | Diagnosis Date . | TEL/AML1 . | Therapy . | CR . | Relapse Date . | WBC . | TEL/AML1 . | Site . | Therapy . | Second CR (yr) . | Relapse Date . | Site . | TEL/AML1 . |
| . | . | . | . | (×109/L) . | . | . | . | (yr) . | . | (×109/L) . | . | . | . | . | . | . | . |
| 87086 | M | 13.9 | c-ALL | 46.0 | 2/18/87 | PCR+ | BFM 86-RG | 3.9 | 1/4/91 | 12.0 | No mat | BM | ALL-REZ 90 | 5.5 | 7/1/96 | BM | PCR+ |
| 88615 | F | 3.5 | c-ALL | 15.6 | 12/14/88 | FISH+ | BFM 86-RG | 3.6 | 7/31/92 | 12.4 | PCR+ | BM | ALL-REZ 90 | 3.9 | 6/25/96 | BM | PCR+ |
| 89100 | M | 3.0 | c-ALL | 84.9 | 3/6/89 | No mat | BFM 86-RG | 4.1 | 3/24/93 | 3.1 | No mat | BM | ALL-REZ 90 | 3.5 | 9/9/96 | BM | PCR+, FISH+ |
| 90568 | M | 5.6 | Pre-B-ALL | 6.1 | 12/20/90 | No mat | BFM 90-SR | 2.5 | 6/28/93 | 3.1 | PCR+ | BM | ALL-REZ 90 | 3.2 | 8/28/96 | BM/T | PCR+, FISH+ |
| 93473 | M | 6.3 | c-ALL | 18.9 | 9/23/93 | PCR+ | BFM 90-MR | 2.9 | 8/21/96 | 8.6 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 94112 | F | 3.4 | c-ALL | 6.7 | 2/14/94 | PCR+ | CoALL-05-92-LR | 2.7 | 10/14/96 | 3.9 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 94565 | F | 3.3 | c-ALL | 5.6 | 10/5/94 | PCR+ | CoALL-05-92-HR | 2.1 | 11/1/96 | 5.0 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 96526 | F | 5.9 | c-ALL* | ND | 1/22/93 | No mat | AML therapy | 3.6 | 9/14/96 | 9.5 | PCR+, FISH+ | BM/CNS | |||||
| 96569 | M | 4.1 | c-ALL | 9.2 | 9/3/93 | No mat | BFM 90-SR | 3.1 | 10/7/96 | 4.3 | PCR+, FISH+ | BM/CNS | ALL-REZ P95 | ||||
| Patient No. . | Sex . | Initial Leukemia . | First Relapse . | Second Relapse . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Age (yr) . | Immunophenotype . | WBC . | Diagnosis Date . | TEL/AML1 . | Therapy . | CR . | Relapse Date . | WBC . | TEL/AML1 . | Site . | Therapy . | Second CR (yr) . | Relapse Date . | Site . | TEL/AML1 . |
| . | . | . | . | (×109/L) . | . | . | . | (yr) . | . | (×109/L) . | . | . | . | . | . | . | . |
| 87086 | M | 13.9 | c-ALL | 46.0 | 2/18/87 | PCR+ | BFM 86-RG | 3.9 | 1/4/91 | 12.0 | No mat | BM | ALL-REZ 90 | 5.5 | 7/1/96 | BM | PCR+ |
| 88615 | F | 3.5 | c-ALL | 15.6 | 12/14/88 | FISH+ | BFM 86-RG | 3.6 | 7/31/92 | 12.4 | PCR+ | BM | ALL-REZ 90 | 3.9 | 6/25/96 | BM | PCR+ |
| 89100 | M | 3.0 | c-ALL | 84.9 | 3/6/89 | No mat | BFM 86-RG | 4.1 | 3/24/93 | 3.1 | No mat | BM | ALL-REZ 90 | 3.5 | 9/9/96 | BM | PCR+, FISH+ |
| 90568 | M | 5.6 | Pre-B-ALL | 6.1 | 12/20/90 | No mat | BFM 90-SR | 2.5 | 6/28/93 | 3.1 | PCR+ | BM | ALL-REZ 90 | 3.2 | 8/28/96 | BM/T | PCR+, FISH+ |
| 93473 | M | 6.3 | c-ALL | 18.9 | 9/23/93 | PCR+ | BFM 90-MR | 2.9 | 8/21/96 | 8.6 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 94112 | F | 3.4 | c-ALL | 6.7 | 2/14/94 | PCR+ | CoALL-05-92-LR | 2.7 | 10/14/96 | 3.9 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 94565 | F | 3.3 | c-ALL | 5.6 | 10/5/94 | PCR+ | CoALL-05-92-HR | 2.1 | 11/1/96 | 5.0 | PCR+, FISH+ | BM | ALL-REZ P95 | ||||
| 96526 | F | 5.9 | c-ALL* | ND | 1/22/93 | No mat | AML therapy | 3.6 | 9/14/96 | 9.5 | PCR+, FISH+ | BM/CNS | |||||
| 96569 | M | 4.1 | c-ALL | 9.2 | 9/3/93 | No mat | BFM 90-SR | 3.1 | 10/7/96 | 4.3 | PCR+, FISH+ | BM/CNS | ALL-REZ P95 | ||||
Abbreviations: ND, not determined; no mat, no material available; BM, bone marrow; T, testis; RG, risk group; SR, standard risk; MR, medium risk; LR, low risk; HR, high risk; ALL-REZ, ALL relapse therapy trials; BFM, Berlin-Frankfurt-Münster therapy trials; CoALL, Cooperative ALL therapy trials.
Immunophenotype of relapse; this patient was diagnosed and treated in Turkey as AML.
Thirty-two of 44 children had a first relapse and 12 a second relapse. The TEL/AML1 rearrangement was detected at first relapse in five patients, whereas the remaining four had a second relapse. From six of nine positive patients, cryopreserved bone marrow from diagnosis and/or first relapse was available. A retrospectively performed RT-PCR or FISH analysis confirmed the presence of the TEL/AML1 fusion gene in all of them (Table 2).
The clinical data at initial diagnosis of the children with TEL/AML1 rearrangement showed a sex ratio of 1.25, a median age at diagnosis of 4.1 years, and a median WBC count of 12.4 × 109/L (Table 3). Flow cytometric data were only available for 13 children, including one positive for TEL/AML1. Only one TEL/AML1-negative patient showed a DNA index more than 1.16 (data not shown). In all of the TEL/AML1-positive patients, BCP-ALL was diagnosed either as common (n = 8) or pre-B-ALL (n = 1), whereas the negative group included two with pre-B-ALL and six with T-ALL.
Clinical Data for TEL/AML1-Positive Children at Relapse Compared With TEL/AML1-Negative Patients With c- and Pre-B-ALL and All Negative Patients at Relapse
| Parameter . | TEL/AML1-Positive . | TEL/AML1-Negative Pre-B-/c-ALL . | Total . |
|---|---|---|---|
| Sex ratio (M:F), P = .99/.7 | 5:4 (1.25) | 12:10 (1.2) | 23:12 (1.9) |
| Age at diagnosis (yr),P = .86/.25 | |||
| n | 9 | 22 | 35 |
| Range | 3-13.94 | 0.7-15.6 | 0.7-16.3 |
| Median | 4.1 | 4.8 | 7.4 |
| WBC initial (×109/L),P = .99/.67 | |||
| n | 8 | 17 | 25 |
| Range | 5.6-84.9 | 2.5-79.4 | 2.5-96.8 |
| Median | 12.4 | 13.6 | 16.4 |
| Risk factor, P = .80/.87 | |||
| n | 5 | 13 | 21 |
| Range | 0.65-1.44 | 0.47-1.81 | 0.47-1.82 |
| Median | 1.05 | 1.07 | 1.07 |
| Risk | |||
| <0.8 | 2 | 2 | 3 |
| ≥0.8 | 3 | 11 | 18 |
| Coexpression at diagnosis, P = .68/.49 | |||
| None | 3 | 11 | 15 |
| 1 marker | 1 | 3 | 6 |
| 2 markers | 1 | 1 | 1 |
| Immunophenotype | |||
| Pro-B | 0 | 2 | |
| Pre-B | 1 | 4 | 4 |
| Common | 8 | 18 | 18 |
| T | 6 | ||
| Unknown | 5 | ||
| WBC relapse (×109/L),P = .88/.74 | |||
| n | 9 | 22 | 32 |
| Range | 3.1-12.4 | 0.8-23.2 | 0.7-77.5 |
| Median | 5.0 | 5.7 | 5.4 |
| Timepoint of relapse,P = .045/.041 | |||
| Very early | 0 | 6 | 11 |
| Early | 1 | 7 | 9 |
| Late | 8 | 9 | 15 |
| First CR (yr), P = .078/.046 | |||
| n | 9 | 22 | 35 |
| Range | 2.1-4.0 | 0.3-9.4 | 0.3-9.9 |
| Median | 3.1 | 2.3 | 2.3 |
| Second CR (yr) | |||
| n | 4 | 4 | 6 |
| Range | 3.2-5.5 | 0.2-4.2 | 0.2-4.2 |
| Median | 3.7 | 2.3 | 3.0 |
| Site of first relapse | |||
| BM | 7 | 11 | 22 |
| BM + CNS | 2 | 7 | 8 |
| BM + testis | 2 | 2 | |
| CNS | 1 | 1 | |
| Testis | 1 | 1 | |
| Unknown | 1 | ||
| Status, P = .31/.24 | |||
| Alive | 7 | 12 | 17 |
| CCR | 8 | 14 | |
| Dead | 2 | 2 | 4 |
| Parameter . | TEL/AML1-Positive . | TEL/AML1-Negative Pre-B-/c-ALL . | Total . |
|---|---|---|---|
| Sex ratio (M:F), P = .99/.7 | 5:4 (1.25) | 12:10 (1.2) | 23:12 (1.9) |
| Age at diagnosis (yr),P = .86/.25 | |||
| n | 9 | 22 | 35 |
| Range | 3-13.94 | 0.7-15.6 | 0.7-16.3 |
| Median | 4.1 | 4.8 | 7.4 |
| WBC initial (×109/L),P = .99/.67 | |||
| n | 8 | 17 | 25 |
| Range | 5.6-84.9 | 2.5-79.4 | 2.5-96.8 |
| Median | 12.4 | 13.6 | 16.4 |
| Risk factor, P = .80/.87 | |||
| n | 5 | 13 | 21 |
| Range | 0.65-1.44 | 0.47-1.81 | 0.47-1.82 |
| Median | 1.05 | 1.07 | 1.07 |
| Risk | |||
| <0.8 | 2 | 2 | 3 |
| ≥0.8 | 3 | 11 | 18 |
| Coexpression at diagnosis, P = .68/.49 | |||
| None | 3 | 11 | 15 |
| 1 marker | 1 | 3 | 6 |
| 2 markers | 1 | 1 | 1 |
| Immunophenotype | |||
| Pro-B | 0 | 2 | |
| Pre-B | 1 | 4 | 4 |
| Common | 8 | 18 | 18 |
| T | 6 | ||
| Unknown | 5 | ||
| WBC relapse (×109/L),P = .88/.74 | |||
| n | 9 | 22 | 32 |
| Range | 3.1-12.4 | 0.8-23.2 | 0.7-77.5 |
| Median | 5.0 | 5.7 | 5.4 |
| Timepoint of relapse,P = .045/.041 | |||
| Very early | 0 | 6 | 11 |
| Early | 1 | 7 | 9 |
| Late | 8 | 9 | 15 |
| First CR (yr), P = .078/.046 | |||
| n | 9 | 22 | 35 |
| Range | 2.1-4.0 | 0.3-9.4 | 0.3-9.9 |
| Median | 3.1 | 2.3 | 2.3 |
| Second CR (yr) | |||
| n | 4 | 4 | 6 |
| Range | 3.2-5.5 | 0.2-4.2 | 0.2-4.2 |
| Median | 3.7 | 2.3 | 3.0 |
| Site of first relapse | |||
| BM | 7 | 11 | 22 |
| BM + CNS | 2 | 7 | 8 |
| BM + testis | 2 | 2 | |
| CNS | 1 | 1 | |
| Testis | 1 | 1 | |
| Unknown | 1 | ||
| Status, P = .31/.24 | |||
| Alive | 7 | 12 | 17 |
| CCR | 8 | 14 | |
| Dead | 2 | 2 | 4 |
P values are given for TEL/AML1-positive v TEL/AML1-negative (pre-B/c-ALL)/TEL/AML1-positive v TEL/AML1-negative (total). The risk factor (RF) used for stratification in the therapy trial ALL-BFM is an estimation of the leukemic cell mass, and is calculated by the equation, RF = 0.2 × log (no. of blasts/μL) + 0.06 × liver (cm*) + 0.04 × spleen (cm*) (*below the costal margin).23
There were no significant differences in clinical features at diagnosis between BCP patients with and without the TEL/AML1 rearrangement (Table 3). However, the interval for first and also second remission was approximately 1 year longer in TEL/AML1-positive children, and relapse occurred not earlier than 2 years after the start of therapy. These findings were significantly different (P = .046) versus those for the total TEL/AML1-negative group, but not compared with those for BCP patients negative for TEL/AML1 (P = .078). Whereas more than half of the children (59%) with BCP-ALL in the TEL/AML1-negative group relapsed early* or very early, only one of nine children with the rearrangement had an early relapse (P = .045/.041). Nearly 80% of relapses in the latter group involved the bone marrow, and only two involved both bone marrow and the central nervous system (CNS). In contrast, the incidence of CNS or testicular relapse as a single or combined relapse was much higher (50%) among the c-/pre-B-ALL patients negative for the t(12; 21) rearrangement.
DISCUSSION
The TEL/AML1 rearrangement has been described as a frequent and specific abnormality associated with childhood BCP-ALL and seems to be correlated with a good prognosis.2-10 Only two reports have described the finding of this abnormality in relapse patients in a very small series, 13 and 16 patients, respectively.8,16 These results are divergent, with the incidence of the TEL/AML1 rearrangement being 31% and 19%, probably due to the small number of patients. In our analysis of bone marrow samples of 49 consecutive children with relapsed ALL, we found a frequency of 19.6%. This incidence is similar to that reported for diagnosis in hitherto retrospective studies, as well as a recent prospective German-Italian analysis.10
On comparing the clinical features at diagnosis, it became obvious that among TEL/AML1-positive children there were no exceptional distinguishing features. All had BCP-ALL, the age was between 2 and 10 years in eight of nine, and they had a low initial blood WBC count (median, 12.4 × 109/L). There were also no differences when compared with the group of relapsed BCP patients without the TEL/AML1 fusion gene or with the published data.
However, the median remission duration was about 1 year longer in TEL/AML1-positive children, and none of them relapsed earlier than 2 years after diagnosis. In contrast, treatment failures in the TEL/AML1-negative group occurred early or very early in more than half of the patients. The difference between TEL/AML1-positive and -negative children is significant (Table 3), but must be verified in a large series of patients. However, similar findings have been reported by others. In the German-Italian study, two of three relapses occurred late,10 and two patients from another Italian study had events after 22 and 31 months, respectively.22 Another three children analyzed at relapse8 had a remission duration completely concordant with our data. The investigators reported one child who was not adequately treated but nevertheless had a complete remission (CR) of 2.7 years, whereas two others had an EFS time of 4.7 and 6.7 years, respectively. Similarly in our study, there was one girl treated initially for AML, but she obtained a CR and had a disease-free interval of 3.6 years.
To date, contradictory results have been reported concerning the prognosis, ranging between an excellent (100% to 92%)2,3,10 and a normal outcome, versus others where two relapses of 11 patients (18%)22 and seven of 24 (29%)16 have been described. However, in all larger studies,2,3 10 a very good prognosis could be demonstrated, whereas the smaller studies have a higher percentage of treatment failures. All of these studies were performed retrospectively and consequently have undergone selection for several criteria.
The reason for the high incidence of rearrangement of TEL/AML1 among patients with relapse in the present study is not yet clear. Contamination of the PCR was excluded by parallel control experiments by FISH in seven of nine, and the remaining two children showed the fusion gene at diagnosis. It is unlikely that the rearrangement was acquired during treatment, since on earlier samples for five of nine patients a retrospective PCR or FISH analysis could be performed and the fusion gene was detected in all of them.
The high frequency of relapses might also be due to insufficient therapy. However, eight of nine patients in this study were treated in one of the German multicenter trials (Table 3): ALL-BFM-86 (n = 3; EFS of the risk group, 75%),23 ALL-BFM-90 (n = 3; EFS of the standard-risk and medium-risk groups, 75%)24 (M. Schrappe, personal communication, May 1992), and CoALL-05-92 (n = 2; EFS of the low-risk group and high-risk group, 85% and 73%)25 (G. Janka-Schaub, personal communication, May 1992; Table 2). The outcome for children treated by these protocols does not differ much from other successful regimens.26,27 Whether differences in therapy protocols, eg, the treatment intensity or the use of cranial irradiation with its known associated morbidity and controversial late effects,28-32 are responsible for the better outcome of TEL/AML1-positive patients is not yet clear. This will only be clarified by performing large long-term prospective studies, which should be critically analyzed before changes in treatment modalities are considered.
The high incidence of TEL/AML1-positive children among relapse patients may imply that the appearance of the TEL/AML1 fusion gene per se is not a good prognostic factor. However, there are no clinical features to identify the patients at risk of relapse. Since relapses in this group appear to be late events, these children should be studied for minimal residual disease using PCR to monitor disease development and consequently to identify relapse at an early stage.5,8,11 22
Nevertheless, if about 20% of all ALL relapses are positive for the TEL/AML1 rearrangement, more long-term prospective studies including a substantial number of patients at diagnosis and relapse are urgently needed. Only after more prognostic information is available should possible changes in treatment strategies for these children be discussed.
ACKNOWLEDGMENT
For excellent technical assistance, we are indebted to Andrea Richter and Christina Böth for the molecular genetics and to Andrea Kretschmann for help with the data management. We thank Dr Rosalyn Slater, Rotterdam, The Netherlands, for critically reviewing the manuscript, and Dr Martin Zimmermann, Hannover, Germany, for the statistical analyses. Finally, we are very grateful to our colleagues from many pediatric oncologic centers outside of Giessen who supplied us with bone marrow samples from their patients.
Supported by the Deutsche Krebshilfe-Mildred-Scheel-Stiftung, Forschungshilfe Station Peiper, and Parents Initiative Giessen.
Address reprint requests to F. Lampert, MD, Department of General Pediatrics/Hematology and Oncology, Justus-Liebig University Giessen, Feulgenstr. 12, D-35392 Giessen, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal