Abstract
The continual recirculation of lymphocytes between the blood, tissues, and lymph is essential for the coordination and dissemination of immune responses. We have compared the functional and phenotypic properties of lymphocytes isolated from blood and lymph, the two major migratory populations. Lymph-borne lymphocytes migrated readily into the lymphatic recirculation pathway, but greater than one third of all peripheral blood lymphocytes (PBLs) were excluded from the lymphatic circuit and showed an enhanced migration to the spleen. Phenotypic analysis showed that most non-recirculating PBLs were B cells. The migration competence of B cells correlated with the surface expression of CD21 and L-selectin; recirculating B cells expressed both of these molecules, whereas non-recirculating B cells lacked both antigens. These results establish that blood contains distinct pools of lymphocytes that differ in their recirculation competence. Clearly, blood sampling is not an efficient method to directly measure the status of the recirculating immune system, and implies important constraints and restrictions in the interpretation of experimental or clinical data that include phenotypic and quantitative analyses of blood lymphocytes.
A SIGNIFICANT PROPORTION of the peripheral lymphocyte pool recirculates continuously between the blood, tissues, and the lymph.1 This traffic of lymphocytes is the physiologic basis of immune surveillance and the dissemination of immunologic memory from a site of local antigen stimulation to distant body parts.2-4 Although data concerning the molecular aspects of lymphocyte migration have emerged from rodent and human models, large animals offer unique opportunities to gain special insights into the physiologic aspects of lymphocyte recirculation.2,3 5
Gowans6 was the first to observe that the progressive decrease in lymphocyte numbers in lymph and blood associated with chronic thoracic duct drainage could be reversed by returning the cells to the blood. Later studies found that lymph nodes were the major site of exchange for recirculating lymphocytes between the blood and the lymph.7 Seminal studies by Hall and Morris demonstrated that the output of lymphocytes in the efferent lymph of lymph nodes approximated 3.0 × 107 cells/hour per gram of lymph node.8,9 In situ labeling techniques established that less than 15% of efferent lymph lymphocytes were derived from afferent lymph input or were cells newly formed by proliferation within the lymph node, and greater than 85% of them migrated directly from blood to lymph.9 Based on the blood flow rates, it was estimated that, to support this cellular output, 1 in 4 lymphocytes passing through the vasculature of the lymph node must cross the endothelium of the postcapillary venules and enter the efferent lymph.10 Differences between the concentration of lymphocyte subsets in blood and lymph then led others to conclude that different subsets crossed the endothelium at different rates.11 12
These deductive approaches were replaced by methods to directly examine the migratory capacity of lymphocytes.13-16 Most of these experiments followed a similar protocol.17 Recirculating lymphocytes were collected directly from lymph, labeled in vitro with a tracking label, and reinjected intravenously. The relative recruitment of labeled cells to various lymph compartments was then compared over periods ranging from several hours to several weeks.13-16,18 Based on data from these sorts of studies, at least 3 distinct pools of lymphocytes migrating preferentially through skin, subcutaneous lymph nodes, or mesenteric tissues have been identified.13-16 These functional differences in the migration of lymphocytes have been correlated to specific cell-surface phenotypes.16,19,20 For example, memory-type CD4+ T cells contribute significantly to the observed tissue specificity of recirculating lymphocytes.21,22 The expression of certain cell-surface adhesion receptors on lymph cells correlates with their presence in the lymph draining specific tissues, suggesting that there may be phenotypically defined pools of lymphocytes that preferentially migrate through distinct tissues.16,23 Based on Gowans' original observations,6 peripheral blood lymphocytes (PBLs) should represent all of these pools.
The above-mentioned studies had two common elements. First, the test cell population was always efferent lymph lymphocytes (ELLs). PBLs were not widely used. Second, most data were interpreted with the tacit assumption that all lymphocytes in the peripheral blood were equally capable of recirculating through the tissues.10 11 Here, we have directly labeled PBLs and observed their migratory behavior in vivo for periods ranging from 8 hours to 18 days. We have found that the peripheral blood contains distinct pools of both recirculating and non-recirculating lymphocytes. About 40% of PBLs were excluded from the lymphatic recirculation pathway during the study period and migrated preferentially through the spleen. This pattern primarily reflected the migratory behavior of a population of CD21− B lymphocytes and correlated with a lack of L-selectin expression by these cells. In view of these data, many previous deductions concerning the rate, magnitude, and specificity of blood-to-lymph migration of lymphocytes may need to be reassessed.
MATERIALS AND METHODS
Experimental Animals
Randomly bred sheep aged from 6 to 12 months were obtained from Versuchsbetrieb Sennweid (Olsberg, Switzerland). Handling and treatment of the animals was according to protocols approved by the regional government authority, the Kantonales Veterinäramt.
Surgical Procedures
Cannulation of afferent popliteal lymphatics, efferent prescapular lymphatics, efferent intestinal lymphatics, efferent popliteal lymphatics, and the thoracic duct was as previously described.17 During general anesthesia, a roentgenography catheter (Becton Dickinson, Franklin Lakes, NJ) attached to a 3-way stopcock was inserted into the jugular vein as previously described.18
Cell Collection and Purification
Lymph. Efferent lymph was collected in sterile nalgene bottles containing a small amount of penicillin/heparin solution in saline as previously described.17 ELLs were harvested by centrifugation at 450g for 7 minutes and used for cell labeling or analysis as described below.17
Blood. Blood was collected in a small amount of 7.5% EDTA via the indwelling jugular cannula. PBLs were isolated by centrifugation over Percoll gradients and labeled as described below.24 For samples intended for fluorescence-activated cell sorting (FACS) analysis, erythrocytes were lysed using 0.16 mol/L Tris/17 mmol/L NH4Cl. Cells were washed twice in phosphate-buffered saline (PBS) and counted on a Coulter ZM cell counter (Coulter, Hialeah, FL).
Cell Labeling
Labeling of lymphocytes with PKH-26 (Sigma, St Louis, MO) and PKH-2 (Sigma) was as previously described.17 Labeling with CFSE (5- and 6-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes, Eugene, OR) was essentially as previously described.25 Cells were washed 3 times in protein-free PBS and were then resuspended at a concentration of 5 × 107 cells/mL in prewarmed PBS containing 1 μg/mL CFSE. Labeling was performed at 37°C for 15 minutes. Labeling was stopped by adding an equal volume of ice-cold PBS containing 1% lymph plasma, and the cells were recovered by centrifugation. For both labeling techniques, cells were washed 2× in PBS containing 1% lymph plasma and resuspended in 20 mL sterile saline for intravenous reinjection. We have found that labeling lymphocytes with these dyes does not significantly alter the composition of the pool from the starting population (unpublished observations). Labeling with the radiolabels Indium-111 (indium oxine; Amersham, Arlington Heights, IL) and Chromium-51 (sodium dichromate; Amersham) was as previously described.17
Cell Tracking Protocol Overview
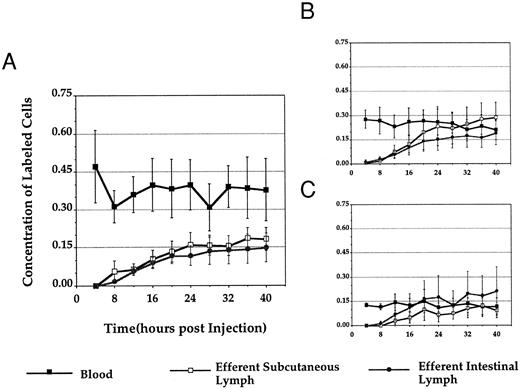
Short-term recirculation experiments. Approximately 15 mL/kg of blood (350 to 500 mL) was withdrawn, and PBLs were isolated as described above. Yields ranged from 3.0 × 108 to 1.4 × 109 cells. Cells were then labeled with either PKH-26 (n = 3) or PKH-2 (n = 3) and resuspended for injection. An equivalent number of ELLs collected from either mesenteric (n = 3) or prescapular (n = 3) lymph nodes were labeled with the complementary label and reinjected at the same time. Lymph and blood samples were collected at 4-hour intervals for the next 40 hours. Lymphocytes were isolated from each sample as described above, fixed in 1% paraformaldehyde in PBS, and stored at 4°C until analyzed by flow cytometry (FACScan; Becton Dickinson). Cells were gated on the basis of forward and side scatter to include only lymphocytes in fluorescence analysis.
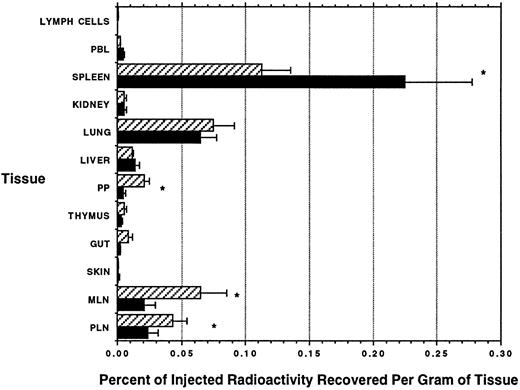
Radiolabel experiments. PBLs (1 × 109) were isolated as described above and labeled with either Indium-111 (3 animals) or Chromium-51 (3 animals) and reinjected intravenously. An equivalent number of ELLs collected from either mesenteric (n = 4) or prescapular (n = 2) lymph nodes were labeled with the complementary label and reinjected at the same time. Cells were allowed to recirculate for either 8 hours (4 animals) or 24 hours (2 animals), and the animal was killed by anesthetic overdose. A variety of tissues (see Fig 2) were removed, weighed, and counted using a gamma counter suitably programmed for dual-isotope analysis. All samples were counted a total of 3 times over several weeks to control for spectral overlap between the two isotopes. Because of the differential decay rates of the two isotopes, the precise contribution of Chromium-51 and Indium-111 to the emission from each sample was calculated.14
Distribution of radiolabeled ELLs and PBLs after intravenous injection. The total radioactivity recovered in each sampled tissue was divided by the weight of the sample (in grams), and then the percentage of injected radioactivity recovered per gram of tissue was calculated. (▨) ELLs; (▪) PBLs. Mean of 4 animals ± SEM. *PBL localization significantly different from ELLs, P < .05. Paired t-test.
Distribution of radiolabeled ELLs and PBLs after intravenous injection. The total radioactivity recovered in each sampled tissue was divided by the weight of the sample (in grams), and then the percentage of injected radioactivity recovered per gram of tissue was calculated. (▨) ELLs; (▪) PBLs. Mean of 4 animals ± SEM. *PBL localization significantly different from ELLs, P < .05. Paired t-test.
Long-term recirculation experiments. Approximately 20 mL/kg of blood (500 to 700 mL) was removed and PBLs were isolated as described above. Cell yields ranged from 5.8 × 108 to 2.5 × 109 cells. Cells were labeled with PKH-26 and reinjected intravenously at time 0. To increase the size of the labeled recirculating pool, thoracic duct lymphocytes (TDLs) were collected cumulatively every 6 to 12 hours for 1 to 3 days after the injection of the blood cells, labeled with CFSE as described above, and reinjected intravenously.
Immunophenotyping
Blood and lymph samples were collected at various time points following injection of labeled PBLs or TDLs. Monoclonal antibodies (MoAbs) against CD4 (MoAb 17D),26 CD8 (MoAb 7C2 stains similarly to other known CD8 antibodies), γδ-TcR (MoAb 86D),27 L-selectin (MoAb 1-29),22 α4 integrin (MoAb FW3-218),22 β1 integrin (MoAb 47),22 CD44 (MoAb 25-32),28 and LFA-1 (MoAb F10-150)21 were used to stain labeled PBLs and labeled ELLs recovered from peripheral blood or subcutaneous efferent lymph, respectively. In addition, two antibodies specific for sheep B cells were used. MoAb 2-8 recognizes all sheep B cells and molecular studies suggest that the target antigen is the sheep homologue of CD72. Immunoprecipitation and N-terminal sequence analysis shows that MoAb 2-128 identifies sheep CD21 (Hein et al, manuscript in preparation). Immunophenotyping was as previously described.21 In the long-term experiments, allophycocyanin-conjugated secondary antibodies were used to identify PKH-26 and CFSE-labeled cells (Molecular Probes). Cells were resuspended in PBS-1% fetal calf serum and analyzed fresh (FACStar; Becton Dickinson). In all other experiments, class-specific fluorescein and phycoerythrin-conjugated antibodies were used (Southern Biotechnology Associates, Birmingham, AL), and the cells were fixed in 1% paraformaldehyde in PBS before analysis. For 3-color analysis, biotinylated MoAb 1-29 (L-selectin) was used, followed by streptavidin Tri-Colour (Caltag Laboratories, South San Francisco, CA), and analyzed fresh. Samples were gated to include only lymphocytes in fluorescence analysis.
Lymphatic Infusion Experiments
After cannulation and for the duration of the experiment, sterile physiologic saline containing 5 U/mL heparin was infused into the popliteal lymph node via the afferent lymphatic. Twenty-four to 48 hours after surgery, when the cell output from the efferent lymphatic had stabilized, 100 mL blood was collected and labeled as described above. Cells were labeled with PKH-26 and resuspended in saline containing 5 U/mL heparin at a concentration of 1 × 108 cells/mL. A sample was retained to confirm the starting proportions of CD21+ and CD21− B cells in the labeled pool. A total of 2 × 108 labeled PBLs were infused over 1 hour into an afferent lymphatic supplying the popliteal lymph node. At 12-hour intervals for a total of 48 hours, efferent popliteal lymph was collected, and the proportion of labeled CD21+ and CD21− B cells were compared in the labeled pool.
Statistics
Paired and unpaired t-tests are according to Glantz.29 Normalization for each set of data is described with each figure.
RESULTS
A Large Proportion of PBLs Do Not Recirculate Via the Lymph in Short-Term Assays
The first series of experiments was designed to examine whether PBLs and ELLs migrated differently in vivo. PBLs were purified and labeled with a red or green fluorescent surface dye and a similar number of ELLs were labeled with the complementary dye. After intravenous reinjection, the concentration of the two types of labeled cells was monitored in blood, efferent prescapular lymph, and efferent intestinal lymph over the next 40 hours.
When PBLs were labeled and reinjected, their concentration always remained higher in the blood than in lymph. Typically, the percentage of labeled PBLs in blood decreased transiently over the first 8 hours and then recovered to a level twofold to fourfold higher than that observed in lymph over the entire 40-hour observation period (Fig 1A). When ELLs were labeled and reinjected, we saw patterns similar to those described previously.13-16 After reinjecting labeled prescapular ELLs, the concentration of labeled cells was always highest in prescapular efferent lymph, whereas, when intestinal efferent lymphocytes were used as input cells, the concentration of labeled cells was highest in intestinal efferent lymph (Fig 1B and C). In direct contrast to the distribution of PBLs, the concentration of labeled ELLs in blood was always equal to or lower than that in lymph by the end of the 40-hour observation period. Control experiments in two animals showed that centrifuging ELLs through Percoll gradients before reinjection, using methods similar to those used for the purification of PBLs, had no effect on the subsequent migration pattern of lymphocytes (data not shown). Therefore, the distinctive migratory properties of PBLs and ELLs after labeling and injection could not be attributed to differences between the methods used to prepare the input cells.
Normalized concentration of labeled cells in blood, efferent subcutaneous lymph, and efferent intestinal lymph after intravenous injection. Figure 1 depicts the distribution of labeled PBLs (A), subcutaneous ELLs (B), and intestinal ELLs (C) in blood, efferent subcutaneous lymph, and efferent intestinal lymph after reinjection. The absolute concentration of labeled cells in each tissue at each time point was divided by the number of cells injected and then multiplied by 109 to give a percentage of labeled cells/109 injected at each point. The results were then pooled between all experimental animals (labeled PBLs, n = 6; labeled subcutaneous ELLs, n = 3; labeled intestinal ELLs, n = 3; all values ± SEM).
Normalized concentration of labeled cells in blood, efferent subcutaneous lymph, and efferent intestinal lymph after intravenous injection. Figure 1 depicts the distribution of labeled PBLs (A), subcutaneous ELLs (B), and intestinal ELLs (C) in blood, efferent subcutaneous lymph, and efferent intestinal lymph after reinjection. The absolute concentration of labeled cells in each tissue at each time point was divided by the number of cells injected and then multiplied by 109 to give a percentage of labeled cells/109 injected at each point. The results were then pooled between all experimental animals (labeled PBLs, n = 6; labeled subcutaneous ELLs, n = 3; labeled intestinal ELLs, n = 3; all values ± SEM).
PBLs and ELLs Home to Different Lymphoid Tissues In Vivo
To search further for differences between the tissue homing patterns of PBLs and ELLs, cells were purified as described above, labeled with either Indium-111 or Chromium-51, and then reinjected intravenously. The distinctive γ-emission spectra of these two isotopes allow for direct measurement of the relative proportions of PBLs and ELLs that migrate into different tissues. The results obtained at 8 hours after injection are shown in Fig 2. No significant difference was observed between the migration of labeled ELLs or PBLs to nonlymphoid tissues such as liver, kidney, gut, or skin. The lung contained a relatively large proportion of both types of labeled cells, but homing was nonspecific. In contrast, as compared with PBLs, ELLs showed a marked preference to migrate back to lymph nodes and Peyer's patches. Labeled PBLs on the other hand migrated twice as effectively to the spleen as did ELLs. Observations made 24 hours after labeling and reinjection differed in the magnitude of cell migration into each tissue, although the above-noted ratios were preserved (data not shown).
Long-Term Analysis of Recirculation
The homing patterns observed above may have resulted from transient physiologic differences between the two types of input cells or they could reflect the differential presence in blood and lymph of pools of cells with stable and distinctive recirculation behaviors. To further resolve this point, a series of long-term experiments was undertaken. PBLs and TDLs were labeled in a complementary manner with either PKH-26 or CFSE (stable dyes that can be used to trace labeled cells for several weeks in vivo), reinjected, and then monitored as before in blood and lymph for periods ranging from 3 to 18 days.18 25 At all times after injection, the concentration of labeled PBLs remained higher in the blood than in lymph, whereas the converse was true for labeled TDLs (Table 1).
Normalized Concentration of Labeled PBLs and TDLs in Blood and Subcutaneous Efferent Lymph 3 to 18 Days After Intravenous Injection
| Time (d) . | Sheep . | % PBL in Blood . | % PBL in Lymph . | % TDL in Blood . | % TDL in Lymph . |
|---|---|---|---|---|---|
| 3 | 5422 | 0.34 | 0.14 | 0.20 | 0.23 |
| 4 | 5432 | 0.80 | 0.26 | 0.10 | 0.15 |
| 4 | 5434 | 0.60 | ND | 0.04 | ND |
| 5 | 5287 | 0.78 | ND | 0.05 | ND |
| 5 | 5422 | 0.30 | 0.13 | 0.17 | 0.22 |
| 6 | 5288 | 0.26 | 0.08 | 0.13 | 0.14 |
| 7 | 5422 | 0.43 | ND | 0.12 | ND |
| 8 | 5432 | 0.71 | 0.24 | 0.09 | 0.16 |
| 8 | 5434 | 0.55 | 0.06 | 0.04 | 0.10 |
| 18 | 5432 | 0.45 | ND | 0.05 | ND |
| Mean ± SE | 0.52 ± 0.06* | 0.15 ± 0.03 | 0.10 ± 0.02† | 0.17 ± 0.02 |
| Time (d) . | Sheep . | % PBL in Blood . | % PBL in Lymph . | % TDL in Blood . | % TDL in Lymph . |
|---|---|---|---|---|---|
| 3 | 5422 | 0.34 | 0.14 | 0.20 | 0.23 |
| 4 | 5432 | 0.80 | 0.26 | 0.10 | 0.15 |
| 4 | 5434 | 0.60 | ND | 0.04 | ND |
| 5 | 5287 | 0.78 | ND | 0.05 | ND |
| 5 | 5422 | 0.30 | 0.13 | 0.17 | 0.22 |
| 6 | 5288 | 0.26 | 0.08 | 0.13 | 0.14 |
| 7 | 5422 | 0.43 | ND | 0.12 | ND |
| 8 | 5432 | 0.71 | 0.24 | 0.09 | 0.16 |
| 8 | 5434 | 0.55 | 0.06 | 0.04 | 0.10 |
| 18 | 5432 | 0.45 | ND | 0.05 | ND |
| Mean ± SE | 0.52 ± 0.06* | 0.15 ± 0.03 | 0.10 ± 0.02† | 0.17 ± 0.02 |
The concentration of labeled cells in each compartment was divided by the number of injected cells to give a percentage per 109 intravenously injected labeled cells at each time point.
Abbreviation: ND, not determined.
Blood concentration of PBLs significantly different than lymph, Student's t-test, P < .001.
Blood concentration of TDLs significantly different than lymph, Student's t-test, P < .05.
Taken together, three sets of conclusions and inferences can be drawn from the data presented so far. (1) Blood and lymph contain pools of lymphocytes with distinctive recirculation properties. Whereas lymph-borne lymphocytes readily re-enter the lymphatic recirculation pathway, blood seemingly contains a pool of lymphocytes that is effectively excluded from this circuit. (2) Although some lymphocytes from both blood and lymph migrate to the spleen, the blood contains a special pool of cells with an enhanced predilection to migrate to this organ. (3) These features probably reflect steady-state differences between the migration properties of different pools of lymphocytes because, at least for lymphatic recirculation, the biased migration remains stable over an 18-day observation period.
Surface Phenotype of Recirculating and Non-Recirculating Lymphocytes
To characterize the surface phenotype of recirculating and non-recirculating lymphocytes, labeled TDLs and PBLs recovered in the long-term experiments summarized in Table 1 were phenotyped for conventional lymphocyte subset markers. Of the labeled PBLs, only recirculating cells would have migrated into the lymph. The relative concentration of each lymphocyte subset within the pool of labeled PBLs recovered in lymph should therefore reflect the composition of the blood-borne recirculating lymphocyte pool (RLP). Using similar logic, the labeled TDLs should have distributed in the peripheral blood in such a way as to reflect the composition of the blood-borne RLP. As seen in Table 2, the composition of labeled TDLs in blood and of labeled PBLs in lymph was virtually identical. Therefore, based on two independent measurements, the blood-borne RLP comprised approximately 25% CD4+ T cells, 5% CD8+ T cells, 40% γδ-T cells, and 25% B cells.
Percentage of Labeled Lymphocyte Subsets in Efferent Lymph and Blood 3 to 18 Days After Injection of Labeled PBLs and Labeled TDLs
| Antibody . | Efferent Lymph . | Blood . | ||
|---|---|---|---|---|
| . | Labeled TDLs . | Labeled PBLs . | Labeled TDLs . | Labeled PBLs . |
| CD4 | 32.7 ± 5.9 | 23.1 ± 5.1 | 27.7 ± 5.4 | 9.9 ± 3.7 |
| CD8 | 8.8 ± 2.1 | 5.1 ± 1.2 | 6.3 ± 0.9 | 9.2 ± 1.5 |
| γδ-TcR | 24.2 ± 4.1 | 38.1 ± 3.7 | 35.6 ± 4.4 | 12.8 ± 3.6 |
| B cells | 24.8 ± 3.5 | 19.2 ± 6.2 | 24.7 ± 4.5 | 62.7 ± 14.3 |
| Antibody . | Efferent Lymph . | Blood . | ||
|---|---|---|---|---|
| . | Labeled TDLs . | Labeled PBLs . | Labeled TDLs . | Labeled PBLs . |
| CD4 | 32.7 ± 5.9 | 23.1 ± 5.1 | 27.7 ± 5.4 | 9.9 ± 3.7 |
| CD8 | 8.8 ± 2.1 | 5.1 ± 1.2 | 6.3 ± 0.9 | 9.2 ± 1.5 |
| γδ-TcR | 24.2 ± 4.1 | 38.1 ± 3.7 | 35.6 ± 4.4 | 12.8 ± 3.6 |
| B cells | 24.8 ± 3.5 | 19.2 ± 6.2 | 24.7 ± 4.5 | 62.7 ± 14.3 |
Data are presented as the mean ± SE.
From the data shown in Table 2, two subsets appeared to have distinctive recirculation properties. First, the majority of labeled PBLs that migrated into the lymph were γδ-T cells, suggesting that this subset may be particularly efficient at recirculation. Secondly, B lymphocytes were substantially enriched among labeled PBLs later recovered from blood, suggesting that a proportion of these cells were unable to recirculate.
The two sets of labeled cells (TDLs and PBLs) were also examined for surface expression of a number of adhesion molecules. No differences were observed between the expression of LFA-1, CD44, or the integrin chains α4 and β1 on the two sets of cells (Fig 3). About 75% of labeled TDLs expressed L-selectin irrespective of whether they were subsequently recovered from blood or lymph. In contrast, labeled PBLs showed markedly different expression of the molecule depending on their migration efficiency. About 70% of labeled PBLs that were recovered from lymph (recirculating cells) expressed L-selectin, whereas 90% of those recovered from blood (non-recirculating cells) were L-selectin−.
Expression of adhesion molecules on labeled PBLs and labeled TDLs recovered from efferent lymph or blood 8 days after labeling and intravenous reinjection. (——) PBLs; (- - -) TDLs. Data from 1 of 3 animals are shown.
Expression of adhesion molecules on labeled PBLs and labeled TDLs recovered from efferent lymph or blood 8 days after labeling and intravenous reinjection. (——) PBLs; (- - -) TDLs. Data from 1 of 3 animals are shown.
These data suggested that blood contained two distinct lymphocyte pools, a RLP that actively recirculated and whose composition was defined above and a non-recirculating pool (NRLP) that was effectively excluded from the lymphatic recirculation circuit. The bulk analysis of labeled cells from these two pools suggested the possibility of a correlation between migration competence and L-selectin expression and that the subset composition of the two lymphocyte pools differed significantly.
Identification of a Pool of Non-Recirculating B Cells
To further resolve the above-mentioned issues, we next examined the expression of L-selectin on individual lymphocyte subsets isolated from blood and lymph. Most γδ-T cells in both blood and lymph were strongly positive for L-selectin (Fig 4). CD4+ T cells, CD8+ T cells, and B cells had broadly comparable profiles of L-selectin expression. Although there was a consistent dominance of positive cells in lymph and of negative cells in blood, L-selectin expression by itself was not especially informative in identifying a particular subset with reduced migration competence.
Flow cytometric analysis of the expression of L-selectin on the surface of lymphocyte subsets collected from peripheral blood or efferent prescapular lymph. Cells were collected, and phenotyped for conventional lymphocyte subsets. Using a second antibody, L-selectin expression could then be compared on each subset normally found in peripheral blood and efferent lymph.
Flow cytometric analysis of the expression of L-selectin on the surface of lymphocyte subsets collected from peripheral blood or efferent prescapular lymph. Cells were collected, and phenotyped for conventional lymphocyte subsets. Using a second antibody, L-selectin expression could then be compared on each subset normally found in peripheral blood and efferent lymph.
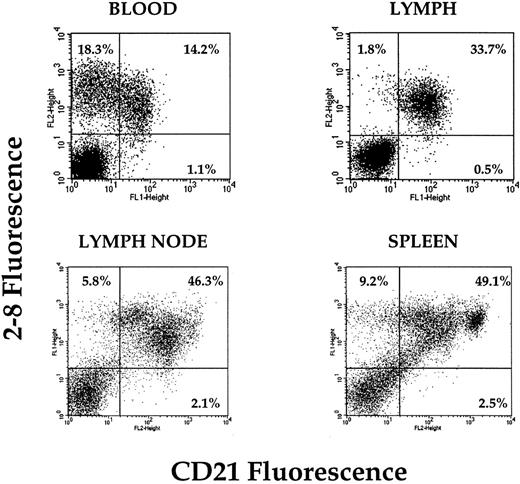
Because the earlier experiments suggested that the NRLP probably contained many B cells, we then focussed on this subset and tested whether the differential expression of a panel of B-cell markers might correlate with recirculation behavior. B cells from all tissues sampled were positive for B220 and surface Ig, and although the levels of expression fluctuated somewhat, there was no consistent pattern (data not shown). However, the expression profiles of CD21 differed significantly and repeatably between different tissues. In lymph nodes and spleen, populations of CD21hi, CD21lo, and CD21− B cells were present (Fig 5). In the animals used in these experiments, approximately 50% of peripheral blood B cells expressed CD21 and the remainder were negative. In striking contrast, all B cells isolated from efferent lymph were consistently CD21+. This suggested a correlation between CD21 expression on B cells and lymphatic migration competence.
Expression of CD21 on the surface of B cells recovered from peripheral blood, lymph, retropharyngeal lymph node, and spleen. Dual-color analysis clearly demonstrates the presence of a pool of CD21− B cells in the peripheral blood that is absent from lymph. A small number of CD21− B cells also appears to be in lymph node and spleen (blood and lymph plots from 1 animal; spleen and lymph node plots from 1 animal).
Expression of CD21 on the surface of B cells recovered from peripheral blood, lymph, retropharyngeal lymph node, and spleen. Dual-color analysis clearly demonstrates the presence of a pool of CD21− B cells in the peripheral blood that is absent from lymph. A small number of CD21− B cells also appears to be in lymph node and spleen (blood and lymph plots from 1 animal; spleen and lymph node plots from 1 animal).
To test this idea, we compared CD21 expression on long-term labeled TDLs and PBLs in a manner analogous to that used previously. Profiles from one animal are shown in Fig 6. Subsets of both labeled TDLs and PBLs that continued to recirculate through the lymphatic system clearly expressed high levels of CD21. Some labeled TDLs harvested from the blood were also CD21hi. In contrast, only a minority of labeled PBLs that were later collected from the blood expressed appreciable levels of CD21. All labeled B cells originally obtained from thoracic duct lymph continued to express CD21 for periods up to 18 days, regardless of whether they were later harvested from blood or lymph (Table 3). All labeled B cells that had originally been obtained from the peripheral blood but had been later harvested from the lymph also remained CD21+. Those labeled B cells that had originally been collected from the blood, and after reinjection were later harvested from the blood, showed a significant increase in the number of CD21− B cells relative to unlabeled peripheral blood. These results directly established a correlation between continued expression of CD21 and recirculation competence of B cells.
Expression of CD21 on the surface of labeled (——) PBLs and (- - -) TDLs harvested from blood and lymph 3 to 18 days after intravenous injection. PBLs and TDLs migrating to efferent prescapular lymph show similar staining patterns. In contrast, CD21 expression is markedly higher on labeled TDLs later recovered from the peripheral blood than on labeled PBLs (representative data from 1 animal).
Expression of CD21 on the surface of labeled (——) PBLs and (- - -) TDLs harvested from blood and lymph 3 to 18 days after intravenous injection. PBLs and TDLs migrating to efferent prescapular lymph show similar staining patterns. In contrast, CD21 expression is markedly higher on labeled TDLs later recovered from the peripheral blood than on labeled PBLs (representative data from 1 animal).
Expression of CD21 on Labeled PBLs and Labeled TDLs Recovered From Blood and Lymph 3 to 18 Days After Labeling and Intravenous Reinjection (n = 5)
| Antibody . | Labeled TDLs . | Labeled PBLs . | ||
|---|---|---|---|---|
| . | Lymph . | Blood . | Lymph . | Blood . |
| B cells | 26.8 ± 6.7 | 24.6 ± 4.4 | 21.6 ± 5.4 | 63.2 ± 13.8 |
| CD21 | 26.8 ± 8.4 | 23.5 ± 3.4 | 22.3 ± 5.8 | 26.1 ± 6.5 |
| Antibody . | Labeled TDLs . | Labeled PBLs . | ||
|---|---|---|---|---|
| . | Lymph . | Blood . | Lymph . | Blood . |
| B cells | 26.8 ± 6.7 | 24.6 ± 4.4 | 21.6 ± 5.4 | 63.2 ± 13.8 |
| CD21 | 26.8 ± 8.4 | 23.5 ± 3.4 | 22.3 ± 5.8 | 26.1 ± 6.5 |
Antibody 2-8 was used to identify all B cells, and antibody 2-128 identified CD21+ lymphocytes. Data are presented as the mean percentage of labeled cells ± SE.
Because the analysis of bulk populations of labeled TDLs and PBLs suggested that the expression of L-selectin may partly determine recirculation competence, we examined its expression on the surface of CD21+ and CD21− B cells in the peripheral blood. The two populations differed significantly in their L-selectin profiles in a coordinated manner, ie, about 70% of CD21+ B cells expressed L-selectin, but 85% of CD21− B cells were L-selectin− (Fig 7).
Expression of L-selectin on the surface of (- - -) CD21+ and (——) CD21− B cells in the peripheral blood (representative plot from 1 of 4 animals).
Expression of L-selectin on the surface of (- - -) CD21+ and (——) CD21− B cells in the peripheral blood (representative plot from 1 of 4 animals).
Selective Lymph Node Entry or Exit?
Two potential mechanisms could account for the differences between the phenotypes of B cells in lymph as compared with blood: (1) a selective entry model whereby only CD21+ cells migrated across high endothelial venules (HEV) into lymph nodes or (2) a selective exit model whereby both CD21− and CD21+ B cells migrated into the node but only CD21+ cells exited via the efferent lymph. We attempted to distinguish between these two possibilities by infusing labeled PBLs containing approximately equal proportions of CD21− and CD21+ B cells directly into the lymph node via the afferent lymphatics. The labeled cells exiting the node in efferent lymph were then phenotyped for CD21 expression. Both CD21+ and CD21− B cells migrated through the node with comparable kinetics. The peak recovery of each cell type was 24 hours after infusion (Fig 8). At all times examined, significant numbers of labeled CD21− B cells were found in the efferent lymph, establishing that, once introduced into the lymph node parenchyme, circulating CD21− B cells could exit the node without upregulating CD21 expression. The fact that under normal circumstances CD21− B cells were undetectable in efferent lymph argued that these cells were unable to migrate across HEV.
Relative recovery of labeled peripheral blood B cells in efferent lymph 0 to 48 hours after intralymphatic injection. The total number of CD21− B cells was calculated by subtracting the number of labeled CD21+ cells from the number of labeled 2-8+ cells recovered at each time point. The percentage of total recovery of each subset at each time point was then calculated, and the results were pooled between experimental animals (mean of 4 animals ± SEM). (○) CD21+ B cells; (▵) CD21− B cells.
Relative recovery of labeled peripheral blood B cells in efferent lymph 0 to 48 hours after intralymphatic injection. The total number of CD21− B cells was calculated by subtracting the number of labeled CD21+ cells from the number of labeled 2-8+ cells recovered at each time point. The percentage of total recovery of each subset at each time point was then calculated, and the results were pooled between experimental animals (mean of 4 animals ± SEM). (○) CD21+ B cells; (▵) CD21− B cells.
DISCUSSION
Experiments aimed at studying lymphocyte traffic have traditionally examined the migratory properties of lymphocytes isolated from either lymph nodes or lymph. Although all recirculating lymphocytes must leave and then re-enter the blood circulation, perhaps for several consecutive cycles, no studies have tested whether the migration competence of blood-borne lymphocytes differs in any respect from lymphocytes in other recirculation compartments. Our identification of two distinct pools of lymphocytes, the RLP and NRLP, emphasizes a previously unrecognized level of functional heterogeneity among blood lymphocytes. The reassortment of lymphocytes into these pools correlates, to a significant degree, with lymphocyte lineage and the expression of maturation and/or adhesion markers.
The initial series of experiments used traditional cell labeling and tracking techniques to follow the migration of PBLs and recirculating lymphocytes harvested from lymph. There were two possible outcomes from these experiments. In the first case, the concentration of labeled PBLs would equilibrate between blood and lymph, implying that all cells in the peripheral blood were equally capable of migration. In the second case, the concentration of labeled cells would remain higher in the blood than in the lymph, indicating that not all cells in the peripheral blood had equal access to the lymphatic recirculation pathway. This is what was observed in both short-term and long-term experiments. The stability of these patterns for periods up to 18 days implies the presence of two stable pools of lymphocytes in the peripheral blood, functionally distinct in their migratory characteristics. Although it is possible and even likely that there is interchange between these pools in vivo, it must occur slowly enough to be undetectable in our experiments.
Of the total lymphocyte component of a mature animal (about 1012 lymphocytes), roughly 10% constitute the RLP.30,31 Because of the methodology used in our experiments, labeled PBLs in the blood must be a mixture of recirculating and non-recirculating lymphocytes. Because labeled recirculating PBLs will distribute throughout the entire RLP, whereas the non-recirculating PBLs will be confined to the blood (1010 lymphocytes) and spleen (3 to 5 × 1010 lymphocytes), labeled PBLs recovered in the blood will be enriched for cells of the NRLP.32 33 Based on the mean concentration of labeled TDLs in blood and lymph, it is possible to calculate the relative sizes of these two pools in the blood.*
If the concentration of labeled TDLs is diluted by the presence of non-recirculating PBLs from 0.17% to 0.10% between the lymph and the blood, it suggests that the RLP represents 0.10/0.17 = 0.588 or about 60% of the peripheral blood (data from Table 1). The NRLP must therefore represent up to 40% of PBLs.
Based on the measured composition of the RLP (Table 2), it is also possible to deduce the composition of the NRLP.† Using this method, we calculate that the NRLP is composed of about 85% B cells, 11% CD8+ T cells, and about 3% CD4+ T cells. Comparable figures can be obtained using either the composition of labeled TDLs in blood or the composition of labeled PBLs in lymph as a base for calculation.
In recent years, a great deal of data have been accumulated regarding the expression of adhesion molecules on the surface of lymphocytes in different tissues.3,5 Although we did not detect any significant differences between the expression of CD44, LFA-1, α4, or β1 integrins on the surface of cells of the RLP and the NRLP, there were clear correlations with L-selectin expression. Because a significant proportion of the RLP was L-selectin−, it is clear that L-selectin is not absolutely required for lymphocyte migration through lymph nodes in vivo. However, it is interesting to note that the NRLP was almost exclusively L-selectin−. Recent studies on L-selectin knockout mice show a similar pattern of lymphocyte migration to that observed here using PBLs as input cells. In these mice, the homing of L-selectin negative lymphocytes to mesenteric lymph nodes, peripheral lymph nodes, and Peyer's patches was markedly reduced, whereas homing to the spleen was increased.34 In contrast to the current level of understanding regarding the molecular basis of lymphocyte migration to lymph nodes, relatively little is known regarding the mechanisms of entry to the spleen.5 Recent data suggest that entry into the red pulp and the white pulp of the spleen is differentially regulated. Entry into the white pulp is pertussis toxin sensitive and is inhibited by fucoidin, suggesting that it has much in common with the entry of lymphocytes to lymph nodes.35 36 In our experiments, both PBLs and ELLs migrated to the spleen, although PBLs demonstrated a clear predilection. This may reflect selective retention within different splenic microenvironments, but our experiments do not provide any direct insight into this possibility.
The analyses of cell subsets showed that the patterns of lymphocyte migration outlined above could in large measure be accounted for by the behavior of two functionally and phenotypically distinct pools of B cells, both of which were represented in the blood. One population (L-selectin+/CD21+) migrated via the lymphatics, whereas the other population (L-selectin−/CD21−) was excluded from this pathway. Given the extended period of time over which our experiments were conducted, CD21 expression emerged as a stable phenotype of recirculating B cells, whereas lack of CD21 marked the non-recirculating B cell pool. The high degree of correlation between the presence or absence of CD21 on B cells with the presence or absence of L-selectin suggests that the expression of these two molecules is coordinately regulated and that both may influence the migratory preferences of B cells. The events that regulate selective B-cell recirculation are mediated, at least in part, at the level of the HEV.
Different levels of expression of CD21 and L-selectin have been reported in a number of other systems. Immature B cells in human bone marrow lack expression of both molecules, although this subset has not been detected in the blood.37,38 It seems unlikely that the CD21−/L-selectin− B cells detected in blood in the present experiments are immature B cells, given their large pool size and phenotypic stability in long-term tracking experiments. In addition, the size of the CD21−/L-selectin− B-cell pool increases with advancing age (unpublished observations). Both L-selectin and CD21 are downregulated on stimulated B cells so that this subset may consist of recently activated or memory B cells.38,39 Indeed, a population of L-selectin− peripheral blood B cells with a surface phenotype indicative of recent activation and seemingly derived from the L-selectin+ pool has been reported in humans.40 In mice, a subset of L-selectin− germinal center B cells homed preferentially to the spleen but not to lymph nodes.41 Other studies showed that memory B cells generated after primary but not secondary immunization lacked L-selectin and did not migrate effectively to lymph nodes.42 A role has also been proposed for complement receptors in the initial localization of B cells in the spleen but not in lymph nodes or Peyer's patches.43
Important functional linkages have been established between the regulation of CD21 and L-selectin that might partly explain the coordinated expression patterns that we observed. One of the ligands for CD21 is the cytokine interferon-α (IFN-α).44 IFN-α induces homotypic adhesion of human B cells and increases the surface expression of L-selectin and Leu-13, a component of the CD21 receptor complex.45,46 Other studies showed that activation through Leu-13 induced the shedding of L-selectin from B cells.47 In vivo, treatment with IFN-α promotes a selective retention of B lymphocytes within lymph nodes.48 It is tempting to speculate that the selective activation of different components of the CD21 receptor complex and coordinated changes in L-selectin levels may regulate the migration of B lymphocytes through a lymph node during an immune response. It is known that there are both fixed and migratory components to B-cell memory and that, after immunization, memory B cells can be detected in lymphoid organs distant from the original site of infection.49 50 CD21− B cells in the peripheral blood may be a reserve pool of non-recirculating memory B lymphocytes. Although we have shown that these cells do not actively recirculate under normal situations, they may be recruited in response to antigenic stimulation.
A small number of CD21− B cells were detected in lymph nodes, although these cells were undetectable in lymph. Immunohistologic analysis showed that the CD21− B cells localized to the dark zone of germinal centers (unpublished observations) and these cells are therefore likely to be resident rather than migratory lymphocytes. However, we cannot exclude the possibility that small numbers of CD21− B cells migrated from lymph nodes in efferent lymph at levels below the threshold of detection during flow cytometry.
Although the antibody response to many antigens can be transfered using thoracic duct lymphocytes, B-cell responses to some antigens reside primarily within a population of splenic B cells.51 The CD21− peripheral blood B cells may therefore be a developmental lineage that reacts primarily with a distinct set of antigens that localize in the spleen. CD8+ T cells and B cells are increased in the peripheral blood of splenectomized humans and rodents, suggesting that similar pools of non-recirculating cells may exist in these species.52,53 Furthermore, one of the immunodeficiencies found in splenectomized mice is an inability to respond to T-independent antigens.54 This suggests a unique role of splenic B cells in normal immune surveillance and responsiveness.
Collectively, these data highlight the degree of heterogeneity in the recirculation properties of blood-borne lymphocytes and identify two distinct pools of B cells with distinctive migration pathways. Although these sorts of experiments have not been performed in other species, the close analogies found between the phenotype and behavior of cells reported here and reports from other experimental systems argue that similar processes probably occur in other animals, including humans. Two important implications follow. First, because not all lymphocytes in blood have an equal probability of migration, many previous estimates of the rate and degree of recirculation of lymphocytes out of the blood and into the tissues are likely to be underestimates and may need to be reassessed. Secondly, given the importance of peripheral blood measurements in the clinical setting, caution needs to be exercised when interpreting PBL counts as a window on the immune system, especially in those conditions in which changes in PBL proportions are not necessarily reflected in peripheral lymphoid tissues.55
ACKNOWLEDGMENT
The authors thank Drs Hans-Reimer Rodewald, Thomas Goebel, and Raul Torres for suggestions in the preparation of this manuscript.
Supported by Hoffmann-LaRoche Ltd (Basel, Switzerland).
Address reprint requests to Alan J. Young, PhD, Basel Institute for Immunology, Grenzacherstrasse 487, Postfach, CH4005 Basel, Switzerland; email: young@bii.ch.
Derivation of the proportion of the RLP and NRLP within the peripheral blood. Assume that PBLs consist of 2 pools of lymphocytes, such that Total PBLs = (RLP + NRLP), where RLP and NRLP represent the relative proportions of recirculating and non-recirculating lymphocytes within the peripheral blood. The lymph only contains recirculating lymphocytes, such that Total ELLs = RLP. The frequencies of labeled cells as measured by FACS analysis give a direct measurement of their overall percentage in blood and lymph (B% and L%, respectively). For a fixed number of labeled cells in lymph, the number of total cells within the RLP will be N1 = L% × RLP. Within the blood, the same number of labeled cells will be given by N2 = B% × (NRLP + RLP). Given that the labeled cells equilibrate such that they are a constant proportion of the RLP in both the blood and the lymph, when N1 = N2 then B% × (RLP + NRLP) = L% × RLP. The expression (RLP)/(RLP + NRLP) gives the proportion of the peripheral blood that is the RLP, and RLP/(RLP + NRLP) = B%/L%. From our measurements, the RLP represents 0.10/0.17 = 0.588 or 58.8% of total PBLs.
Equation to derive the proportions of lymphocyte subsets within the recirculating and non-recirculating lymphocyte pools. The calculations are based on the assumption that all blood-borne γδ-T cells are in the RLP. The proportion of each other subset relative to γδ-T cells must therefore remain constant whether the recirculating pool is measured in the blood or the lymph. The composition of the RLP measured as the concentration of labeled PBLs recovered from lymph as presented in Table 2 is used for the following calculations. If there are 12.8% γδ-T cells in the blood and the ratio of B cells to γδ-T cells in the lymph is 19.2%/38.1%, then the overall percentage of recirculating B cells in the labeled pool in the peripheral blood must be (19.2%/38.1%) × 12.8% = 6.5%. The difference between the total number of labeled B cells and the recirculating component must be non-recirculating B cells. Therefore, of the total labeled PBLs in the blood, about 62.7% − 6.5% = 56.2% are non-recirculating B cells. Similar calculations derive that 7.5% of the labeleled PBLs in the blood are non-recirculating CD8+ T cells and 2.1% non-recirculating CD4+ T cells. Therefore, the total percentage of the labeled PBLs in the blood that are non-recirculating is 56.2% + 7.5% + 2.1% = 65.8%. The percentage of non-recirculating lymphocytes that are B cells is then 65.2%/65.8% × 100% = 85.4%. Similarly, about 11.4% are CD8+ T cells, and at most 3.2% CD4+ T cells. Similar values can be obtained using the concentration of labeled TDLs in blood as the base for the calculation.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal