Abstract
Microvascular endothelial cells (EC) have multiple functions in inflammatory responses, including the production of chemoattractants that enhance leukocyte transmigration into tissues. Chemotactic protein, 10 kD (CP-10), is an S100 protein with potent chemotactic activity for myeloid cells in vitro and in vivo and is expressed in neutrophils and lipopolysaccharide (LPS)-activated macrophages. We show here that CP-10 is induced in murine endothelioma cell lines (bEnd-3, sEnd-1, and tEnd-1) after activation with LPS and interleukin-1 (IL-1) but not tumor necrosis factor α (TNFα) or interferon γ (IFNγ). Induction was not mediated by endogenous release of IL-1 or TNFα and was not directly upregulated by phorbol myristate acetate, calcium ionophore, or vitamin D3. EC were exquisitely sensitive to IL-1 activation (3.4 U/mL) and CP-10 mRNA induction with IL-1 occurred earlier (8 hours) than with LPS (12 hours). Furthermore, some microvessels and capillaries in delayed-type hypersensitivity lesions expressed cytoplasmic CP-10. Responses to LPS and not IL-1 in vitro were regulated by the degree of cell confluence and by TNFα costimulation. The related MRP-14 mRNA had a different induction pattern. Monomeric and homodimeric CP-10 upregulated by activation was predominantly cell-associated. EC-derived CP-10 may contribute to amplification of inflammatory processes by enhancing leukocyte shape changes and transmigration in the microcirculation.
ENDOTHELIAL CELLS (EC) have a multifunctional role in inflammation. Factors such as lipopolysaccharide (LPS), interleukin-1 (IL-1), or tumor necrosis factor α (TNFα) present at inflammatory sites stimulate/induce many endothelial functions, including adhesion molecule expression, release of proinflammatory cytokines, expression of tissue factor and other procoagulants, nitric oxide release, and production of proliferative and angiogenic mediators (reviewed in Mantovani et al1,2 ). Furthermore, activated EC contribute to leukocyte recruitment and accumulation by production of chemoattractants, including the chemokines monocyte chemoattractant protein-1 (MCP-1), IL-8, interferon-inducible protein-10 (IP-10), gro, macrophage inflammatory protein-1β (MIP-1β), and RANTES (reviewed in Mantovani et al2 and Introna et al3 ) and other chemotactic factors such as platelet-activating factor (PAF) and transforming growth factor β1 (TGFβ1; reviewed in Diaz-Flores et al4 ) and by expression of adhesion molecules. Some chemoattractants are specific, or more potent, for particular leukocyte populations, and their regulation and kinetics of induction could influence the composition of the inflammatory exudate.
CP-10 is an S100 protein with potent chemotactic activity for polymorphonuclear leukocyte (PMN) and monocytes in vitro5,6 and in vivo.6,7 Since our original observation, two additional members of the S100 family have been reported to have chemotactic activity (S100L, which is chemotactic for eosinophils, and S100A7, which is chemotactic for lymphocytes).8,9 Like TGFβ, CP-10 is considered a pure chemoattractant, because it activates myeloid cell migration without stimulating respiratory burst, enzyme release, or calcium ion flux.10 CP-10–activated neutrophils and macrophages are large, with increased polymerised actin within extended pseudopodia. Morphologic changes indicate increased stiffening and reduced deformability, suggesting that CP-10 may cause retention of leukocytes in microcapillaries during the early phase of adhesion-independent capillary blockage.10 Intradermal injection of CP-10 initiates an infilitration of neutrophils over 4 to 8 hours,7,11 followed by a more sustained recruitment of mononuclear cells for over 24 hours.6,11 The kinetics of leukocyte infiltration are more prolonged than with other chemoattractants, such as IL-8, which elicits PMN infiltration that resolves within 2 to 8 hours.12
CP-10 is expressed constitutively in neutrophils13 and is inducible in macrophage-like RAW cells and elicited murine macrophages after stimulation with LPS.14 CP-10 produced locally during acute inflammatory activation could therefore amplify leukocyte recruitment. Our preliminary experiments indicated CP-10 on the endothelial lining of small blood vessels of LPS-injected mouse footpads together with recruited CP-10–positive neutrophils,7 but the source of CP-10 was undefined. The closely related human neutrophil protein, MRP-8 (migration inhibitory factor [MIF ]-related protein, 8 kD), was also detected on EC in inflammatory lesions but was assumed to be deposited on EC by transmigrating leukocytes.15 CP-10 has been claimed as the murine homologue of human MRP-8,16 but the latter is not chemotactic for human leukocytes.6
We provide here the first evidence of an S100 protein induced in EC. CP-10 was expressed in microvascular EC after direct stimulation with LPS or IL-1, whereas responses to TNFα were more complex. We propose that CP-10 production by microcapillary EC, together with its ability to stimulate leukocyte chemotaxis and shape change, may significantly amplify and perpetuate the inflammatory response and contribute to vascular blockages that cause tissue ischaemia.
MATERIALS AND METHODS
Reagents. Dulbecco's modified Eagle's medium (DMEM) containing high glucose (4.5 g/L) was from Cytosystems (Sydney, Australia); bovine calf serum (BCS) was from Hyclone (Logan, UT); penicillin/streptomycin was from Sigma (St Louis, MO); and plastic tissue culture flasks and plates were from Falcon (Lincoln Park, NJ). LPS, Escherichia coli, 055:B5 was from DIFCO (Detroit, MI). Recombinant murine TNFα (endotoxin content, 0.51 endotoxin units [EU]/mg; specific activity, 1.2 × 107 U/mg) and recombinant murine interferon γ (IFNγ; 0.032 EU/mg; specific activity, 0.5 × 107 U/mg) were gifts from Dr G.R. Adolf (Ernst-Boehringer Institut für Arzneimittelforschung, Vienna, Austria). Recombinant murine IL-1β (specific activity, 3.5 × 107 U/mg), murine IL-1α (specific activity, 1.5 × 108 U/mg), murine IL-6 (specific activity, 3.3 × 108 U/mg), and purified human TGFβ1 (specific activity, 5 × 105 U/mg) were from Genzyme (Cambridge, MA). Recombinant CP-10 was E coli-derived as described.13 Human IL-1 receptor antagonist (IL-1RA; specific activity, 1,000 U/mL) was from Boehringer Mannheim GmbH (Mannheim, Germany). Neutralizing hamster antimurine TNFα monoclonal antibody (MoAb; 10 to 100 ng neutralized 1 U mouse TNFα in vitro) was a generous gift from Dr Ian Clarke (Australian National University, Canberra, Australia). IL-1RA and the anti-TNFα MoAb had no detectable endotoxin. Isotype control hamster antibody from PharMingen (San Diego, CA) was used at the same concentration.
Calcium ionophore A23187 (ICN-Flow, Costa Mesa, CA) was reconstituted in absolute ethanol to 1 mmol/L and diluted for use in DMEM at 10 μmol/L. PMA (Sigma), 2 mg/mL in absolute ethanol, was used at 10 ng/mL. Vitamin D3 (1-α, 25-dihydroxycholecalciferol) from Roche (Basel, Switzerland) was diluted and stored in argon according to instructions and used at the concentration indicated. Cycloheximide (CHX; Sigma) was a 2 mg/mL stock in distilled water. Actinomycin D (Calbiochem, La Jolla, CA), 5 mg/mL in absolute ethanol, was kept protected from light and used at 0.1 to 1 μg/mL.
Cell culture. Murine endothelioma cell lines derived from brain (bEnd-3), thymus (tEnd-1), and skin (sEnd-1) hemangiomas and characterized as described17 were generous gifts from Dr Werner Risau (Max-Planck Institute, Bad Neuheim, Germany) and were maintained in tissue culture flasks (Falcon) in high glucose DMEM supplemented with 10% heated (56°C for 30 minutes) BCS, 100 U/mL penicillin, and 100 mg/mL streptomycin. DMEM, antibiotics, and CHX were sterilized by filtration through Zetapore 0.2-μm membranes (Cuno, Meriden, CT) to remove contaminating traces of endotoxin. All reagents and media had less than 1 ng/mL endotoxin assayed using the chromogenic limulus amoebocyte lysate assay (Cape Cod Assoc, Woods Hole, MA). Cells subcultured from flasks onto 100-mm diameter tissue culture plates (Falcon) were fed every second day with fresh serum-containing medium and grown to confluence (4 to 5 days) at 37°C in 5% CO2 in air. Confluence determined by visual inspection was defined as greater than 95% of cells in contact with adjacent cells; postconfluence was 2 days and late postconfluence was 4 days after confluence. Cells were replenished with fresh serum-containing medium before stimulation for up to 48 hours with the agents indicated.
RNA isolation and Northern analysis. Total RNA from approximately 107 cells was isolated as described,14 size fractionated on a 1% agarose-2.2 mol/L formaldehyde gel, transferred onto Hybond-N+ membrane (Amersham, Buckinghamshire, UK), and alkali-fixed in 0.05 mol/L NaOH. Hybridizations were performed overnight at 42°C in formamide-containing buffer with [α-32P]-UTP (Amersham)-labeled riboprobes synthesized from a 330-bp fragment corresponding to the full-length cDNA of murine CP-10, as described,13 or a 0.7-kb fragment of murine MCP-1 cDNA cloned into pBLUESCRIPT SK. The MRP-14 riboprobe synthesized from a 400-bp polymerase chain reaction fragment was derived as detailed by Iismaa et al.13 Membranes were washed at room temperature for 15 minutes followed by washes at 65°C with increasing stringency in SSPE/0.1% sodium dodecyl sulfate. Blots were stripped according to the manufacturer's instructions and reprobed with (1) a 20-bp 5′-end-labeled oligonucleotide to the unique hinge region sequence of CP-10 (5′-TTTTCGATATTTATATTCTG-3′) hybridized at 35°C and (2) a 30-bp 5′-end-labeled oligonucleotide to rat 18S rRNA (5′-CGGCGTGTATTAGCTCTAGAATTACCACAG-3′) hybridized at 53°C. mRNA signals were quantitated using the MacBas program and a Bio-Imaging Analyzer BAS 1000 (Fujix, Tokyo, Japan), and relative magnitudes of expression were normalized to values for 18S RNA on the same blot.
Identification of CP-10. A double-sandwich enzyme-linked immunosorbent assay (ELISA) for detection of CP-10 in supernatants or cell lysates using rabbit polyclonal anti–CP-10 IgG was performed as described.14 18 Monomeric recombinant CP-10 (rCP-10; 1 to 50 ng/mL) standards were used for quantitation. The same procedure was used to perform a cell-surface ELISA with bEnd-3 monolayers grown to late postconfluence on flat-bottomed 96-well ELISA plates and fixed in 1% glutaraldehyde permeabilized with methanol/acetone after stimulation.
Identification of CP-10–positive endothelial cells in vivo. Quackenbush-Swiss female mice (6 to 8 weeks old) were sensitized in the rear flank with 300 μg ovalbumin in Complete Freund's Adjuvant and challenged in the corresponding rear footpad 20 days later with 10 μg ovalbumin in 20 μL normal saline. Sham-injected controls received 20 μL saline in the rear footpad. After 30 hours, footpads were dissected, fixed overnight in 95% methanol/5% glacial acetic acid, and then transferred to 70% methanol overnight. Tissue was embedded in paraffin and 4-μm sections were blocked for endogenous peroxidase with 0.3% peroxide/40% methanol in Tris buffered saline, pH 7.5, potential nonspecific binding blocked with normal goat serum (Kirkegaard and Perry, Gaithersburg MD) and an avidin-biotin blocking kit (Vector Labs, Inc, Burlingame, CA). Sections were stained with 1.8 μg/mL rabbit anti–CP-10 IgG and reactivity detected with biotinylated goat antirabbit IgG and horseradish peroxidase-conjugated streptavidin (both from Kirkegaard and Perry) and diaminobenzidine (Sigma). Counterstaining was with Harris's hemotoxylin. To verify specificity of anti–CP-10 reactivity, 10 μg/mL rCP-10 was added to 1.8 μg/mL antibody before application to the tissue sections.
Protein purification. Approximately 3 × 107 bEnd-3 cells, unstimulated or stimulated with LPS (100 ng/mL) or IL-1 (340 U/mL) for 12, 16, and 24 hours, were lysed in phosphate-buffered saline (PBS)/0.2% Triton X-100 (Sigma) containing protease inhibitors (Complete Protease Cocktail Tablets; Boehringer). Insoluble material was pelleted by high speed centrifugation and the supernatant was applied directly to rabbit anti–CP-10 IgG purified by elution from an rCP-10 affinity column coupled to CNBr-Sepharose (Pharmacia) as described.13 Reaction proceeded for 16 hours at 4°C on a rotating wheel and unbound protein was removed by washing sequentially with 2 × 10 mL PBS/10% glycerol, 2 × 10 mL PBS/10% glycerol/0.2 mol/L NaCl, and 2 × 10 mL PBS. Bound protein was eluted with 2 × 2 mL washes of 0.1 mol/L glycine, pH 2, concentrated (Centricon 10; Amicon, Beverley, MA), and analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining or Western blot analysis using polyclonal anti–CP-10 IgG as described.13
RESULTS
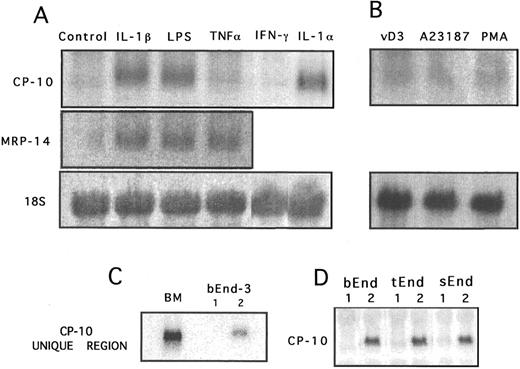
CP-10 gene expression in activated bEnd-3 cells. Confluent unstimulated bEnd-3 cells had no detectable CP-10 mRNA by Northern analysis, whereas it was markedly upregulated by LPS (100 ng/mL), IL-1α (150 U/mL), and IL-1β (340 U/mL) after 24 hours (Fig 1A). In distinct contrast, no CP-10 mRNA was induced by TNFα (12 U/mL), IFNγ (100 U/mL; Fig 1A), IL-6 (500 U/mL), TGF-β1 (1 ng/mL), or rCP-10 (10−8 mol/L; not shown). MRP-14 is coexpressed with CP-10 in murine neutrophils13 and blots rehybridized with MRP-14 riboprobe indicated upregulation in EC activated for 24 hours with LPS, IL-1β, and TNFα, whereas CP-10 was not induced by TNFα (Fig 1A). Functional activity of TNFα was confirmed by its ability to induce tissue factor within 4 hours17 of stimulation of these cells (not shown). Because of the highly conserved nature of the S100 proteins,19 particularly within the two calcium-binding domains, specificity of CP-10 mRNA induction in LPS-activated (Fig 1C) and IL-1β–activated (not shown) and bEnd-3 mRNA was confirmed with an oligonucleotide probe specific for the unique hinge region. Some S100 proteins are regulated by activation of protein kinase C, calcium/calmodulin, or steroid hormone signal transduction pathways but CP-10 mRNA was not upregulated by PMA, the calcium ionophore A23187, or vitamin D3 (Fig 1B). Endothelioma cells derived from thymus and spleen, cultured under identical conditions to bEnd-3, expressed comparable levels of CP-10 mRNA after activation with LPS (100 ng to 1 μg/mL; Fig 1D).
Northern blot analysis of CP-10 and MRP-14 mRNA expression by bEnd-3 cells after 24 hours of stimulation. (A) Control unstimulated, IL-1β (340 U/mL), LPS (100 ng/mL), TNFα (12 U/mL), IFNγ (100 U/mL), and IL-1α (150 U/mL). (B) After 24 hours of culture with vitamin D3 (100 nmol/L), calcium ionophore 23187 (10 μmol/L), or PMA (10 ng/mL). (C) RNA from murine bone marrow cells (BM) and 24-hour (1) unstimulated or (2) LPS-activated (100 ng/mL) bEnd-3 cells was hybridized with the 32P-labeled CP-10 hinge region oligonucleotide probe. (D) CP-10 mRNA expression by bEnd-3, tEnd-1, and sEnd-1 cells cultured alone (1) or with LPS (100 ng/mL) for 24 hours. Membranes from (A) and (B) were rehybridized with a rat 18S RNA probe. Results are representative of at least three experiments.
Northern blot analysis of CP-10 and MRP-14 mRNA expression by bEnd-3 cells after 24 hours of stimulation. (A) Control unstimulated, IL-1β (340 U/mL), LPS (100 ng/mL), TNFα (12 U/mL), IFNγ (100 U/mL), and IL-1α (150 U/mL). (B) After 24 hours of culture with vitamin D3 (100 nmol/L), calcium ionophore 23187 (10 μmol/L), or PMA (10 ng/mL). (C) RNA from murine bone marrow cells (BM) and 24-hour (1) unstimulated or (2) LPS-activated (100 ng/mL) bEnd-3 cells was hybridized with the 32P-labeled CP-10 hinge region oligonucleotide probe. (D) CP-10 mRNA expression by bEnd-3, tEnd-1, and sEnd-1 cells cultured alone (1) or with LPS (100 ng/mL) for 24 hours. Membranes from (A) and (B) were rehybridized with a rat 18S RNA probe. Results are representative of at least three experiments.
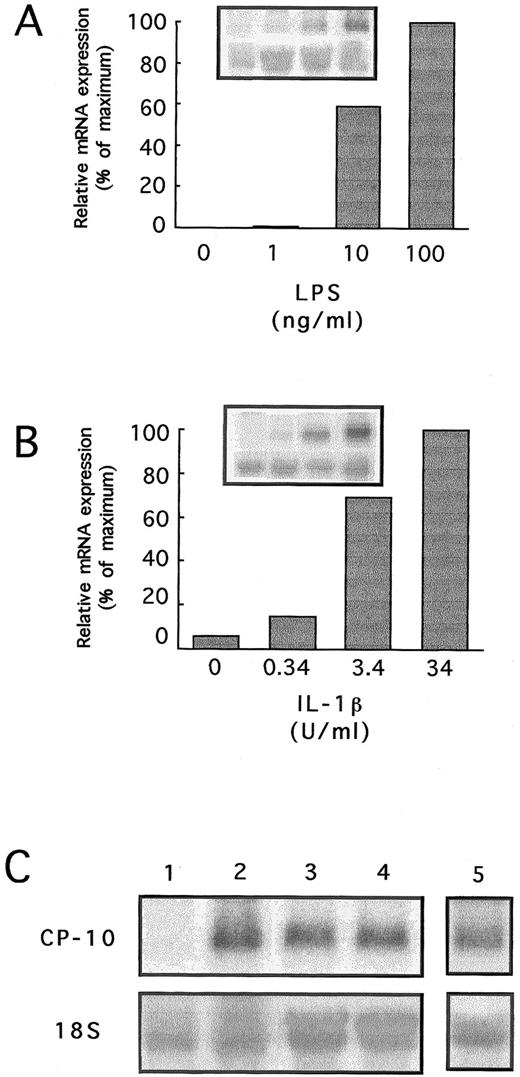
CP-10 mRNA in bEnd-3 cells stimulated for 24 hours with 10 ng/mL LPS almost doubled when 100 ng/mL was used (Fig 2A). These cells were particularly sensitive to IL-1β, with CP-10 mRNA first apparent with 0.34 U/mL; linear increases totalled approximately sevenfold with 3.4 U/mL, with little additional effect at higher concentrations (Fig 2B).
(A and B) Dose-response of CP-10 mRNA induction in bEnd-3 cells cultured for 24 hours with LPS or IL-1β. Insets show Northern blots hybridized with CP-10 and 18S probes. Quantitation densitometry of CP-10 mRNA is given. (C) Northern blot analysis of bEnd-3 cells stimulated for 24 hours as follows: lane 1, control, unstimulated; lane 2, LPS (100 ng/mL); lane 3, LPS and control IgG (0.5 μg/mL); lane 4, LPS and neutralizing anti-TNFα MoAb (0.5 μg/mL); and lane 5, LPS and IL-1RA (100 ng/mL). The blot was rehybridized with a rat 18S RNA probe. Results are representative of two experiments.
(A and B) Dose-response of CP-10 mRNA induction in bEnd-3 cells cultured for 24 hours with LPS or IL-1β. Insets show Northern blots hybridized with CP-10 and 18S probes. Quantitation densitometry of CP-10 mRNA is given. (C) Northern blot analysis of bEnd-3 cells stimulated for 24 hours as follows: lane 1, control, unstimulated; lane 2, LPS (100 ng/mL); lane 3, LPS and control IgG (0.5 μg/mL); lane 4, LPS and neutralizing anti-TNFα MoAb (0.5 μg/mL); and lane 5, LPS and IL-1RA (100 ng/mL). The blot was rehybridized with a rat 18S RNA probe. Results are representative of two experiments.
EC activation with LPS or IL-1 triggers an array of intermediary responses including the autocrine release of IL-120 and TNFα21 but IL-1RA, and anti-TNFα antibody did not alter mRNA levels induced by these reagents (Fig 2C).
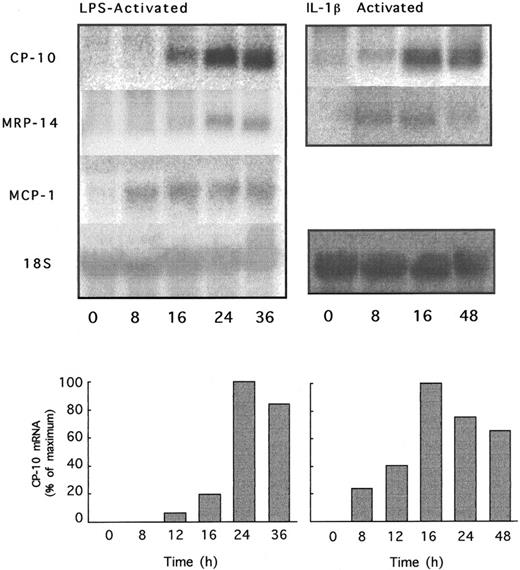
The kinetics of CP-10 gene expression in bEnd-3 cells activated with LPS and IL-1β indicated CP-10 mRNA 12 hours after the addition of LPS, peaking at 24 hours and sustained over 36 hours. Induction by IL-1β was earlier, with detectable signal by 8 hours and optimal expression after 16 hours, declining to 75% of maximum by 48 hours (Fig 3). CP-10 induction kinetics markedly contrasted with MCP-1 mRNA expression, which was strongly positive 4 hours after LPS stimulation (not shown) and was maintained at high levels over 36 hours (Fig 3). MRP-14 mRNA was evident earlier, ie, at 4 hours, than CP-10 mRNA in LPS-activated EC (not shown) and at 8 hours in IL-1–activated bEnd-3 cells (Fig 3).
Time course of CP-10, MRP-14, and MCP-1 mRNA induction by LPS or IL-1β. bEnd-3 cells were cultured with LPS (1 μg/mL) or IL-1β (340 U/mL) for the indicated times and total RNA was analyzed by Northern blotting using CP-10, MRP-14, and MCP-1 riboprobes and the 18S rat RNA probe. Separate experiments with IL-1 and LPS are given. RNA was quantitated by densitometry and values expressed as detailed in the Materials and Methods. Results are representative of two experiments.
Time course of CP-10, MRP-14, and MCP-1 mRNA induction by LPS or IL-1β. bEnd-3 cells were cultured with LPS (1 μg/mL) or IL-1β (340 U/mL) for the indicated times and total RNA was analyzed by Northern blotting using CP-10, MRP-14, and MCP-1 riboprobes and the 18S rat RNA probe. Separate experiments with IL-1 and LPS are given. RNA was quantitated by densitometry and values expressed as detailed in the Materials and Methods. Results are representative of two experiments.
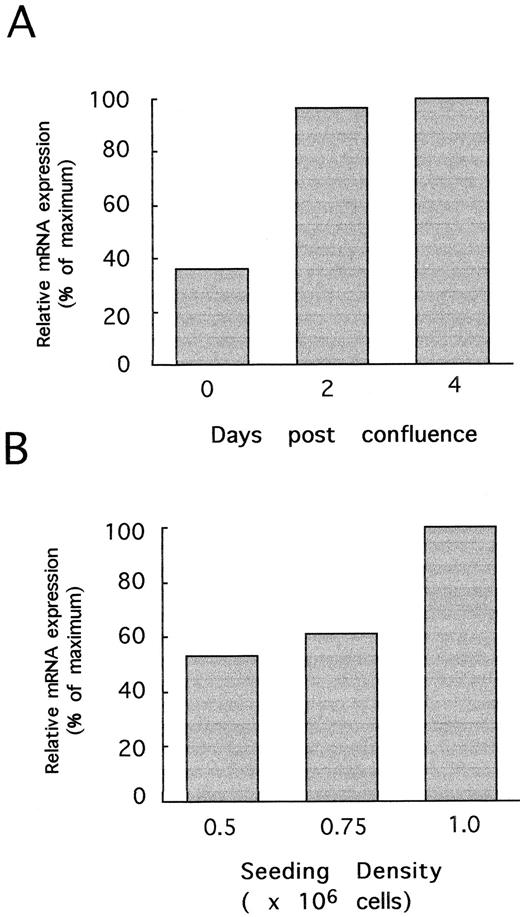
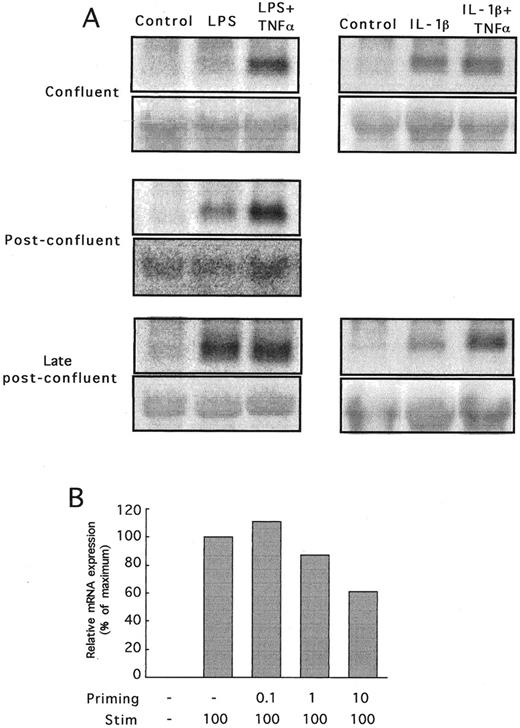
Culture conditions determine CP-10 expression. Induction of CP-10 mRNA was initially variable, suggesting that culture conditions might be important. Preconfluent cells activated with LPS failed to express CP-10 mRNA (not shown), whereas bEnd-3 cells cultured to confluence (day 0), postconfluence (day 2), or late postconfluence (day 4) (times corresponding to days 3, 5. and 7 after subculture) and stimulated with LPS for 24 hours expressed CP-10 mRNA (Fig 4A). The low levels induced by LPS in confluent cells increased some threefold in postconfluent cells, with little change thereafter. The effect of cell-cell contact contributed to the confluence-dependent induction by LPS, because CP-10 mRNA levels were greatest in bEnd-3 cells seeded at higher densities despite equal periods of subculture/cell doublings (Fig 4B). TNFα did not directly induce CP-10 mRNA at any state of cell confluence tested, but acted in synergy with LPS to markedly upregulate mRNA in confluent monolayers (Fig 5A). TNFα enhancement was reduced in postconfluent cells, and in late postconfluence cells, the high mRNA levels induced by LPS were not upregulated further by TNFα. In marked contrast, IL-1β induced CP-10 mRNA expression in EC regardless of their state of confluence (Fig 5A), and induction was not influenced by TNFα costimulation (Fig 5A).
The state of confluence of bEnd-3 cells influences CP-10 mRNA expression. (A) mRNA from confluent, postconfluent, and late postconfluent bEnd-3 cells (days 0, 2, and 4, respectively) stimulated with LPS (1 μg/mL) for 24 hours. Quantitation by densitometry of CP-10 mRNA is detailed in the Materials and Methods. (B) bEnd-3 cells were subcultured onto 100-mm tissue culture plates at the densities given and grown for 4 days before stimulating with LPS (1 μg/mL) for 24 hours and mRNA was processed for Northern analysis. Results are representative of two experiments.
The state of confluence of bEnd-3 cells influences CP-10 mRNA expression. (A) mRNA from confluent, postconfluent, and late postconfluent bEnd-3 cells (days 0, 2, and 4, respectively) stimulated with LPS (1 μg/mL) for 24 hours. Quantitation by densitometry of CP-10 mRNA is detailed in the Materials and Methods. (B) bEnd-3 cells were subcultured onto 100-mm tissue culture plates at the densities given and grown for 4 days before stimulating with LPS (1 μg/mL) for 24 hours and mRNA was processed for Northern analysis. Results are representative of two experiments.
(A) Northern analysis showing CP-10 mRNA expression in bEnd-3 cells activated for 24 hours with LPS (1 μg/mL) or IL-1β (3.4 U/mL) with and without TNFα (12 U/mL). Confluent (day 0), postconfluent (day 2), and late postconfluent (day 4) monolayers were used. Blots were rehybridized with 18S probe as shown. Results are representative of two experiments. (B) CP-10 mRNA expression in bEnd-3 cells after LPS priming (0.01 to 10 ng/mL) for 16 hours, followed by stimulation with LPS at 100 ng/mL for 24 hours. CP-10 mRNA levels determined by densitometry are expressed relative to the nonprimed LPS-stimulated mRNA level (100%).
(A) Northern analysis showing CP-10 mRNA expression in bEnd-3 cells activated for 24 hours with LPS (1 μg/mL) or IL-1β (3.4 U/mL) with and without TNFα (12 U/mL). Confluent (day 0), postconfluent (day 2), and late postconfluent (day 4) monolayers were used. Blots were rehybridized with 18S probe as shown. Results are representative of two experiments. (B) CP-10 mRNA expression in bEnd-3 cells after LPS priming (0.01 to 10 ng/mL) for 16 hours, followed by stimulation with LPS at 100 ng/mL for 24 hours. CP-10 mRNA levels determined by densitometry are expressed relative to the nonprimed LPS-stimulated mRNA level (100%).
Serum replenishment of quiescent cells did not result in CP-10 mRNA induction without activation (Table 1). Although serum-starved bEnd-3 cells expressed low levels of CP-10 when activated with LPS in medium containing 1% serum, Table 1 shows greater expression by cells activated in 10% serum. Synchronization of cells to G0 phase by serum starvation as described22 before LPS activation in 10% serum did not affect the high CP-10 mRNA expression. Thus, activation under conditions of optimal growth appeared critical for CP-10 mRNA induction by bEnd-3 cells.
CP-10 mRNA Levels Measured in 24-Hour LPS-Activated bEND-3 After and During Exposure to Medium Containing 1% or 10% Serum
| . | Serum-Starved . | Serum-Growing . | ||
|---|---|---|---|---|
| . | Control . | LPS . | Control . | LPS . |
| 1% serum | Nil | + | ND | ND |
| 10% serum | Nil | +++ | Nil | ++++ |
| . | Serum-Starved . | Serum-Growing . | ||
|---|---|---|---|---|
| . | Control . | LPS . | Control . | LPS . |
| 1% serum | Nil | + | ND | ND |
| 10% serum | Nil | +++ | Nil | ++++ |
bEnd-3 were grown to late postconfluence as described in the Materials and Methods. Monolayers were washed and refed fresh DMEM containing 1% BCS (serum-starved) or DMEM containing 10% BCS (serum-growing) and incubated for 24 hours before use. Medium was then removed and monolayers were replenished with serum-starved or serum-growing medium as indicated and activated with LPS (100 ng/mL) for 24 hours before harvesting RNA for Northern analysis. Representative results of CP-10 mRNA expression are shown.
Abbreviation: ND, not done.
Regulation of CP-10 mRNA expression. Successive priming and stimulation with LPS can positively or negatively influence CP-10 mRNA expression in various macrophage populations.14 Figure 5B shows that priming late postconfluent cells with a low dose of LPS (10 ng/mL) reduced mRNA levels compared with those induced by the challenge dose alone. LPS priming and challenge of subconfluent or confluent EC did not induce CP-10 mRNA at any priming dose (from 10 to 100 ng/mL) tested (not shown).
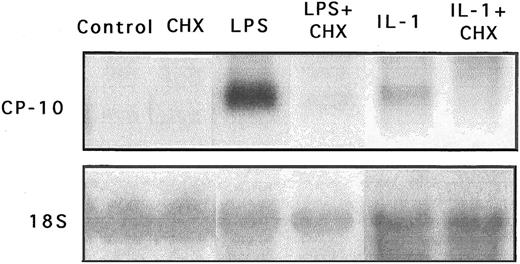
CP-10 gene expression is dependent on de novo protein synthesis. CHX did not superinduce CP-10 mRNA when incubated with bEnd-3 cells, and coincubation with LPS or IL-1β completely abolished gene expression (Fig 6), indicating involvement of de novo protein synthesis in CP-10 mRNA induction. Stabilization of mRNA transcripts induced by LPS or IL-1 could not be tested, because steady-state levels of constitutive CP-10 mRNA in untreated cells were below the level of detection by Northern analysis. The stability of LPS-induced or IL-1–induced CP-10 mRNA transcripts were not compared, because actinomycin D was lethal to bEnd-3 cells cultured for periods greater than 6 to 8 hours, even using as little as 0.1 μg/mL (not shown).
Expression of CP-10 mRNA is dependent on de novo protein synthesis. Northern blot analysis of CP-10 mRNA levels in bEnd-3 cells incubated for 24 hours without stimulation (control), with CHX (0.5 μg/mL), with LPS alone (100 ng/mL), with LPS and CHX, with IL-1β alone (30 U/mL), and with IL-1β and CHX. Blots were reprobed with the 18S RNA probe. Note that the results of different experiments with LPS and IL-1 are given. Results are representative of two experiments.
Expression of CP-10 mRNA is dependent on de novo protein synthesis. Northern blot analysis of CP-10 mRNA levels in bEnd-3 cells incubated for 24 hours without stimulation (control), with CHX (0.5 μg/mL), with LPS alone (100 ng/mL), with LPS and CHX, with IL-1β alone (30 U/mL), and with IL-1β and CHX. Blots were reprobed with the 18S RNA probe. Note that the results of different experiments with LPS and IL-1 are given. Results are representative of two experiments.
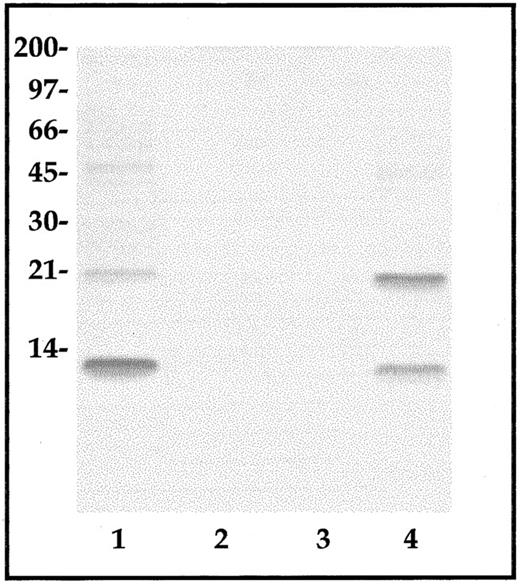
Expression of CP-10 protein. CP-10 was not detected by Western blot analysis of lysates of stimulated bEnd-3 cells applied directly to gels. Lysates from 3 × 107 cells were absorbed to an affinity-purified anti–CP-10 antibody support, bound protein concentrated, and examined by Western blot analysis using anti–CP-10 or anti–MRP-14 IgG. CP-10 monomer and homodimers were detected in 24-hour LPS-stimulated bEnd-3 cell lysates (Fig 7), whereas barely detectable levels were found in IL-1–stimulated cell lysates were harvested after 12 to 16 hours (not shown) or 24 hours (Fig 7). Fractionation of immunoaffinity-isolated proteins from LPS-stimulated cell lysates on C4 RP-HPLC indicated CP-10 monomer (eluted at 19.5 to 20.0 minutes) and homodimer (eluted at 19.8 to 20.2 minutes) as the major components. Retention times were typical of pure native CP-10 and the covalently linked homodimer and peaks with similar retention times were obtained with IL-1–stimulated cell lysates, although these represented only about 20% of the amounts found in LPS-activated cells (not shown). MRP-14 was not detected in any of the samples (not shown). In accordance with these results, unstimulated bEnd-3 lysates did not contain CP-10 by ELISA (detection limit, 10−11 mol/L), whereas 56.5 ± 18.5 pmol/L were estimated in LPS-activated lysates and 45 ± 15 pmol/L were estimated in IL-1–activated lysates. No CP-10 was detected in supernatants harvested 12 to 24 hours after activation or on the surface of LPS-stimulated monolayers.
CP-10 protein from control, LPS-activated, or IL-1–activated EC lysates. CP-10 was enriched from lysates of control (lane 2), IL-1–activated (lane 3), and LPS-activated (lane 4) bEnd-3 cells by anti–CP-10 polyclonal antibody affinity chromatography. Eluted protein was examined by Western blot analysis as described in the Materials and Methods. Similar results were obtained in three experiments. Recombinant CP-10 (lane 1) was used as a positive control and the positions of molecular weight standards are shown.
CP-10 protein from control, LPS-activated, or IL-1–activated EC lysates. CP-10 was enriched from lysates of control (lane 2), IL-1–activated (lane 3), and LPS-activated (lane 4) bEnd-3 cells by anti–CP-10 polyclonal antibody affinity chromatography. Eluted protein was examined by Western blot analysis as described in the Materials and Methods. Similar results were obtained in three experiments. Recombinant CP-10 (lane 1) was used as a positive control and the positions of molecular weight standards are shown.
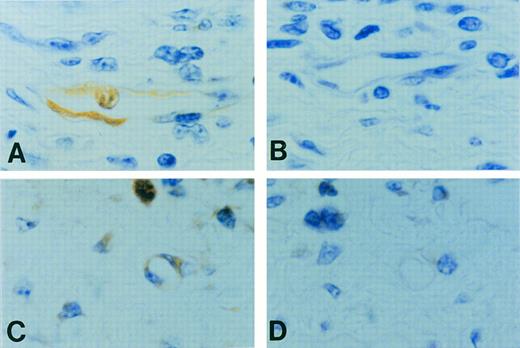
Preliminary immunohistochemistry studies indicated CP-10 distributed throughout the cytoplasm of activated bEnd-3 cells (not shown). Footpad sections obtained 30 hours after ovalbumin challenge of sensitized mice indicated areas of intense leukocyte infiltration. In contrast to the negative reactivity of control IgG (Fig 8B), anti–CP-10 reacted with neutrophils. Expression of CP-10 by EC was mainly confined to approximately 17% of microvessels (Fig 8A and C); reactivity of EC lining large vessels accounted for only approximately 1.5%. No CP-10–positive EC were evident in footpad sections from normal mice challenged with ovalbumin (not shown). Reactivity in microvessels was apparent in the absence of luminal PMN (strongly CP-10–positive), indicating that CP-10 was not deposited by transmigrating leukocytes. Competition studies showed that EC reactivity with anti–CP-10 antibody was totally eliminated by including 5.6-fold excess rCP-10 in the staining procedure (Fig 8D). Reactivity of this antibody was not competed by a recombinant preparation of the closely related human S100 protein, MRP8 (not shown).
Positive CP-10 staining (A) of microvessels and capillaries (n = 3, 4 fields/footpad) was obtained in 18 of 110 vessels counted when footpads from sensitized mice were challenged with ovalbumin and sections were stained with affinity-purified anti–CP-10 IgG. Note the positive staining of the neutrophil within the vessel. No reactivity occurred with control rabbit IgG (B). Serial sections show CP-10–positive staining of a small blood vessel and PMN (C) with anti–CP-10, whereas the same vessel was negative (D) when reactivity was competed with rCP-10. Original magnification × 1,000.
Positive CP-10 staining (A) of microvessels and capillaries (n = 3, 4 fields/footpad) was obtained in 18 of 110 vessels counted when footpads from sensitized mice were challenged with ovalbumin and sections were stained with affinity-purified anti–CP-10 IgG. Note the positive staining of the neutrophil within the vessel. No reactivity occurred with control rabbit IgG (B). Serial sections show CP-10–positive staining of a small blood vessel and PMN (C) with anti–CP-10, whereas the same vessel was negative (D) when reactivity was competed with rCP-10. Original magnification × 1,000.
DISCUSSION
Postcapillary EC are important sites of leukocyte extravasation in inflammation. Our studies show that the chemotactic factor CP-10 was induced in microvascular EC after activation with proinflammatory mediators, further supporting its role in inflammation. In contrast to MCP-1 or IL-8, which are also produced by activated EC17,23 and which provoke relatively acute responses in vivo, CP-10 elicits a leukocyte infiltrate typical of the progression of a delayed-type hypersensitivity (DTH) response.6,7,11 Furthermore, the macrophage phenotype recruited expresses high levels of CD11b/CD18 and scavenger receptors11 that may play a major role in atherogenesis and other inflammatory responses. CP-10 and MRP-14 are expressed constitutively in neutrophils, but only CP-10 is induced in elicited macrophages by LPS.14
Murine brain, thymus, and skin microvascular endothelioma cell lines did not express CP-10 constitutively, but high levels of mRNA were induced with LPS (Fig 1A and D) and upregulation was independent of IL-1 or TNFα produced by these cells (Fig 2C). Various EC types display functional heterogeneity24 and, although the cells used here were transformed, they have been used extensively to study the regulation of IL-1, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF ), macrophage colony-stimulating factor (M-CSF ), and MCP-1.17 LPS activation of EC is dependent on factors such as soluble CD14 in serum,25 and CP-10 mRNA expression in LPS-activated bEnd-3 cells was optimal in serum (Table 1) but not evident in serum-deprived and replenished cells, suggesting that induction was not directly regulated by this stress response.
IL-1α and IL-1β were potent activators of the CP-10 gene, whereas TNFα, IFNγ (Fig 1A), IL-6 (500 to 5,000 U/mL), TGFβ1 (1 ng/mL), and rCP-10 (10−8 mol/L; not shown) were inactive. Moreover, PMA, calcium ionophore, or vitamin D3 failed to upregulate CP-10 mRNA (Fig 1B), indicating distinct pathways of induction for CP-10 and MRP-8 and some other S100 proteins that are regulated by protein kinase C and/or calcium in other cell types (reviewed by Fano et al26 and Zimmer et al27 ).
CP-10 induction by LPS occurred at levels typically found in the circulation during bacteraemia (10 to 100 ng/mL).28 Moreover, in marked contrast to the relatively high levels of IL-1β (200 U/mL) required to upregulate M-CSF and MCP-1 mRNA in eEnd-1 and tEnd-1 cell lines,17 bEnd-3 cells were exquisitely sensitive to IL-1β (3.4 U/mL; Fig 2B), suggesting that CP-10 may be one of the earliest chemotactic factors induced by low local concentrations of IL-1 after initial inflammatory triggers. LPS-induced CP-10 mRNA was detected after 12 hours and maximal at 24 hours, whereas the response provoked by IL-1 was evident after 8 hours (Fig 3). In contrast, RANTES, which contributes to recruitment of monocytes and CD4+ memory T lymphocytes in DTH, is not induced in EC by IL-1.29 The kinetics of CP-10 mRNA expression was delayed compared with that of MCP-1, which was evident by 4 hours (Fig 3 and Bussolino et al17 ), and with other chemokines.23 Different chemokines expressed at inflammatory foci at various times may represent one means of regulating the composition of the tissue infiltrate and may profoundly affect the progression of the inflammatory response. Although CP-10 mRNA expression occurred 12 hours after LPS stimulation in vitro, subcutaneous injection of LPS caused myeloid cell infiltration with CP-10 prominent on small vessel endothelium after 4 hours,7 suggesting additional influences active in vivo. Figure 8A and C shows CP-10 expression predominantly in microvessels of a DTH lesion. Although the mechanism of CP-10 induction is unknown, IL-1 is produced in large amounts in DTH lesions and may contribute to amplification of leukocyte recruitment by activating CP-10 expression in the microcirculation.
TNFα activates proinflammatory and prothrombotic responses in EC that largely overlap with those induced by IL-1 and LPS,30 although selective regulation by TNFα, but not IL-1, has been reported.31,32 TNFα did not induce CP-10 mRNA in bEnd-3 cells under any growth conditions but, in addition to LPS and IL-1, directly upregulated MRP-14 mRNA in postconfluent cells (Fig 1A). MRP-14 is expressed in high amounts in PMN16 and was recently purified as an activation product of murine spleen cells.33 The direct induction of MRP-14, but not CP-10, by TNFα in EC and the absence of MRP-14 expression by LPS-activated macrophages14 show the striking differences in expression patterns of these related S100 proteins and contrasts with the generally coordinated expression of MRP-8/MRP-14 in human myeloid cells.34
TNFα acted as a costimulator with LPS, but not with IL-1 (Fig 5A), to induce CP-10 mRNA in confluent EC, and expression declined with increasing confluence when CP-10 mRNA was strongly upregulated by LPS alone. Changes in confluence alone did not activate CP-10 expression, although cell-cell contact regulates S100β, which increases 20-fold when glial cells reach confluence.35 Furthermore, the inflammatory phenotype of EC may be influenced by cell-cell interactions, particularly nitric oxide synthase36 and TGF-β,37 which are more prominent in nonconfluent cells. In distinct contrast to responses provoked by LPS, CP-10 mRNA was consistently induced by IL-1 irrespective of confluence (Fig 5A). These mechanisms may provide an additional level of control in inflammatory conditions such as DTH, in which neovascularisation and proliferation of immature EC occur. Low levels of IL-1 could provoke CP-10 expression in the microvasculature, irrespective of EC growth stage, to initiate widespread leukocyte recruitment.
LPS induces CP-10 in murine macrophages, whereas LPS challenge of primed cells causes downregulation,14 typical of the tolerization response of macrophages38 and of human umbilical vein EC in which tissue factor39 and E-selectin31 are reduced by this process. Similarly, late postconfluent bEnd-3 cells sequentially primed and challenged with LPS had reduced amounts of CP-10 mRNA (Fig 5B). Although transcriptional events were not examined, cycloheximide blocked CP-10 gene induction by LPS and IL-1 (Fig 6), indicating a requirement for de novo protein synthesis. Sequence and preliminary promoter deletion analysis of the CP-10 gene indicates that it is under complex control and expression in LPS-activated RAW cells is regulated by positive and negative cis-acting elements, including consensus motifs for NF-κB, a pivotal regulator of a number of LPS-inducible genes in EC and macrophages located upstream and downstream of the transcription initiation start site (Hu et al, unpublished observations).
The majority of CP-10 induced in bEnd3 cells was cell-associated, with more in LPS–activated than in IL-1–activated cells (Fig 7 and ELISA) and undectable amounts (<10−11 mol/L) in supernatants. This contrasts with the high levels of secreted and cell-associated CP-10 after LPS activation of macrophages.14 The optimal concentration of CP-10 required for chemotaxis of myeloid cells is between 10−12 and 10−13 mol/L and release of picomolar amounts by activated EC cannot be excluded. The potential contribution of such low levels of CP-10 to the chemotactic activity of supernatants would require verification as other chemokines are also produced (Fig 3). In addition, activation of target cells by juxtacrine signaling (reviewed by Zimmerman et al40 ) by membrane-associated CP-10 cannot be excluded. Enrichment of CP-10 purified by immunoaffinity indicated monomeric and homodimeric components (Fig 7) in LPS-stimulated bEnd-3 cell lysates. MRP-14 was not specifically isolated and was not obvious using anti–CP-10 affinity and Western blotting, procedures used to copurify MRP-14 as a heteromeric complex with CP-10 from bacterial abscess fluid.18 Interactions with other S100 proteins or with unrelated binding proteins, particularly those associated with the cytoskeleton,41-43 may complicate affinity purification and identification. Although S100 proteins are generally active extracellularly as complexes,44-46 monomeric CP-10 is chemotactic6 and interactions between MRP-14 and CP-10 and their function in activated EC remain to be elucidated.
S100 proteins are generally expressed in a tissue- and cell-specific manner (reviewed in Fano et al26 and Zimmer et al27 ), but our studies indicate common pathways of regulation in macrophages and microvascular EC. Localized CP-10 produced by activated EC of the microvascular circulation may contribute to leukocyte recruitment and leukocyte-EC plugging to exacerbate inflammatory responses. Furthermore, CP-10–recruited macrophages have a distinct inflammatory phenotype11 that may mediate vessel wall diseases such as atherogenesis, deep venous thrombosis, and inflammatory-related vasculitides as well as micro-ischaemic events typical of chronic tissue inflammation.
ACKNOWLEDGMENT
The authors are grateful to Dr W. Risau for the gift of the endothelial cells, to C. Cornish and D. Lubach (Heart Research Institute, Sydney, Australia) for the ELISA determinations, and to W. Lau and S. Thliveris for help with DTH experiments.
Supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation. T.Y. is a National Health and Medical Research Council Postgraduate scholar.
Address correspondence to Tina Yen, MB, BS, School of Pathology, Faculty of Medicine, The University of New South Wales, Kensington, New South Wales 2052, Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal