Abstract
To evaluate the usefulness of magnetic resonance imaging for the quantitative determination of hepatic iron, we examined 43 patients with thalassemia major and assessed the influence of pathologic changes in the liver on the precision of estimates of the hepatic iron concentration. Tissue signal intensities were measured from magnetic resonance T1-weighted images derived from gradient-echo (GE) pulse sequences and the ratio of the signal intensity of liver to muscle calculated. By excluding patients (n = 9) having a signal intensity ratio (SIR) less than or equal to 0.2, a linear relationship with hepatic iron was found and subsequent analyses were limited to these 34 patients. In 27 patients with hepatic fibrosis, an overall correlation of −0.848 was found between hepatic iron and SIR. By contrast, in the seven patients with no fibrosis, the correlation coefficient (−0.993) was significantly greater (P < .0001). Despite the differences in correlation, the regression line between hepatic iron and SIR for the patients with no fibrosis did not differ significantly with respect to either slope or intercept from that of the patients with fibrosis. Thus, the presence of fibrosis did not seem to affect the pattern of the relationship between hepatic iron and the SIR, but rather to increase the variability of the relationship. Clinically, the presence of fibrosis makes estimates of hepatic iron derived from magnetic resonance imaging so variable as to be of little practical use in the management of transfusional iron overload.
RECENT PROSPECTIVE studies of patients with thalassemia major treated with transfusion and iron chelation therapy have found that the magnitude of the body iron burden was the major determinant of the risk of clinical complications and of early death.1,2 In patients with thalassemia major treated by allogeneic bone marrow transplantation, the available data suggest that each of the risk factors for increased morbidity and mortality with bone marrow transplantation,3,4 hepatomegaly, portal fibrosis, and inadequate previous iron-chelation therapy, is in turn related to the extent of iron overload.4,5 These results have reemphasized the importance of accurate determination of the body iron burden in the management of patients with thalassemia major. Measurement of the hepatic storage iron concentration provides the most quantitative means of evaluating body iron stores in patients with transfusional iron overload.1,6 7 The reference method for quantitative determination of hepatic iron is chemical analysis of tissue obtained by biopsy.
Although recent studies have documented the safety and feasibility of liver biopsy in the evaluation of patients with thalassemia major,8 the discomfort and risk of biopsy have led to efforts to devise other means of assessing the body iron. At present, the most widely used method for clinical purposes is measurement of the concentration of ferritin in plasma or serum. The serum ferritin concentration is influenced by the body iron, but the concentration is also altered by other factors common in patients with thalassemia major, such as inflammation, infection, hepatic dysfunction, ascorbate deficiency, hemolysis, and ineffective erythropoiesis. Typically, only about half of the variability in serum ferritin is explained by variation in hepatic iron.9 As a result, clinical reliance on the serum ferritin alone can lead to inaccurate assessment of the body iron load in individual patients. In patients with iron overload, determination of hepatic magnetic susceptibility provides direct measurements of liver storage iron that are quantitatively equivalent to those obtained by chemical analysis of tissue obtained by liver biopsy,1,10 but the superconducting quantum interference device (SQUID) susceptometer used to make these measurements is not generally available.1,11 Equipment for magnetic resonance imaging is widely accessible and the striking changes in the proton resonance behavior of tissue water produced by the presence of iron have led to repeated efforts to use this technique for quantitative determinations of the hepatic iron. Recently, significant correlations between estimates of hepatic iron obtained by magnetic resonance imaging and chemical measurements of hepatic iron have been reported.12-22 Although studies of correlation are informative, statistical analyses of regression are also needed to examine the ability of measures obtained by magnetic resonance imaging to predict the hepatic iron concentration with sufficient accuracy for clinical application. Moreover, the numbers of patients studied in most of these series have been small and few patients with thalassemia major and transfusional iron overload were included. To provide a more extensive evaluation of the clinical usefulness of magnetic resonance imaging for determination of liver iron, we examined a number of patients with thalassemia major and assessed the influence of pathologic changes in the liver on the variability of the estimates of the hepatic iron concentration.
MATERIALS AND METHODS
Patients. Our study protocol and procedures for obtaining informed consent were approved by the Ethic Committee of the Pesaro Hospital; written informed consent was obtained from each patient or parent. The population examined consisted of 43 patients (18 women, 25 men), 20 with thalassemia major and 23 patients who had undergone allogeneic bone marrow transplantation for thalassemia major. Table 1 summarizes the clinical characteristics of these patients.
Clinical Characteristics of Patients Studied Before or After Allogeneic BMT for Thalassemia Major
| . | Studied Before Transplantation . | Studied After Transplantation . | All Patients . |
|---|---|---|---|
| N | 20 | 23 | 43 |
| Age (yr) | 17 ± 6 | 17 ± 4 | 17 ± 5 |
| Years after BMT | — | 5 ± 3 | — |
| Years of transfusion therapy | 17 ± 6 | 10 ± 5* | 13 ± 6 |
| Age at first transfusion (mo) | 10 ± 8 | 23 ± 30 | 18 ± 24 |
| Transfusions received | 361 ± 153 | 164 ± 108* | 243 ± 160 |
| Serum ferritin ( μg/L) | 2,699 ± 1,962 | 1,391 ± 1,718 | 1,914 ± 1,904 |
| Anti-HCV antibody positive | 14 | 17 | 31 |
| . | Studied Before Transplantation . | Studied After Transplantation . | All Patients . |
|---|---|---|---|
| N | 20 | 23 | 43 |
| Age (yr) | 17 ± 6 | 17 ± 4 | 17 ± 5 |
| Years after BMT | — | 5 ± 3 | — |
| Years of transfusion therapy | 17 ± 6 | 10 ± 5* | 13 ± 6 |
| Age at first transfusion (mo) | 10 ± 8 | 23 ± 30 | 18 ± 24 |
| Transfusions received | 361 ± 153 | 164 ± 108* | 243 ± 160 |
| Serum ferritin ( μg/L) | 2,699 ± 1,962 | 1,391 ± 1,718 | 1,914 ± 1,904 |
| Anti-HCV antibody positive | 14 | 17 | 31 |
Values shown are the mean ± SD.
Abbreviation: BMT, bone marrow transplantation.
Before bone marrow transplantation.
Clinical and pathological evaluation. Clinical and pathological investigations were done in the Division of Hematology and the Center for Bone Marrow Transplantation of the Pesaro Hospital. Serum ferritin, total transferrin and unbound transferrin were measured in blood samples from each patient. The serum ferritin concentration was measured by an enzyme-linked immunosorbent assay (Eurogenetics, Turin, Italy) and expressed as μg/L. Serum transferrin and unbound iron binding capacity were determined by a nephelometric method and the iron saturation of transferrin calculated. All patients underwent liver biopsies for clinical indications and all laboratory tests were done within 3 days before or after liver biopsy. Tissue was obtained from the right lobe of the liver by the subcostal route under ultrasound guidance using a 16 gauge tru-cut needle. The tissue obtained at biopsy was considered evaluable if a minimum of three portal spaces were present. The techniques used for slide preparation and the evaluation criteria for the biopsy specimens have been previously described in detail.5 In brief, biopsy specimens were evaluated independently in a double-blind manner by two pathologists from Pesaro Hospital with regard to iron grade, hepatitis, and fibrosis. Iron overload was graded on sections stained with Prussian Blue using a previously described method5 based on the number and density of hemosiderin granules in parenchymal and mesenchymal sites. Liver fibrosis and lymphocytic infiltration were evaluated on sections stained with hematoxylin-eosin, periodic acid-Schiff (PAS), diastases-PAS, Mallory trichrome stain, and Gomori silver impregnation. Chronic hepatitis was classified as nonspecific, persistent, or active.5 Fibrosis was graded in five categories as absent, mild, moderate, severe, or cirrhosis.5 Paraffin-embedded liver specimens were deparaffinized and the iron content determined by atomic absorption spectroscopy.23 The mean weight (± standard deviation [SD]) of liver specimens studied was 1 ± 0.3 mg dry weight. The dry weights of the specimens included in this study were all greater than 0.4 mg, in accordance with the recommendation of Olynyk et al.24
Radiological studies. Magnetic resonance imaging studies were performed in the Department of Radiology of Ancona University within 3 days of the biopsy procedure. All patients were studied with a magnetic resonance imaging unit (Siemens Magnetom Impact, Erlangen, Germany) operating at 1 T. Magnetic resonance imaging of the liver was acquired according to a protocol routinely used in the Ancona University School of Medicine for upper abdomen imaging. In particular, the following types of sequences were used in all patients: (1) for a T1-weighted image, a gradient-echo (GE) pulse sequence was used with repetition time TR/TE = 110/6 msec, Flip Angle = 70°, using a single excitation with breath hold of 16 seconds; matrix 128*256, and slice thickness 10 mm. The field of view (FOV) was 30 or 40 cm in axial plane. (2) For a T2-weighted image a turbo spin-echo (TSE) pulse sequence was used with repetition time TR/TE = 5100/112 msec, matrix 240*256 and slice thickness 10 mm. Two acquisitions were performed with the time of acquisition 2 minutes and 48 seconds and a turbo factor = 15. The total time of imaging for each patient was about 5 minutes. The signal intensity (SI) of the liver, paraspinous muscle, and noise were measured to obtain a signal intensity ratio (SIR) value.19 Signal intensities were measured with the use of operator-defined regions of interest (ROIs), always greater than 50 pixels. For each sequence, the ROIs examined were obtained on the same image to avoid variations in signal intensity. Liver signal intensity was averaged from three ROIs measured in the right lobe of the liver. A single ROI was measured in the paraspinous muscle. The signal intensity of the liver was calculated with respect to that of the muscle as indicated by Johnston et al.12 All quantitative evaluations were performed independently in a double-blind manner by two radiologists from Ancona University; mean values of the two measurements were used for the calculations reported below.
Statistical analysis. Results for chemical and magnetic resonance measurements of hepatic iron and for measurements of serum ferritin concentration are expressed as means ± the standard error of the mean (SEM), unless otherwise indicated. For data from patients considered as a single group and for subgroups of patients, the linear relationship between chemical and magnetic resonance measurements of hepatic iron was assessed using Pearson's coefficient of correlation and linear regression analysis. Tests that two or more independent correlation coefficients were estimates of the same true correlation coefficient were performed.25 Residual analyses were conducted to examine the assumption of the simple linear regression model. The analysis of variance (ANOVA) F-test for equality of regression lines across groups was applied. Under the assumption that the linear models were appropriate, the F-test for variances was used to compare the variance in the errors of prediction for two different regression lines, with corresponding residual degrees of freedom. The coefficient of determination was used to estimate the proportion of variation in magnetic resonance measurements that could be accounted for by variation in hepatic iron stores. Regression analysis was also used to determine 95% prediction intervals for the hepatic iron concentration, given the results of magnetic resonance studies. Multivariate analyses with stepwise regression and all possible subsets regression were used to assess the contribution of selected variables to the prediction of hepatic iron concentration from the results of magnetic resonance studies.26 The BMDP (BMDP Statistical Software, Inc., Los Angeles, CA, 1992) and S-PLUS (Statistical Sciences, Inc, Version 3.3 for Windows, Seattle, WA, 1995) statistical computer packages were used for computations. All statistical tests were two-tailed and a significance level of 0.05 was used.
RESULTS
Ratios of signal intensity of liver with respect to muscle. The SIR of liver with respect to that of muscle in each patient was determined both from T1-weighted images derived from GE pulse sequences and from T2-weighted images derived from TSE pulse sequences. The correlations between the ratios of signal intensity estimated by the two independent radiologists were 0.998 (95% confidence interval, 0.997 to 0.999) and 0.959 (95% confidence interval, 0.925 to 0.978) for the GE and TSE pulse sequences, respectively. The mean ratios of signal intensity obtained from liver were 0.69 ± 0.07 (SEM) for GE and 0.72 ± 0.07 for TSE sequences.
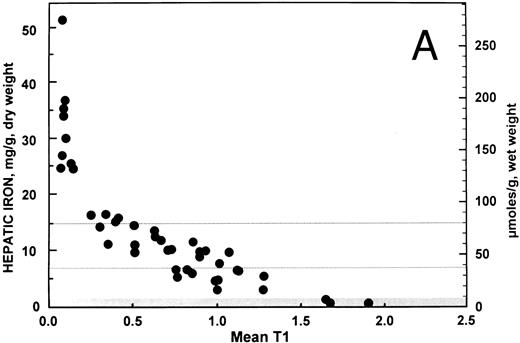
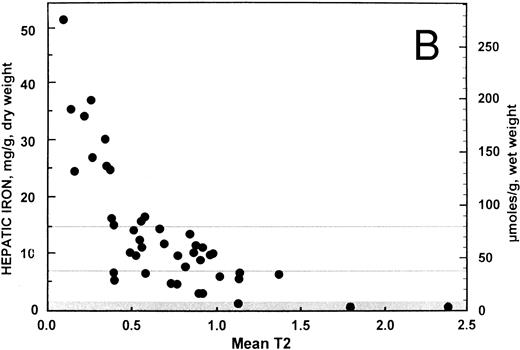
Correlation and simple linear regression analysis. For all 43 patients, the overall relationship between hepatic iron concentration and the ratio of the signal intensity of the liver to muscle with T1-weighted images derived from GE pulse sequences is shown in Fig 1A. Similarly, the overall relationship between the hepatic iron concentration and the SIR with T2-weighted images derived from TSE pulse sequences is shown in Fig 1B. Both scatter plots show a curvilinear pattern. By excluding patients (n = 9) having a SIR with T1-weighted images less than or equal to 0.2, a more linear pattern was evident with the remaining 34 patients (r = −0.877; 95% confidence interval, −0.937 to −0.765). The correlation between hepatic iron concentration and the ratio of the signal intensity of liver to muscle with T1-weighted images was significantly different from zero (P < .0001). By contrast, no improvement was found in the correlation (r = −0.625; 95% confidence interval, −0.795 to −0.364) between hepatic iron and the SIRs for T2-weighted images for these 34 patients. Accordingly, all subsequent analyses reported here were performed using the ratio of the signal intensity of liver to muscle with T1-weighted images derived from GE pulse sequences only for those 34 patients with this ratio greater than 0.2.
Relationship between hepatic iron concentration and the SIR of liver to muscle with (A) T1-weighted images derived from GE pulse sequences and with (B) T2-weighted images derived from TSE pulse sequences. To facilitate assessment of the clinical use of magnetic resonance imaging as a means of measuring hepatic iron, hepatic iron concentration is plotted on a scale that encompasses the entire range typically found in patients with transfusional iron overload, from normal to more than 50 mg iron per gram liver, dry weight. The normal range is indicated by a gray band extending up to about 1.6 mg iron per gram liver, dry weight. A horizontal gray line at a concentration of 7 mg iron per gram liver, dry weight, indicates the upper limit of the “optimal” range in patients with transfusional iron overload.28 Another horizontal gray line at a concentration of 15 mg iron per gram liver, dry weight indicates a “threshold” for the development of cardiac disease and early death in patients with thalassemia major and transfusional iron overload.1
Relationship between hepatic iron concentration and the SIR of liver to muscle with (A) T1-weighted images derived from GE pulse sequences and with (B) T2-weighted images derived from TSE pulse sequences. To facilitate assessment of the clinical use of magnetic resonance imaging as a means of measuring hepatic iron, hepatic iron concentration is plotted on a scale that encompasses the entire range typically found in patients with transfusional iron overload, from normal to more than 50 mg iron per gram liver, dry weight. The normal range is indicated by a gray band extending up to about 1.6 mg iron per gram liver, dry weight. A horizontal gray line at a concentration of 7 mg iron per gram liver, dry weight, indicates the upper limit of the “optimal” range in patients with transfusional iron overload.28 Another horizontal gray line at a concentration of 15 mg iron per gram liver, dry weight indicates a “threshold” for the development of cardiac disease and early death in patients with thalassemia major and transfusional iron overload.1
These 34 patients with SIRs from T1-weighted images greater than 0.2 were then grouped by the presence and severity of fibrosis found in the liver biopsy specimen. The estimated correlations between the hepatic iron concentration and the ratio of the signal intensity of liver to muscle with T1-weighted images are summarized in Table 2. A significant difference in correlation coefficient was found between the four subgroups with (1) no fibrosis, (2) mild fibrosis, (3) moderate fibrosis, or (4) severe fibrosis or frank cirrhosis (χ2 = 8.66, degrees of freedom [df ] = 3, P = .034). In the 27 patients with fibrosis, no significant differences in correlation coefficients were found between the three subgroups with (1) mild fibrosis, (2) moderate fibrosis, or (3) severe fibrosis or frank cirrhosis (χ2 = 0.54, df = 2, P = .765), thus a combined estimate of the correlation coefficient was computed (r = −0.848; 95% confidence interval, −0.937 to 0.658). By contrast, in the seven patients with no fibrosis, the correlation (r = −0.993) was significantly stronger than the combined correlation estimate found from 27 patients with fibrosis (z = −2.802, P = .005).
Correlations Between Hepatic Iron and the SIR of Liver to Muscle for Patients With SIRs < 0.2
| . | No. . | Pearson's r (lower, upper 95% confidence limits) . | |
|---|---|---|---|
| . | . | . | . |
| Fibrosis | |||
| Absent | 7 | −0.993* | −0.993* |
| (−0.999, −0.954) | (−0.999, −0.954) | ||
| Mild | 8 | −0.789 | |
| (−0.960, −0.190) | |||
| Moderate | 12 | −0.839 | −0.848 |
| (−0.954, −0.512) | (−0.937, −0.658) | ||
| Severe or cirrhosis | 7 | −0.914 | |
| (−0.987, −0.518) | |||
| Chronic hepatitis | |||
| Absent | 9 | −0.884 | |
| (−0.975, −0.532) | |||
| Nonspecific | 11 | −0.906 | −0.877 |
| (−0.976, −0.669) | (−0.937, −0.765) | ||
| Persistent | 6 | −0.824 | |
| (−0.980, −0.037) | |||
| Active | 8 | −0.892 | |
| (−0.980, −0.503) | |||
| . | No. . | Pearson's r (lower, upper 95% confidence limits) . | |
|---|---|---|---|
| . | . | . | . |
| Fibrosis | |||
| Absent | 7 | −0.993* | −0.993* |
| (−0.999, −0.954) | (−0.999, −0.954) | ||
| Mild | 8 | −0.789 | |
| (−0.960, −0.190) | |||
| Moderate | 12 | −0.839 | −0.848 |
| (−0.954, −0.512) | (−0.937, −0.658) | ||
| Severe or cirrhosis | 7 | −0.914 | |
| (−0.987, −0.518) | |||
| Chronic hepatitis | |||
| Absent | 9 | −0.884 | |
| (−0.975, −0.532) | |||
| Nonspecific | 11 | −0.906 | −0.877 |
| (−0.976, −0.669) | (−0.937, −0.765) | ||
| Persistent | 6 | −0.824 | |
| (−0.980, −0.037) | |||
| Active | 8 | −0.892 | |
| (−0.980, −0.503) | |||
See text.
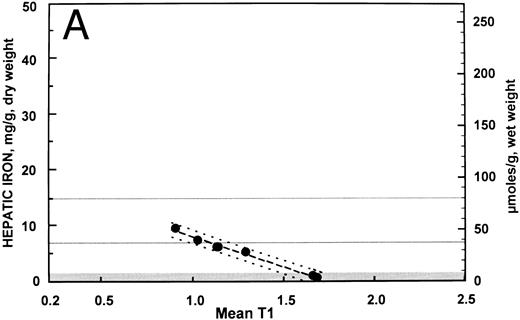
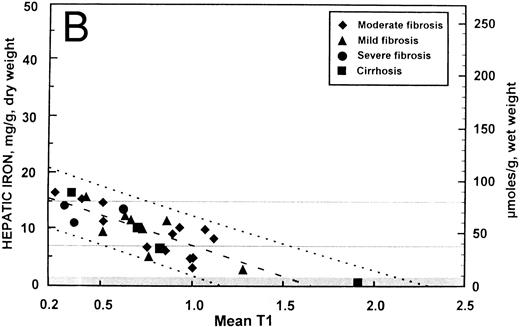
These data are displayed graphically in Fig 2A and B with the fitted regression line and the 95% prediction intervals for the hepatic iron concentration, given the SIR. Despite the differences in correlation, the regression line for the patients with no fibrosis (y = 18.8 − 10.8 [SIR]) did not differ significantly from that of the patients with fibrosis (y = 17.4 − 10.5 [SIR]) with respect to either slope or intercept. Regression analysis indicated that for patients with no fibrosis, over 98% of the variation in SIR could be explained by variability in hepatic iron concentration. In patients with fibrosis, variability in hepatic iron concentration accounted for only about 70% of the variation in SIR. These differences were also apparent in the width of the 95% prediction intervals (ie, an interval of predicted hepatic iron concentrations that for a given SIR will include the actual hepatic iron concentration with 95% confidence) shown in Fig 2A and 2B. Assuming a linear relationship between hepatic iron concentration and SIR, the variance in the errors of prediction for patients with no fibrosis (standard error of the regression = 0.42 mg iron per gram liver) was significantly less than that of patients with fibrosis (standard error of the regression = 2.46 mg iron per gram liver) (F = 34.3, df = [5, 25], P < .0001).
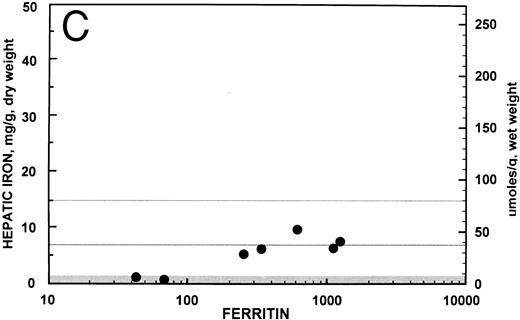
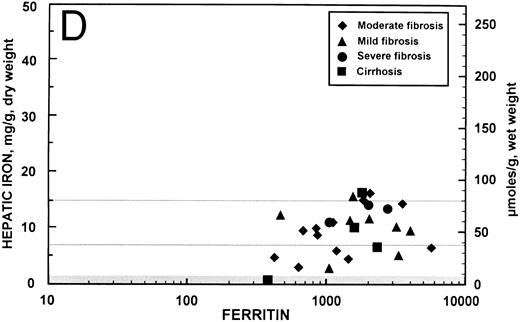
Relationship between hepatic iron concentration and the SIR of liver to muscle with T1-weighted images derived from GE pulse sequences for patients with SIR greater than 0.2 for patients with no fibrosis (A) or with fibrosis (B). For comparison, the relationship between hepatic iron concentration and the serum ferritin concentration is shown for the same groups of patients without fibrosis (C) and with fibrosis (D).
Relationship between hepatic iron concentration and the SIR of liver to muscle with T1-weighted images derived from GE pulse sequences for patients with SIR greater than 0.2 for patients with no fibrosis (A) or with fibrosis (B). For comparison, the relationship between hepatic iron concentration and the serum ferritin concentration is shown for the same groups of patients without fibrosis (C) and with fibrosis (D).
Similar analyses of the correlations between the ratio of the signal intensity of the liver to muscle with T1-weighted images and the hepatic iron concentration were performed with these 34 patients stratified (1) for the presence of chronic hepatitis in the liver biopsy specimen (Table 2), (2) for the presence and severity of iron deposition in hepatic reticuloendothelial cells (data not shown), and (3) for the presence and severity of iron deposition in hepatic parenchymal cells (data not shown). No significant differences in correlation coefficients were found with respect to chronic hepatitis or the site or grade of iron deposition.
Multiple regression analyses. Multivariate analyses were performed to determine if the prediction of hepatic iron concentration from SIRs could be improved by considering hepatitis, site and severity of iron deposition, serum alanine transaminase, or the presence of anti-HCV (hepatitis C) antibody, using stepwise regression and all the possible subsets regression. None of these variables contributed significantly to the regression models.
Mean hepatic iron concentration in patients with and without fibrosis. The mean hepatic iron concentration of the 27 patients with fibrosis, 9.61 ± 0.9 (SEM) mg iron per gram liver, dry weight (51.6 ± 4.6 μmol iron per gram of liver, wet weight), was higher (P = .02) than that of the seven patients with no fibrosis, 5.3 ± 1.3 mg iron per gram liver, dry weight (28.3 ± 6.7 μmol iron per gram of liver, wet weight).
Relationship between serum ferritin and hepatic iron concentration. In the 34 patients with SIRs from T1-weighted images greater than 0.2, the overall correlation coefficient between the serum ferritin, expressed logarithmically, and the hepatic iron concentration was r = 0.566 (95% confidence interval, 0.281 to 0.759; P < .001). These data are shown graphically for the seven patients with no hepatic fibrosis in Fig 2C (r = 0.876; 95% confidence interval, 0.363 to 0.982; P = .007) and for the 27 patients with fibrosis in Fig 2D (r = 0.353; 95% confidence interval, −0.032 to 0.646; P = .07). For comparison, the overall correlation coefficient between the serum transferrin saturation and the hepatic iron concentration in these 34 patients was r = 0.410 (95% confidence interval, 0.084 to 0.657; P = .02).
DISCUSSION
Our studies of the usefulness of magnetic resonance imaging as a noninvasive measure of hepatic iron examined patients with thalassemia major with iron concentrations that spanned the entire range that is typically found in patients with transfusional iron overload, from normal to more than 50 mg iron per gram of liver dry weight (approximately 270 μmol iron per gram of liver, wet weight, assuming that liver is about 70% water). In addition, the specimens of liver obtained at biopsy from these patients exhibited the full extent of histopathologic changes found in thalassemia major; most had hepatitis and hepatic fibrosis of various degrees of severity. Using magnetic resonance imaging to obtain the ratio of the signal intensity of liver to muscle with T1-weighted images derived from a GE pulse sequence, a close correlation has been found with the hepatic iron concentration as determined by chemical analysis of tissue obtained by biopsy, provided that two conditions were met: (1) the SIR was greater than 0.2, and (2) no fibrosis was present.
Although the exact upper limit varies, the first limitation has been recognized in almost all previous reports12-20 27 because with increasing iron concentration the signal intensity of liver is reduced to such an extent that discrimination between different concentrations becomes impossible. In our study, all patients with an SIR less than 0.2 had hepatic iron concentrations that were greater than 20 mg iron per gram of liver dry weight (about 108 μmol iron per gram of liver, wet weight). In clinical terms, this finding means that magnetic resonance imaging cannot be used during treatment to follow patients with the most severe iron loading. In this study, about 20% of those examined had hepatic iron concentrations in excess of those that could be measured by magnetic resonance, but the proportion subject to this limitation would depend on the population examined.
The second limitation, the presence of fibrosis, has not been previously identified as a factor restricting the precision of estimates of the hepatic iron concentration by magnetic resonance imaging. In this study, the presence of fibrosis did not seem to affect the pattern of the relationship between hepatic iron and the SIR; no significant difference was found in either the slope or the intercept of the regression equation between these factors in patients with or without fibrosis. Instead, the effect of fibrosis seemed to be to increase the variability of the relationship. Assuming a linear relationship between hepatic iron concentration and SIR, we found that the variance in the errors of prediction for patients with no fibrosis was significantly less than that for patients with fibrosis (P < .0001). In those patients without fibrosis, regression analysis showed that over 98% of the variation in SIR could be explained by variability in hepatic iron concentration. By contrast, in patients with fibrosis of any degree, from mild fibrosis to frank cirrhosis, variability in hepatic iron concentration accounted for only about 70% of the variation in SIR. As shown in Table 2, if fibrosis were present, an increasing severity of fibrosis had no significant effect on the coefficient of correlation between hepatic iron and SIR. Although all the correlations shown in Table 2 are statistically significantly different from zero (P < .025), for all to be of quantitative clinical usefulness, a correlation close to 1.0 is needed.
The clinical consequences of the limitation imposed by the presence of fibrosis on the precision of magnetic resonance imaging studies may be appreciated by examining the 95% prediction intervals shown in Fig 2A and B. For example, for a patient with a SIR of about 1.0 and no fibrosis, the prediction interval extends from about 6.5 to 9.0 mg iron per gram liver, dry weight (35 to 48 μmol iron per gram liver, wet weight) and could be interpreted clinically to indicate that the liver iron concentration is near the upper limit of the “optimal” range.28 By contrast, if the patient with a SIR of about 1.0 has hepatic fibrosis, the prediction interval is then widened considerably to about 1.7 to 12.0 mg iron per gram liver, dry weight (9 to 65 μmol iron per gram liver, wet weight). This prediction interval, from near normal to the upper portion of the range of hepatic iron concentration associated with an increased risk of clinical complications of iron overload, is so broad as to be of little clinical use. In an individual patient, in the absence of a biopsy to determine whether or not fibrosis is present, the prediction interval for an estimate of the hepatic iron derived from magnetic resonance imaging must be assumed to be so broad as to be of little practical assistance in the management of transfusional iron overload. In this population of patients, the serum ferritin concentration was also of little practical use as a means of estimating body iron (Fig 2C and D).
In this study, each of the seven patients without fibrosis had a hepatic iron less than about 10 mg iron per gram of liver, dry weight (54 μmol iron per gram liver, wet weight). Additional patients with thalassemia major without fibrosis, but with hepatic iron concentration between 10 and 20 mg iron per gram of liver, dry weight (54 to 108 μmol iron per gram liver, wet weight), will need to be examined to determine if the same correlation between hepatic iron and SIR will also extend into this higher range. With multivariate analyses, none of the other factors examined in this study, including hepatitis, the histologic site and grade of iron deposition, serum alanine transaminase, or the presence of anti-HCV antibody, seemed to significantly improve the correlation between hepatic iron concentration and SIR.
The effect of fibrosis on the variability of the relationship between hepatic iron and the SIR has not been previously recognized, both because of the restricted numbers of patients in whom direct comparisons have been made between chemical measurements of hepatic iron and the results of magnetic resonance imaging and because of technical differences in the methods used. For example, both chemical and magnetic resonance determinations were made in only nine patients with iron overload (eight patients with hereditary hemochromatosis, one patient with thalassemia major) in the study by Kaltwasser et al,22 in only 10 patients (all with thalassemia major) in the investigation by Gomori et al,13 in only seven patients (all with thalassemia major) in the report by Dixon et al,17 and in only 11 patients (all with hereditary hemochromatosis) in the report by Engelhardt et al.18 A larger number of patients (n = 67, including 55 with homozygous or heterozygous hemochromatosis and 12 with increased liver iron considered related to alcoholic liver disease) were studied by Gandon et al,19 who concluded that neither fibrosis nor cirrhosis altered the accuracy of estimates of the hepatic iron concentration derived from magnetic resonance imaging. In their report, the overall correlations observed between hepatic iron concentration and the results of magnetic resonance studies were much weaker (eg, −0.66 for patients with hepatic iron between about 8 and 16 mg iron per gram liver, dry weight). As a result of the weaker correlations between hepatic iron and magnetic resonance studies found with their procedure, their method could not have detected the decrease in correlation with fibrosis from −0.993 to −0.837 that is reported here in a population of patients with thalassemia major and a high prevalence of hepatic fibrosis.
Because the resonance behavior that results from the application of the oscillating magnetic fields used in magnetic resonance imaging is incompletely understood, the physical basis for the effect of fibrosis on the variability of the ratio of the signal intensity of liver to muscle in magnetic resonance studies is uncertain. The very high correlation (0.998) between the ratios of signal intensity estimated by the two independent radiologists makes it unlikely that sampling error of the magnetic resonance studies is responsible. It should be emphasized that the estimate of hepatic iron derived from magnetic resonance imaging is indirect, resulting from the effect of ferritin and hemosiderin iron on the proton resonance behavior of tissue water. This interaction is complex, involving factors such as the number and sizes of the iron cores in ferritin and hemosiderin, the distribution of iron and water within the tissue examined, tissue hydration, and the water diffusion coefficient, as well as the applied field strength, the repetition time used in the imaging sequences, and other technical aspects of measurements procedure.17,19,20,22,29 In particular, hepatic fibrosis may increase the extent of microheterogeneity in iron and water distribution within the liver and thereby exaggerate measurement variability. Measurement variability may also be increased by differences between livers in the pattern of microheterogeneity in iron and water distribution associated with iron-loading and fibrosis of various etiologies and degrees of severity. In addition, the use of the ratio of the signal intensity of the liver to muscle suggests that the iron concentration in muscle is invariant with respect to hepatic iron, but earlier studies of African iron overload indicated that the nonheme iron concentration in muscle increases with increasing body iron.30 The mean hepatic iron in patients with fibrosis in this study (9.61 ± 0.9 [SEM] mg iron per gram liver, dry weight [51.6 ± 4.6 μmol iron per gram liver, wet weight]) was significantly higher than that of patients without fibrosis (5.3 ± 1.3 mg iron per gram liver, dry weight [28.3 ± 6.7 μmol iron per gram liver, wet weight]). In this study, quantitative determination of hepatic iron by chemical analysis of tissue was used as the reference method, but this technique has its own variability24 31 that may be increased in the presence of fibrosis.
Overall, the results of our study emphasize the limitations of presently available methods for the use of magnetic resonance imaging as a noninvasive means of measuring the hepatic iron concentration and the need for caution in their clinical application and interpretation. In this series of patients with thalassemia major, estimates of the hepatic iron concentrations derived from magnetic resonance studies were sufficiently precise for clinical purpose only if the hepatic iron was less than about 20 mg iron per gram of liver, dry weight (about 108 μmol iron per gram liver, wet weight) and no hepatic fibrosis were present, conditions met for only about 16% (7 of 43) of the patients with thalassemia major examined. Despite these clinical limitations, these results document the potential feasibility of noninvasive measurements of hepatic iron using magnetic resonance techniques, but indicate the need for an improved understanding of the effects of iron on the proton resonance behavior of tissue water.
Supported in part by Fondazione Berloni for the fight against Thalassemia and by Grants No. RO1 DK49108 and RO1 HL57507 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Emanuele Angelucci, MD, Divisione Ematologica di Muraglia, Azienda Ospedale di Pesaro, 61100 Pesaro, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal