Abstract
Chemotherapy produces extended remissions, and potential cures, in a small minority of patients with acute myeloid leukemia (AML). We explored whether potentially cured patients were at increased risk of subsequent invasive cancer and were able to return to work. Potentially cured patients were defined as those in first or second complete remission (CR) for at least 3 years based on hazard rates for recurrence or death in CR, which declined sharply after this time. Patients who received allogeneic marrow transplant were excluded. We used questionnaires, phone contact, and chart review to obtain information about subsequent cancer and work status. The number of patients who developed invasive cancer was compared with the number expected based on age, gender, and years of follow-up using the Connecticut Tumor Registry. A total of 215 patients met our criteria for potential cure: 203 in first CR and 12 in second CR (of 1,663 treated between 1965 and December, 1992). At a median of 9.2 years from first or second CR, 163 (76%) remain alive in CR. Fifteen patients developed 18 invasive cancers (expected number of patients, 8.8; observed/expected, 1.70; 95% CI, 0.96 to 2.84; P = .06). Patients initially treated between 1973 to 1979, patients above the potentially cured cohort's median age of 40 years, and those who presented with abnormalities of chromosomes 5 and/or 7 were more likely to develop subsequent cancer, whereas the observed/expected ratio for younger patients was 1.05 (95% CI, 0.13 to 3.80; P = .56). Seventy-four percent of the patients who were working full-time and who were under age 50 at time of treatment for AML have been working full-time in the last 6 months. Only 17 of 56 patients who are currently not working cited physical limitation as the reason. Patients with potentially cured AML are likely to be able to return to work, and at least if younger do not, on average, have an increased risk of invasive cancer.
CHEMOTHERAPY PRODUCES extended remissions in 10% to 30% of patients with newly-diagnosed acute myeloid leukemia (AML), depending on such characteristics as age and leukemia cell karyotype.1-6 Long remissions more rarely occur in patients who at first relapse, and thereafter, receive chemotherapy without transplantation, although this phenomenon is essentially limited to patients with relatively lengthy first remissions.7 It is unclear if the risk of recurrence in either newly-diagnosed or second complete remission (CR) patients in long-term remission becomes equivalent to the normal population's risk of acquisition of AML, thus meeting a formal definition of cure. Nonetheless, the existence of patients in remission many (eg, 3, 5, 10, and 20) years after diagnosis suggests that potential cure of AML is not an unreasonable concept.
The implications of cure of AML have not received extensive attention. Here we focus on two such implications. First, we examine the risk of second malignancies in patients treated at M.D. Anderson (MDA; Houston, TX) whom we consider potentially cured of AML. Many authors have documented an increased incidence of AML in patients successfully treated for lymphoma,8,9 breast cancer,10,11 ovarian cancer,12 etc, and our AML patients not infrequently ask about the risk of AML chemotherapy inducing another cancer. Second, we examine whether potentially cured patients return to work, an issue with obvious implications for patients and society.
PATIENTS AND METHODS
A total of 1,892 patients received treatment for newly-diagnosed AML at MDA between 1965 and May, 1995. We accepted the criteria for diagnosis of AML extant at MDA at the various times during this 30 year period, eg, the French-American-British (FAB) criteria since 1975. Cases called AML before 1975 could very well be called ALL using current classification systems. The patients' median age was 54 years. Twenty-eight percent had a documented abnormality in blood count for ≥1 month before MDA presentation, suggestive of a prior myelodysplastic syndrome. Thirteen percent had a prior malignancy. Although AML and ALL patients were treated on common protocols until 1979, all such patients received ara-C from 1967 to 1979 and anthracyclines from 1973 to 1979. Beginning in 1979, AML patients received anthracycline + ara-C ± other drugs.
Definition of potentially cured AML. To establish this definition, we examined the annual rate of failure (recurrence or death in CR) in the patients in the above-described cohort in whom a first or second CR occurred, but who did not receive an allogeneic transplant. We used the method of Simes and Zelen13 to calculate the hazard rates. A total of 1,085 of the 1,892 newly diagnosed patients (57%) obtained a first CR. In addition, there were 302 second CRs. Twenty patients received an allogeneic transplant in first CR and 41 in second CR. Excluding these gave totals of 1,065 first CR and 261 second CR patients. For reasons outlined in Results, we defined potentially cured patients to be those alive in CR 3 years after either a first or second CR. A total of 215 patients met this criterion (203 in first and 12 in second remission), thus constituting our study group.
Data collection. One hundred seventy members of our study group are alive. We sent questionnaires asking about second cancers and current, compared with pretreatment, work status in these 170. Regarding work status, the questionnaire asked seven questions: (1) Were you working before the diagnosis of leukemia? (2) If so, were you working full or part-time? (3) What was your occupation? (4) If you were not working, were you retired or did you have a physical limitation? (5) Have you been working in the last 6 months? (6) If so, full or part-time? (7) If not, are you retired or do you have a physical limitation? Homemaking and childrearing were not included as work reflecting that, invariably, when people noted they were engaged in these activities, they did not consider them as work on the questionnaires. We did not pretest questionnaires. One hundred fourteen of the living patients (67%) returned their questionnaires. Thirty-three of the 56 remaining patients were contacted by phone. In 8 cases information was provided by the patients' local physicians (in 9 cases both the physician and the questionnaire provided information about subsequent cancer and work status; there was concordance between the questionnaire and physician in each case). In 15 of the 170 cases (9%) known to be alive we were unsuccessful at contacting either the patient or the local physician. Here we used information from the medical chart. Forty-five patients in the potentially cured cohort had died at the time of this analysis. Information about cancer and work status before death could be obtained for 28 of these 45 from letters that had been sent annually to the patients by our Department of Epidemiology. For all patients in whom a second cancer was known to have been diagnosed outside MDA we obtained verification using outside diagnostic slides (62%), pathology reports (25%), or verbal confirmation from the attending physician (13%). We considered patients in whom acute leukemia reappeared to have recurrent disease rather than a subsequent cancer. We did not investigate the incidence of myelodysplasia. We established causes of death from information provided either by the MDA Cancer Registry, by phone call to the outside physician, or from the MDA chart in cases where death occurred here.
Cancer risk analysis. To determine whether the number of patients in our potentially cured cohort who developed malignancies after the diagnosis of AML was excessive, we computed standardized incidence ratios (SIRs). These, essentially, are the ratio of the number of patients who developed subsequent invasive (excluding nonmelanoma skin cancer) cancers in our potentially cured population compared with the number of people in the general population who would be expected to develop primary invasive cancer. The latter number was determined using age, sex, and calendar-year specific incidence rates from the Connecticut Tumor Registry from 1936 to 1985 applied to the relative person-years at risk. The computer program cohort analysis for genetic epidemiology (CAGE) was used.14 This program counts patients developing cancer rather than the number of cancers developed, with patients with multiple malignancies scored only at the time the initial cancer was diagnosed. Accordingly, we followed the same scheme in our calculations. For SIRs, person-years at risk were calculated from time of entry into potentially cured cohort to the date of the second cancer diagnosis, death, or date of last contact, whichever came first. The 95% confidence intervals and P values for the SIRs were determined by assuming a Poisson distribution for the observed number of patients with subsequent cancers. A two-sided test was used to test the equality of the observed and expected number of patients with cancer. SIRs were also calculated for subsets of the data as described below.
RESULTS
Potentially cured patients. Table 1 (top) gives the failure rate as a function of the number of years elapsed from first CR.13 Because this rate became relatively low after 3 years in CR we defined first CR patients potentially cured once they were in CR for 3 years. A total of 203 patients met this criterion. Although the initial failure rate was higher than in first CR patients, the rate again became relatively low after 3 years in CR when we examined outcome in the 261 patients entering second CR between 1965 and 1995 (Table 1, bottom). This led us to define second CR patients as potentially cured once they too were in CR for 3 years. Twelve patients met this criterion. Thus, the potentially cured cohort consisted of 215 patients. Disease has recurred in 28 of these 215 patients (26 first CR and 2 second CR) at a median of 1.19 years (range, 2 weeks to 14 years) after entering the cohort for the first CR patients and at 19 weeks and 4.8 years for the second CR patients, recalling that entry into the cohort occurs after 3 years in continuous CR. Twenty-one of the 28 are dead. Twenty-four members of the potentially cured group have died in CR (23 in first CR and 1 in second CR) at a median of 9.4 years (range, 10 weeks to 19 years) after entering the cohort. One hundred sixty-three patients (76%) remain alive in CR at a median of 6.2 years (range, 9 weeks to 23.2 years) from entry into the cohort, ie, 9.2 years from first or second CR. One hundred seventy patients remain alive (Fig 1). Given these data, we believe it is justifiable to call patients potentially cured once they are in first or second CR for 3 consecutive years. Of the 245 patients whose initial treatment date was 1965 to 1972, 6.9% fell into the potentially cured cohort compared with 12.8% of 422 patients treated initially between 1973 and 1979, 14.0% of 458 patients treated initially between 1980 and 1986, and 14.9% of 538 patients treated initially between 1987 and December 31, 1992.
Failure Rate as Function of Years Elapsed From First (Top) or Second (Bottom) CR
| . | Patients . | Failures . | Censored . | Failure Rate* . |
|---|---|---|---|---|
| Years from first CR | ||||
| 0-1 | 1,065 | 491 | 11 | 60 |
| 1-2 | 563 | 238 | 36 | 56 |
| 2-3 | 289 | 65 | 21 | 26 |
| 3-4 | 203 | 11 | 17 | 6 |
| 4-5 | 175 | 8 | 22 | 5 |
| 5-6 | 145 | 35 | 12 | 4 |
| 6-7 | 128 | 1 | 11 | 1 |
| 7-8 | 116 | 3 | 6 | 3 |
| 8-9 | 107 | 2 | 8 | 2 |
| 9-10 | 97 | 1 | 6 | 1 |
| 10-11 | 90 | 1 | 4 | 1 |
| Years from second CR | ||||
| 0-1 | 261 | 169 | 21 | 102 |
| 1-2 | 71 | 34 | 7 | 67 |
| 2-3 | 30 | 14 | 4 | 67 |
| 3-4 | 12 | 2 | 2 | 18 |
| 4-5 | 10 | 0 | 10 | 0 |
| . | Patients . | Failures . | Censored . | Failure Rate* . |
|---|---|---|---|---|
| Years from first CR | ||||
| 0-1 | 1,065 | 491 | 11 | 60 |
| 1-2 | 563 | 238 | 36 | 56 |
| 2-3 | 289 | 65 | 21 | 26 |
| 3-4 | 203 | 11 | 17 | 6 |
| 4-5 | 175 | 8 | 22 | 5 |
| 5-6 | 145 | 35 | 12 | 4 |
| 6-7 | 128 | 1 | 11 | 1 |
| 7-8 | 116 | 3 | 6 | 3 |
| 8-9 | 107 | 2 | 8 | 2 |
| 9-10 | 97 | 1 | 6 | 1 |
| 10-11 | 90 | 1 | 4 | 1 |
| Years from second CR | ||||
| 0-1 | 261 | 169 | 21 | 102 |
| 1-2 | 71 | 34 | 7 | 67 |
| 2-3 | 30 | 14 | 4 | 67 |
| 3-4 | 12 | 2 | 2 | 18 |
| 4-5 | 10 | 0 | 10 | 0 |
Failures per 100 years patient follow-up.
Subsequent Cancers in Patients Potentially Cured of AML
| Patient No. . | Subsequent Cancer . | Age at Diagnosis Subsequent Cancer . | Sex . | Years From Entry Into Potentially Cured Cohort to First Diagnosis of Subsequent Cancer* . | Cytogenetics at Leukemia Diagnosis . | Leukemia Treatment Initial/Salvage . |
|---|---|---|---|---|---|---|
| 1 | Colon-adenocarcinoma | 68 | M | 2 | Normal | ROAP |
| 2 | Colon-adenocarcinoma | 77 | M | 9 | Complex-7 | ADOAP/ADOAP |
| 3 | Lung-carcinoid/colon/breast | 69 | F | 14 | Normal (unbanded) | ADOAP |
| 4 | Lung-small cell carcinoma | 62 | F | 18 | Insufficient | ADOAP |
| 5 | Lung-adenocarcinoma | 65 | F | 1 | Normal (unbanded) | ROAP |
| 6 | Pancreas/melanoma | 68 | M | 4 | Normal | AMSA + OAP |
| 7 | Prostate | 60 | M | 2 | Inv 16 | IDA + HDAC |
| 8 | Prostate | 73 | M | 4 | t(8; 21) | AMSA + OAP |
| 9 | Breast | 51 | F | 2 | Inv 16 | AMSA + OAP |
| 10 | Endometrial | 82 | F | 17 | Insufficient | ADOAP |
| 11 | Endometrial | 58 | F | 11 | t(8; 21) (unbanded) | ADOAP/ADOAP |
| 12 | Penile-squamous | 49 | M | 14 | 47XY + G (unbanded) | ARA-C |
| 13 | Lymphoma-NHL | 48 | M | 13 | Normal | ADOAP |
| 14 | Lymphoma-DLCL | 63 | M | 3 | Inversion 5, +8, +4 | HDAC/FLUDARABINE + ARA-C |
| 15 | Bladder-TCC | 58 | M | 5 | +8 (unbanded) | ADOAP |
| Patient No. . | Subsequent Cancer . | Age at Diagnosis Subsequent Cancer . | Sex . | Years From Entry Into Potentially Cured Cohort to First Diagnosis of Subsequent Cancer* . | Cytogenetics at Leukemia Diagnosis . | Leukemia Treatment Initial/Salvage . |
|---|---|---|---|---|---|---|
| 1 | Colon-adenocarcinoma | 68 | M | 2 | Normal | ROAP |
| 2 | Colon-adenocarcinoma | 77 | M | 9 | Complex-7 | ADOAP/ADOAP |
| 3 | Lung-carcinoid/colon/breast | 69 | F | 14 | Normal (unbanded) | ADOAP |
| 4 | Lung-small cell carcinoma | 62 | F | 18 | Insufficient | ADOAP |
| 5 | Lung-adenocarcinoma | 65 | F | 1 | Normal (unbanded) | ROAP |
| 6 | Pancreas/melanoma | 68 | M | 4 | Normal | AMSA + OAP |
| 7 | Prostate | 60 | M | 2 | Inv 16 | IDA + HDAC |
| 8 | Prostate | 73 | M | 4 | t(8; 21) | AMSA + OAP |
| 9 | Breast | 51 | F | 2 | Inv 16 | AMSA + OAP |
| 10 | Endometrial | 82 | F | 17 | Insufficient | ADOAP |
| 11 | Endometrial | 58 | F | 11 | t(8; 21) (unbanded) | ADOAP/ADOAP |
| 12 | Penile-squamous | 49 | M | 14 | 47XY + G (unbanded) | ARA-C |
| 13 | Lymphoma-NHL | 48 | M | 13 | Normal | ADOAP |
| 14 | Lymphoma-DLCL | 63 | M | 3 | Inversion 5, +8, +4 | HDAC/FLUDARABINE + ARA-C |
| 15 | Bladder-TCC | 58 | M | 5 | +8 (unbanded) | ADOAP |
Abbreviations: NHL, non-Hodgkin's lymphoma; DLCL, diffuse large-cell lymphoma; TCC, transitional cell carcinoma; ADOAP, adriomycin, ara-C, vincristine, prednisone; ROAP, rubidazone, ara-C, vincristine, prednisone; HDAC, high-dose ara-C.
Add 3 years to determine time from initial AML therapy to subsequent cancer.
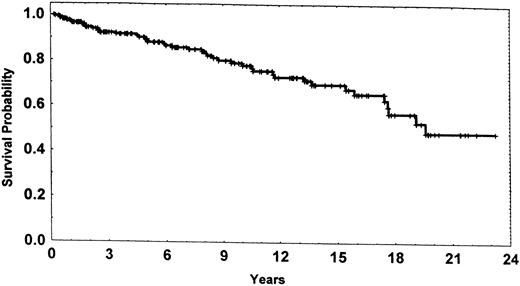
Survival probability for the 215 patients in the potentially cured cohort dated from entry into the cohort, ie, once 3 years had elapsed from first or second CR.
Survival probability for the 215 patients in the potentially cured cohort dated from entry into the cohort, ie, once 3 years had elapsed from first or second CR.
Second cancers and causes of death. Once a patient enters the potentially cured cohort, it becomes reasonable to inquire about the risk of second cancer possibly induced by treatment for AML. Twenty-two patients in our 215-patient potentially cured group developed subsequent cancers. Seven developed noninvasive basal or squamous cell skin cancer, whereas 15 developed invasive cancers (Table 2). The overall incidence of subsequent invasive cancer was 1 patient per 107.8 years of follow-up after entry into the potentially cured cohort. Multiple cancers after treatment for AML occurred in 2 of the 15 (Table 2, patients 3 and 6), giving a total of 18 invasive cancers. These 18 included 3 cases of lung cancer, 3 of colon cancer, 2 of breast cancer, 2 of prostate cancer, 2 of endometrial cancer, 2 of lymphoma, and 1 each of melanoma, penile cancer, pancreatic cancer, and transitional cell bladder cancer. Patients 1 and 12 had had another cancer before the diagnosis of AML (respectively, cancer of the tongue treated with radiation and chemotherapy 3 years before development of AML and diffuse histiocytic lymphoma treated with cyclophosphamide, doxorubicin, vincristine, prednisone, and bleomycin 1 year before onset of AML) giving each of these patients a total of 3 cancers.
The median interval between entry into the potentially cured cohort and diagnosis of a subsequent invasive cancer was 5.0 years (range, 1 to 18 years), considering only the 15 patients who developed a subsequent invasive cancer. Considering all 215 patients in the potentially cured cohort, with time zero corresponding to time of entry into the cohort and considering patients censored at time of death or last follow-up, the median time to development of subsequent invasive cancer has not been reached. As seen in Table 3, 7 of the 15 patients who developed invasive cancer are known to have died of cancer. Of the 7, 3 had also suffered a recurrence of their AML, whereas 4 died in CR. Of the 8 patients who remain alive with cancer, AML remains in CR in 7.
The 15 patients in the 215 member potentially cured cohort who developed invasive cancer subsequent to AML diagnosis compares with an expected number of 8.8 patients for an SIR of 1.74. The 95% confidence interval for this SIR is 0.96 to 2.84, with P = .06 for the hypothesis of no difference between the potentially cured cohort and a normal population (Table 4). We examined whether particular subsets of the cohort might be at higher risk. The median age of the cohort was 40 years. After adjusting for follow-up time, patients in the cohort age 40 years and above were 8.7-fold more likely to develop a subsequent invasive cancer than younger patients (P = .002). The SIR for the members of the cohort age ≥40 years was 1.96, with a 95% CI of 1.02 to 3.27 and P = .03 for the hypothesis of no difference with a normal population age ≥40 years (Table 4). One hundred seventy patients in the potentially cured cohort had banded cytogenetic studies performed at the initial diagnosis of AML. Of the 170, 5 had abnormalities of chromosomes 5 and/or 7. Two of the 5 developed subsequent invasive cancer versus an incidence of 6 of 165 in the patients without chromosome 5 and/or 7 abnormalities (P = .018 after adjusting for follow-up time). Despite the suggestion that the small number of patients with chromosomes 5 or 7 abnormalities in the potentially cured cohort may be at considerably increased risk of subsequent invasive cancer, the small number of patients (n = 5) with these abnormalities in the potentially cured cohort precludes computation of SIRs. Similarly, only 11 patients in the cohort had a malignancy before development of AML, of whom 2, as noted above, developed another invasive cancer subsequent to development of AML. The incidence, adjusted for follow-up time, of subsequent cancer in these 11 patients was 3.0-fold higher than in the 204 potentially cured patients without a malignancy before development of AML, although the P value for the comparison was only .330, possibly reflecting the small number (n = 11) of patients with secondary AML who met our criterion for potential cure. Of 105 patients in the potentially cured cohort under age 40 years at treatment of AML, 4 had history of prior malignancy versus an incidence of prior malignancy of 7 of 110 in patients ≥40 years of age (P = .39). Therefore, we did not adjust the prior malignancy comparison for age. Finally, we computed SIRs for patients in the potentially cured cohort according to when treatment for AML began. None of the 17 patients potentially cured after beginning treatment between 1965 and 1972 developed subsequent invasive cancer compared with 9 of the 54 (17%) who began treatment between 1973 and 1979, 5 of the 64 (8%) who began treatment between 1980 and 1986 and 1 of the 80 (1%) who began treatment between 1987 and December 31, 1992. The SIR ratios were 2.11 (95% CI, 0.97 to 4.01; P = .06) for the 1973 to 1979 cohort, 1.35 (95% CI, 0.44 to 3.15; P = .31) for the 1980 to 1986 cohort, and 0.47 (95% CI, 0.01 to 2.62; P = .88) for the 1987 to December 31, 1992 cohort. Because there were no subsequent cancers in the 1965 to 1972 potentially cured cohort, an SIR could not be computed. There was no difference in age at time of initiation of treatment between the 1965 to 1972, 1973 to 1979, 1980 to 1986, and 1987 to December 31, 1992 potentially cured cohorts (P = .959 Kruskal-Wallis test, medians 40, 38, 40, and 42 years, respectively). The 5 patients in the potentially cured cohort with chromosome 5 and/or 7 abnormalities were treated after 1980.
Observed v Expected Incidence of Invasive Subsequent Cancers
| Patients . | No. of Patients . | No. of Patients . | SIR = Observed/Expected (O/E) . | 95% CI . | P . |
|---|---|---|---|---|---|
| . | Who Developed Cancer . | Expected to Develop Cancer . | . | . | . |
| 215 (total) | 15 | 8.8 | 1.70 | 0.96-2.84 | .06 |
| 105 patients age <40 yr | 2 | 1.9 | 1.05 | 0.13-3.80 | .56 |
| 110 patients age ≥40 yr | 13 | 6.8 | 1.91 | 1.02-3.27 | .03 |
| Patients . | No. of Patients . | No. of Patients . | SIR = Observed/Expected (O/E) . | 95% CI . | P . |
|---|---|---|---|---|---|
| . | Who Developed Cancer . | Expected to Develop Cancer . | . | . | . |
| 215 (total) | 15 | 8.8 | 1.70 | 0.96-2.84 | .06 |
| 105 patients age <40 yr | 2 | 1.9 | 1.05 | 0.13-3.80 | .56 |
| 110 patients age ≥40 yr | 13 | 6.8 | 1.91 | 1.02-3.27 | .03 |
Return to work. Information about work status was available in 181 of the 215 patients in the potentially cured cohort (including 163 of the 170 patients who are alive and 28 of the 45 who are dead). Among 124 patients under age 50 when first treated for AML, 97 were working full-time before treatment (Table 5). Of these 97, 89 are currently alive. Sixty-six of the 89 (74%) have been working full time in the last 6 months. Six of the 8 patients who were working full time before treatment and who died after entering the potentially cured cohort had returned to full-time work at some time after treatment, although it is unclear whether this occurred before or after entry into the cohort. Among 57 patients over age 50 at time of AML diagnosis, 31 were working full time before diagnosis. Of these patients, 20 are currently alive. Six of these 20 have been working full time in the last 6 months. Five of the 11 dead patients who were working full time before treatment had returned to full-time work at some time after treatment (again, we do not know if this occurred before or after entry into the potentially cured cohort). Twelve of the 29 younger patients who, although alive, are not working cited physical limitation; 7 of the remaining 17 are homemakers, 4 are retired, 1 is a student, 1 is incarcerated, and the remaining 4 did not specify why they are not working. Five of the 27 older patients who although alive are not working cited physical limitation, 17 were retired, 2 were homemakers, and 3 did not cite a reason. Thus, only 17 of the 153 living patients (11%) in whom we have information cited physical limitation as a reason for being unable to work (12 of 116 under age 50 and 5 of 37 age ≥50 years). The proportion of those not working who cite physical limitation is 17/56 (30%).
Comparison of Pre- and Post-Treatment Work Status in Potentially Cured Patients
| After Treatment . | Before Treatment for AML . | Total . | ||
|---|---|---|---|---|
| for AML . | Working Fulltime . | Working Parttime . | Not Working . | . |
| Age <50 yrs5-150 | ||||
| Working fulltime | 66/65-151 | 8/0 | 3/0 | 77/6 |
| Working parttime | 8/0 | 2/0 | 0/0 | 10/0 |
| Not working | 15/2 | 3/0 | 11/0 | 29/2 |
| Total | 89/8 | 13/0 | 14/0 | 116/8 |
| Age ≥50 yr5-150 | ||||
| Working fulltime | 6/5 | 0/0 | 0/0 | 6/5 |
| Working parttime | 3/3 | 1/0 | 0/0 | 4/3 |
| Not working | 11/3 | 2/0 | 14/9 | 27/12 |
| Total | 20/11 | 3/0 | 14/9 | 37/20 |
| After Treatment . | Before Treatment for AML . | Total . | ||
|---|---|---|---|---|
| for AML . | Working Fulltime . | Working Parttime . | Not Working . | . |
| Age <50 yrs5-150 | ||||
| Working fulltime | 66/65-151 | 8/0 | 3/0 | 77/6 |
| Working parttime | 8/0 | 2/0 | 0/0 | 10/0 |
| Not working | 15/2 | 3/0 | 11/0 | 29/2 |
| Total | 89/8 | 13/0 | 14/0 | 116/8 |
| Age ≥50 yr5-150 | ||||
| Working fulltime | 6/5 | 0/0 | 0/0 | 6/5 |
| Working parttime | 3/3 | 1/0 | 0/0 | 4/3 |
| Not working | 11/3 | 2/0 | 14/9 | 27/12 |
| Total | 20/11 | 3/0 | 14/9 | 37/20 |
Age at start of therapy for AML.
The 66 refers to patients currently alive; the 6 refers to patients who have died.
DISCUSSION
Our results suggest that, upon entering the potentially cured cohort, one AML patient will develop invasive cancer for every 107.8 years of follow-up. The true SIR comparing the potentially cured AML population and the normal population could, using the conventional 95% confidence interval, be as low as 0.96 or as high as 2.84. However, given our multiple significance, testing this interval should be assumed to be wider; alternatively, the nominal P value of .06 for the hypothesis of no difference between the AML and normal population should be adjusted upward. Our data indicate that whatever increased risk exists is likely confined to specific groups such as patients initially treated between 1973 and 1979, patients age 40 years and older at time of treatment for AML, and, particularly, patients with abnormalities of chromosomes 5 and/or 7, again subject to the constraint of multiple significance testing. Given the small number of potentially cured patients with abnormalities of chromosomes 5 and/or 7, our finding of a difference in the risk of subsequent invasive cancer between these and other patients was of interest; however, the small numbers prevented computation of an SIR for these patients. It is unclear why the risk of subsequent invasive cancer appears greatest for potentially cured patients initially treated between 1973 and 1979, even after accounting for their longer follow-up versus the later cohorts. This does not appear to reflect differences in age or obvious differences in therapy. Our data also suggest that should they enter the potentially cured cohort, younger patients are likely to return to full-time work, whereas the major bar to return to full-time work in older patients is the desire to retire rather than physical limitation.
Our purpose was not to estimate the risk of subsequent cancers in all patients treated for AML but only in patients potentially cured of the disease. We believe that the risk of subsequent cancers is of relevance only for patients who are relatively unlikely to die of AML. Clearly the emphasis must be on potential in the phrase potentially cured, because disease recurs in many patients in our potentially cured cohort (Table 1) and their survival expectations (Fig 1) are unlike those of a normal population. Other definitions of potentially cured could be used, eg, a CR lasting more than 4 years, 5 years, etc (Table 1). However, it is not certain that the risk of failure ever becomes equivalent to the risk that a normal individual will develop AML.1
A tumor registry appropriate to the diverse population from which our patients come does not exist. The Connecticut Tumor Registry, based largely on a white population, has been used in many studies.15 16 We considered this Registry's rates to be the most representative available, given the cultural and geographic heterogeneity of our patients and, particularly, the range of calendar years of observation. This registry does not consider myelodysplasia as cancer and, as noted above, we did not investigate the incidence of subsequent myelodysplasia in our patients. Although the registry does consider acute leukemia as cancer and although we considered our patients in whom acute leukemia reappeared to have recurrent rather than new disease, the proportion of cancer represented by acute leukemia in the general population is sufficiently low that this consideration should not influence our results.
There has been little systematic investigation of the risk of subsequent cancer after treatment for AML. de Nully Brown et al17 reported that 6 of 174 patients aged 17 to 65 years treated for newly diagnosed AML developed a total of 7 secondary malignancies. Three of the 7 were diagnosed simultaneously with the diagnosis of AML, and 1 was diagnosed at autopsy 8 months after beginning treatment for AML. Considering the 3 remaining tumors, which the investigators believe are the only ones that could be attributed to treatment for AML, the SIR was 1.46 (95% CI; 0.47 to 4.57) ie, not demonstrably different than the risk in the normal Danish population. Unlike de Nully Brown et al,17 our cohort included patients over age 65 years and included only potentially cured patients; as stated above, we believe such patients are the clinically relevant group. Because our report focuses only on such patients, ie, only on patients in CR for at least 3 years, it is impossible to compare the risk of subsequent cancer in our patients with the risk after allogeneic marrow transplantation because the patients described in studies of subsequent cancer after transplantation18 19 include all patients at risk from the initiation of transplantation. We should also note that the intensities of today's regimens are greater than those used for many years during the period of this study and, therefore, that our results may not predict future outcomes, although it was interesting to note that the risk of subsequent invasive cancer appears greatest for patients treated between 1973 and 1979. We hope our data allow physicians to inform patients that, should they be cured of AML with chemotherapy, they are likely to be able to return to work and, at least if younger and without abnormalities of chromosomes 5 and/or 7, do not have an increased risk of subsequent cancer.
ACKNOWLEDGMENT
The authors thank Soon Woo for expert secretarial assistance.
Address correspondence to Elihu Estey, MD, Department of Hematology Box 61, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal