Abstract
The safety and efficacy of filgrastim as an adjunct to acute myeloid leukemia (AML) induction and consolidation therapy was assessed in this prospective double-blind, randomized, placebo-controlled, multicenter trial. A total of 521 consecutive de novo AML patients aged 16 or more years were randomized to receive filgrastim (5 μg/kg/d subcutaneously) or placebo after standard induction as well as consolidation chemotherapy. Blinded study drug was given from 24 hours after chemotherapy until the absolute neutrophil count was ≥1.0 × 109/L for 3 consecutive days. The overall complete remission rate was 68%. After a median follow-up of 24 months (range 5 to 40) the median disease-free survival was 10 months (95% confidence interval [CI], 8.7 to 10.8) and the median overall survival was 13 months (95%CI, 12.2 to 14.6). These did not differ between treatment groups. Patients receiving filgrastim experienced neutrophil recovery 5 days earlier after induction 1 than those receiving placebo (P < .0001). This was accompanied by reductions in the duration of fever (7 v 8.5 days; P = .009), parenteral antibiotic use (15 v 18.5 days; P = .0001), and hospitalization (20 v 25 days; P = .0001). Similar reductions were seen after induction 2 and the consolidation courses. There was a significant reduction in the number of patients requiring systemic antifungal therapy in the filgrastim group during induction treatment (34% v 43%; P = .04). In conclusion, filgrastim is safe in that it had no negative impact on the prognosis of the AML patients. In addition, it effectively reduced the duration of neutropenia, leading to significant clinical benefits by reducing the duration of fever; requirement for parenteral anti-infectives, specifically amphotericin B; and the duration of hospitalization.
REMISSION INDUCTION and consolidation treatment for acute myeloid leukemia (AML) is associated with considerable morbidity and mortality.1-3 A significant component of the morbidity is related to the prolonged, severe neutropenia resulting from the disease as well as the intensive chemotherapy. The outcome of treatment is dependent, in part, on the ability of patients to tolerate the myelosuppression. The proportion of patients achieving complete remission (CR) decreases with advancing age.4 5
Filgrastim (recombinant methionyl human granulocyte colony-stimulating factor [r-metHuG-CSF ]) reduces the duration of neutropenia and associated complications after chemotherapy for solid tumors.6-9 After high-dose chemotherapy and bone marrow transplantation, filgrastim reduces the time to neutrophil recovery as well as the requirements for supportive care and hospitalization.10 11
The application of myeloid growth factors in AML has been limited by the theoretical concerns about their ability to stimulate the growth of myeloid leukemia. These concerns stemmed from in vitro studies showing that the myeloid blasts from AML patients expressed receptors for hematopoietic growth factors including granulocyte colony-stimulating factor (G-CSF ) and granulocyte macrophage colony-stimulating factor (GM-CSF ).12 This finding necessitated controlled studies to examine the influence of the growth factor on disease response and outcome as well as on neutropenia and infection-related parameters.
A number of studies have been reported in which myeloid growth factors have been used as an adjunct to AML chemotherapy (for review see Schiffer,13 Rowe and Liesveld,14 Büchner et al,15 and Terpstra and Löwenberg16 ). Different strategies have been followed in these trials in that the growth factors were given after chemotherapy to ameliorate the myelotoxicity and/or during chemotherapy to reduce the chemoresistance of the leukemic blasts. In eight randomized trials recombinant human granulocyte colony-stimulating factor (rHuGM-CSF )17-24 was used, and in another four trials rHuG-CSF25-28 was used. The majority of these studies were confined to elderly populations or patients with relapsed/refractory disease with a high risk of treatment-related mortality. These trials have produced inconsistent results with respect to the impact of the growth factor on disease outcome, hematopoietic recovery, and frequency of infections.
The primary objective of this study was to investigate the influence of filgrastim on disease outcome of de novo AML patients and to address theoretical concerns about potential stimulation of a myeloid leukemia by a myeloid growth factor. This study was not confined to elderly patients, but was open to all patients legally able to give informed consent. Filgrastim was used both after induction as well as after consolidation therapy including a high-dose cytarabine-based postremission regimen in younger patients.
PATIENTS AND METHODS
Patients. Patients aged 16 or older with de novo AML, as defined by the French-American-British (FAB) classification system,29 and an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 were eligible and entered the trial in a consecutive manner. Those with previously diagnosed myelodysplasia (MDS) or with a blast crisis of chronic myeloid leukemia (CML) were excluded, as were patients who had received previous treatment for AML or had a previous malignancy. All patients gave written informed consent to the study. The study was approved by ethics committees in each country or hospital as appropriate.
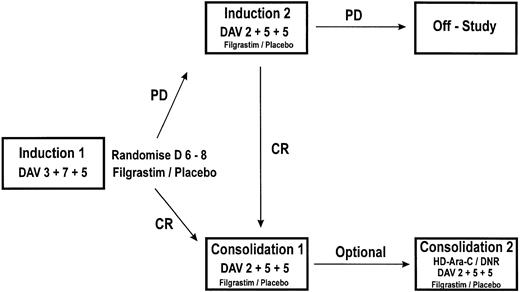
Schematic representation of the study design. PD, persistent disease; CR, complete remission. Induction 1: daunorubicin 45 mg/m2 (3 days), cytosine-arabinoside 200 mg/m2 (7 days), and etoposide 100 mg/m2 (5 days). Induction 2: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days). Consolidation 1: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days). Consolidation 2: (optional) <50 years: cytosine-arabinoside 3 g/m2 12 hourly (6 days), daunorubicin 30 mg/m2 (2 days); ≥50 years: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days).
Schematic representation of the study design. PD, persistent disease; CR, complete remission. Induction 1: daunorubicin 45 mg/m2 (3 days), cytosine-arabinoside 200 mg/m2 (7 days), and etoposide 100 mg/m2 (5 days). Induction 2: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days). Consolidation 1: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days). Consolidation 2: (optional) <50 years: cytosine-arabinoside 3 g/m2 12 hourly (6 days), daunorubicin 30 mg/m2 (2 days); ≥50 years: daunorubicin 45 mg/m2 (2 days), cytosine-arabinoside 200 mg/m2 (5 days), and etoposide 100 mg/m2 (5 days).
Demographics and Disease Characteristics
| . | Filgrastim . | Placebo . |
|---|---|---|
| No. of patients | 259 | 262 |
| Age | ||
| <50 yr | 40% | 44% |
| ≥50 yr | 60% | 56% |
| 50-70 yr | 49% | 49% |
| ≥70 yr | 11% | 7% |
| Median (yr) | 54 | 54 |
| Range | 16-89 | 16-88 |
| Sex | ||
| Male | 54% | 54% |
| Female | 46% | 46% |
| ECOG status | ||
| 0 | 24% | 25% |
| 1 | 59% | 57% |
| 2 | 17% | 18% |
| FAB subtype | ||
| M0 | 3% | 3% |
| M1 | 21% | 23% |
| M2 | 27% | 24% |
| M3* | 1% | 3% |
| M4 | 24% | 27% |
| M5 | 17% | 13% |
| M6 | 4% | 4% |
| M7 | 1% | 2% |
| Not assessed | 2% | 1% |
| Cytogenetics† | ||
| Favorable | 3% | 4% |
| Normal | 37% | 43% |
| Unfavorable | 30% | 29% |
| Not assessed | 30% | 24% |
| . | Filgrastim . | Placebo . |
|---|---|---|
| No. of patients | 259 | 262 |
| Age | ||
| <50 yr | 40% | 44% |
| ≥50 yr | 60% | 56% |
| 50-70 yr | 49% | 49% |
| ≥70 yr | 11% | 7% |
| Median (yr) | 54 | 54 |
| Range | 16-89 | 16-88 |
| Sex | ||
| Male | 54% | 54% |
| Female | 46% | 46% |
| ECOG status | ||
| 0 | 24% | 25% |
| 1 | 59% | 57% |
| 2 | 17% | 18% |
| FAB subtype | ||
| M0 | 3% | 3% |
| M1 | 21% | 23% |
| M2 | 27% | 24% |
| M3* | 1% | 3% |
| M4 | 24% | 27% |
| M5 | 17% | 13% |
| M6 | 4% | 4% |
| M7 | 1% | 2% |
| Not assessed | 2% | 1% |
| Cytogenetics† | ||
| Favorable | 3% | 4% |
| Normal | 37% | 43% |
| Unfavorable | 30% | 29% |
| Not assessed | 30% | 24% |
These patients had no previous treatment with ATRA.
Favorable: t(15,17), t(8,21) and inv(16). Unfavorable: monosomies 5, 7, trisomy 8, 11q(23), complex and multiple abnormalities.
Number of Patients by Course
| . | Filgrastim . | Placebo . |
|---|---|---|
| Induction 1 | 259 | 262 |
| Induction 2 | 60 (23%) | 67 (26%) |
| Consolidation 1 | 162 (63%) | 157 (60%) |
| Consolidation 2 | 81 (31%) | 84 (32%) |
| DAV (2 + 5 + 5) | 60 (23%) | 58 (22%) |
| HD-Ara-C/DNR | 21 (8%) | 26 (10%) |
| . | Filgrastim . | Placebo . |
|---|---|---|
| Induction 1 | 259 | 262 |
| Induction 2 | 60 (23%) | 67 (26%) |
| Consolidation 1 | 162 (63%) | 157 (60%) |
| Consolidation 2 | 81 (31%) | 84 (32%) |
| DAV (2 + 5 + 5) | 60 (23%) | 58 (22%) |
| HD-Ara-C/DNR | 21 (8%) | 26 (10%) |
Abbreviations: DAV: daunorubicin, cytosine-arabinoside, etoposide; HD-Ara-C/DNR; high-dose cytosine-arabinoside/daunorubicin.
Treatment plan. Induction 1 consisted of daunorubicin 45 mg/m2 for 3 days, cytosine arabinoside 200 mg/m2 for 7 days, and etoposide 100 mg/m2 for 5 days (daunorubicin, cytosine-arabinoside, etoposide [DAV] 3 + 7 + 5; Fig 1). Patients were centrally randomized after 6 days of chemotherapy and were stratified by center and age group (age <50 years and age ≥50 years). Blinded study drug (filgrastim 5 μg/kg/d or matched placebo) was given subcutaneously (sc) from 24 hours after the last dose of chemotherapy until the absolute neutrophil count (ANC) was ≥1.0 × 109/L for 3 consecutive days or ≥10 × 109/L for 1 day. A maximum of 28 days of the study drug was permitted. Patients failing to achieve a CR after a single course had blinded study medication discontinued immediately and received a second induction course of daunorubicin 45 mg/m2 for 2 days, cytosine arabinoside 200 mg/m2 for 5 days, and etoposide 100 mg/m2 for 5 days (DAV 2 + 5 + 5) followed by the study drug. Patients not in CR after a second induction were considered to have completed the study. Patients in CR received consolidation 1 chemotherapy (DAV 2 + 5 + 5) followed by the study drug. A second consolidation course was optional and varied according to institutional policy. This was standardized and consistently applied within centers. Patients received either a further course of DAV 2 + 5 + 5 or if aged <50 years could receive high-dose treatment with cytosine arabinoside 3 g/m2, 12 hourly, for 6 days and daunorubicin 30 mg/m2 for 2 days (HD-Ara-C/DNR) followed by the study drug (Fig 1). Subsequent therapy was given according to institutional policy and was not specified in the protocol.
Management of fever and infection. Patients received prophylactic oral quinolone antibiotics from the start of each course of chemotherapy until the ANC had recovered to 0.5 × 109/L. Those experiencing an oral temperature of ≥38°C or with other clinical signs of infection received treatment with empiric broad-spectrum parenteral antibacterials. In the event of culture-negative fevers these were discontinued when the patient remained afebrile for 48 hours. All other supportive care was according to institutional practice.
Deaths in Induction
| . | Filgrastim . | Placebo . |
|---|---|---|
| Number of patients | 259 | 262 |
| Number of deaths | 21 | 25 |
| Primary cause | ||
| Infection related | 9 | 18 |
| Hemorrhage | 6 | 3 |
| Chemotherapy toxicity | 1 | 0 |
| Renal failure | 2 | 0 |
| Persistent disease | 3 | 3 |
| Unknown | 0 | 1 |
| Infection Related Deaths During Induction: | ||
| All infection related deaths | 9 | 18 |
| Age ≤50 | 1 | 6 |
| >50 <60 | 1 | 1 |
| >60 <70 | 3 | 8 |
| >70 | 4 | 3 |
| Types of infection | ||
| Bacteremia/sepsis | 3 | 6 |
| Pneumonia | 3 | 6 |
| Fungal infection | 3 | 6 |
| . | Filgrastim . | Placebo . |
|---|---|---|
| Number of patients | 259 | 262 |
| Number of deaths | 21 | 25 |
| Primary cause | ||
| Infection related | 9 | 18 |
| Hemorrhage | 6 | 3 |
| Chemotherapy toxicity | 1 | 0 |
| Renal failure | 2 | 0 |
| Persistent disease | 3 | 3 |
| Unknown | 0 | 1 |
| Infection Related Deaths During Induction: | ||
| All infection related deaths | 9 | 18 |
| Age ≤50 | 1 | 6 |
| >50 <60 | 1 | 1 |
| >60 <70 | 3 | 8 |
| >70 | 4 | 3 |
| Types of infection | ||
| Bacteremia/sepsis | 3 | 6 |
| Pneumonia | 3 | 6 |
| Fungal infection | 3 | 6 |
Remission Rate by Age and Cytogenetics
| . | Filgrastim . | Placebo . | Total . |
|---|---|---|---|
| Overall | 178/259 (69%) | 177/262 (68%) | 355/521 (68%) |
| Age | |||
| <50 yr | 79/104 (76%) | 82/115 (71%) | 161/219 (74%) |
| ≥50 yr | 99/155 (64%) | 95/147 (65%) | 194/302 (64%) |
| Cytogenetics | |||
| Unfavorable | 51/78 (65%) | 42/75 (56%) | 93/153 (61%) |
| Normal/favorable | 78/104 (75%) | 98/123 (80%) | 176/227 (78%) |
| Not assessed | 49/77 (64%) | 37/64 (58%) | 86/141 (61%) |
| . | Filgrastim . | Placebo . | Total . |
|---|---|---|---|
| Overall | 178/259 (69%) | 177/262 (68%) | 355/521 (68%) |
| Age | |||
| <50 yr | 79/104 (76%) | 82/115 (71%) | 161/219 (74%) |
| ≥50 yr | 99/155 (64%) | 95/147 (65%) | 194/302 (64%) |
| Cytogenetics | |||
| Unfavorable | 51/78 (65%) | 42/75 (56%) | 93/153 (61%) |
| Normal/favorable | 78/104 (75%) | 98/123 (80%) | 176/227 (78%) |
| Not assessed | 49/77 (64%) | 37/64 (58%) | 86/141 (61%) |
Unfavorable: monosomies 5, 7; trisomy 8; 11q(23); complex and multiple abnormalities. Favorable: t(15; 17), t(8; 21), inv(16).
Remission assessment. Remission status was assessed after the study drug had been discontinued for a minimum of 3 days. CR was defined as ≤5% blasts in the bone marrow in the presence of trilineage regeneration. Diagnostic and remission-assessment bone-marrow aspirate samples were centrally reviewed blinded to the study drug.
Statistical methods. The study was designed to detect a 15% change in CR rate from the anticipated remission rate of 65% in the placebo group. Interim analyses were scheduled when the remission status of each group of 60 randomized patients became known. The double-triangular test30 sequential trial plan was used so that, allowing for these interim analyses, a 15% difference would be detected with a power of 90% at the a = 0.05 (5%) level of significance. An independent data monitoring committee reviewed all interim analyses.
The safety endpoints were CR rate, disease-free survival, and overall survival. Efficacy endpoints were the duration of neutropenia, the incidence and duration of fever and intravenous (IV) anti-infective therapy, the incidence of documented infections, and the duration of hospitalization.
The main statistical comparisons for the infection-related endpoints were made in induction course 1 to avoid the possible patient selection bias affecting later courses. However, comparisons for subsequent courses are also presented. The endpoints of fever, IV anti-infective therapy, documented infections, and hospitalization in first induction were counted from day 6 of chemotherapy.
CR rates were compared between the treatment groups by using the χ2 test adjusted for the interim analyses using sequential trial plan methods.30 Effects of prognostic factors and covariates were assessed by logistic regression. Disease-free survival, overall survival and time to ANC recovery were analyzed using the log-rank test, and the effect of prognostic factors and covariates were assessed by Cox's proportional hazards modelling. The incidences of fever, IV anti-infectives, and infections were analyzed using the χ2 test with the effects of prognostic factors being assessed by logistic regression. The durations of fever, IV anti-infectives, and hospitalization were analyzed using the Wilcoxon rank sum test. The effects of prognostic factors and covariates were assessed by transforming the durations and using linear regression.
Disease-free survival of the filgrastim (n = 178) and of the placebo group (n = 177). After a median follow-up of 24 months the median disease-free survival was 10.1 months for the filgrastim group and 9.4 months for the placebo group with a probability of disease-free survival at 3 years of 20% in the filgrastim versus 21% in the placebo group.
Disease-free survival of the filgrastim (n = 178) and of the placebo group (n = 177). After a median follow-up of 24 months the median disease-free survival was 10.1 months for the filgrastim group and 9.4 months for the placebo group with a probability of disease-free survival at 3 years of 20% in the filgrastim versus 21% in the placebo group.
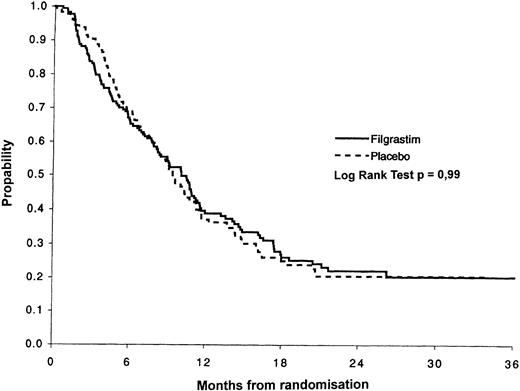
Overall survival of the filgrastim (n = 259) and the placebo patients (n = 262). The median survival time of the filgrastim patients was 12.5 months versus 14.0 months in the placebo group. The probability of survival after 36 months was 21% in the filgrastim versus 19% in the placebo group.
Overall survival of the filgrastim (n = 259) and the placebo patients (n = 262). The median survival time of the filgrastim patients was 12.5 months versus 14.0 months in the placebo group. The probability of survival after 36 months was 21% in the filgrastim versus 19% in the placebo group.
The prognostic factors considered in the multivariate analyses were center, age, performance status, FAB subtype, cytogenetics, evidence of pre-existing MDS (determined from central review of bone marrow aspirate slides), and use of prophylactic IV anti-infective therapy. The covariates considered were the incidence of neutropenia, fever, nonprophylactic IV anti-infective therapy, or a microbiologically defined infection between days 1 and 5 of induction course 1.
All analyses, with the exception of disease-free survival and analysis of courses subsequent to induction course 1, were performed in an intent-to-treat manner, ie, including all randomized patients in the treatment groups assigned at randomization. The analysis of disease-free survival only included patients who achieved a CR.
RESULTS
Patients. A total of 521 patients were randomized from March 1992 to October 1994. Three patients had their diagnosis amended to CML after randomization and were withdrawn, and 3 further patients had a history of prior malignancy. All patients were included in the analysis.
Two hundred fifty-nine patients received filgrastim and 262 received the placebo. The groups were well balanced for age, sex, ECOG performance status, FAB subtype, and cytogenetics (Table 1). The number of patients starting each course of chemotherapy is given in Table 2.
A total of 62 patients died during the study period (ie, within 30 days of the last dose of blinded study medication). There were 46 deaths before remission status was determined after the first induction treatment (21 filgrastim, 25 placebo). The primary cause of death was determined from patient summaries prepared before unblinding and are listed in Table 3. The observed differences between the treatment groups for the primary causes of death are not statistically significant.
Of the 355 patients who entered remission, 319 continued and received consolidation chemotherapy. Twenty-seven patients were withdrawn by investigators after the first induction course to receive alternative salvage chemotherapy (17 filgrastim, 10 placebo). Other patient withdrawals during induction chemotherapy were because of adverse events (12 filgrastim, 15 placebo), withdrawal of patient consent (4 filgrastim, 9 placebo), or other reasons including deaths (35 filgrastim, 39 placebo). Sixty-one patients left the study because of failure to enter remission (29 filgrastim, 32 placebo). No difference was seen between the groups.
Thirty-five patients were withdrawn during consolidation chemotherapy for the following reasons: adverse events (3 filgrastim, 1 placebo), withdrawal of patient consent (2 placebo), disease progression (12 filgrastim, 5 placebo), death (3 filgrastim, 4 placebo), and other (3 filgrastim, 2 placebo). No differences between treatment groups were seen in the reasons for withdrawal.
Disease outcome. The overall CR rate was 68% (95% confidence interval [CI], 64% to 72%) and did not differ between the treatment groups (69% filgrastim, 68% placebo), either with (P = .47) or without (P = .77) adjustment for the interim analyses. Fifty-six percent of patients achieved remission with one course of induction chemotherapy.
Age and cytogenetic risk factors significantly influenced the probability of achieving CR. Table 4 shows remission rates by age and cytogenetics. After adjusting for these factors, there remained no effect of treatment with filgrastim (P = .83).
Disease-free and overall survival. At the time of this analysis, the median follow-up was 24 months (range 5 to 40). Disease-free survival was defined as the time from the first bone marrow documentation of CR until either the date of the first positive bone-marrow aspirate after CR or the date of death if there was no evidence of disease progression. The Kaplan-Meier (KM) disease-free survival curves are shown in Fig 2 for patients who achieved CR. There was no evidence of a difference between the treatment groups (P = .99). The median disease-free survival in the filgrastim group was 10.1 months (95% CI, 8.2 to 11.4) compared with 9.4 months (95% CI, 8.2 to 11.1) in the placebo group.
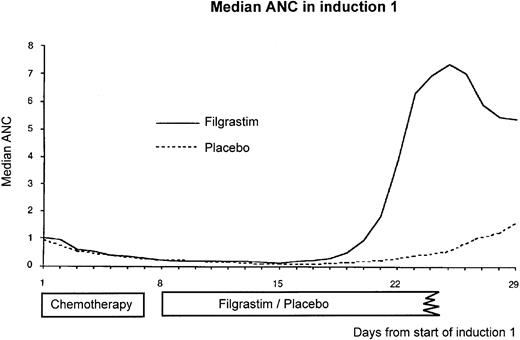
Median absolute neutrophil count (×109/L) per day starting from day 1 of the first induction course. Filgrastim was administered for a median of 13 days and placebo for a median of 20 days. There was no detectable effect of filgrastim on median absolute neutrophil count until day 17/18.
Median absolute neutrophil count (×109/L) per day starting from day 1 of the first induction course. Filgrastim was administered for a median of 13 days and placebo for a median of 20 days. There was no detectable effect of filgrastim on median absolute neutrophil count until day 17/18.
Incidence of Fever, IV Anti-Infectives, and Infection in Induction Course 1
| . | Filgrastim . | Placebo . | . |
|---|---|---|---|
| Fever | 91% | 92% | P = .505-150 |
| Anti-infective | 95% | 96% | P = .815-150 |
| Antibacterial | 95% | 96% | |
| Antifungal | 37% | 47% | |
| Antiviral | 12% | 10% | |
| Microbiologically defined infection | 37% | 36% | P = .855-150 |
| . | Filgrastim . | Placebo . | . |
|---|---|---|---|
| Fever | 91% | 92% | P = .505-150 |
| Anti-infective | 95% | 96% | P = .815-150 |
| Antibacterial | 95% | 96% | |
| Antifungal | 37% | 47% | |
| Antiviral | 12% | 10% | |
| Microbiologically defined infection | 37% | 36% | P = .855-150 |
Incidences counted from day 6.
χ 2 test.
The KM overall survival curves are shown in Fig 3 for all randomized patients. There was no evidence of a difference in survival between the two treatment groups (P = .83). The median survival in the filgrastim group was 12.5 months (95% CI, 10.9 to 14.4) compared with 14.0 months (95% CI, 12.2 to 15.6) in the placebo group. The factors affecting overall survival were age, performance status, and FAB subtype. After adjusting for these factors, there remained no effect of filgrastim (P = .94). The most important factor affecting survival, apart from the prognostic factors evaluated before randomization, was remission status. The median survival for patients not achieving remission was 4.4 months compared with 18.2 months for patients who achieved remission. The median survival for the 47 patients who received HD-Ara-C/DNR for the second consolidation course was 27.7 months compared with 15.9 months in the 21 younger patients receiving standard (DAV 2 + 5 + 5) treatment.
Duration of Fever and Intravenous Anti-Infective Therapy in Induction Course 1
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Fever (days) | |||
| Median | 7.0 | 8.5 | P = .0096-150 |
| Quartiles | 3.0-13.0 | 5.0-15.0 | |
| Range | 0-38 | 0-38 | |
| Number of patients | 259 | 262 | |
| Intravenous anti-infectives (days) | |||
| Median | 15.0 | 18.5 | P = .00016-150 |
| Quartiles | 10.0-20.0 | 13.0-27.0 | |
| Range | 0-54 | 0-54 | |
| Number of patients | 259 | 262 |
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Fever (days) | |||
| Median | 7.0 | 8.5 | P = .0096-150 |
| Quartiles | 3.0-13.0 | 5.0-15.0 | |
| Range | 0-38 | 0-38 | |
| Number of patients | 259 | 262 | |
| Intravenous anti-infectives (days) | |||
| Median | 15.0 | 18.5 | P = .00016-150 |
| Quartiles | 10.0-20.0 | 13.0-27.0 | |
| Range | 0-54 | 0-54 | |
| Number of patients | 259 | 262 |
Durations counted from day 6.
Wilcoxon Rank Sum Test.
Infection Related Endpoints in Subsequent Courses
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Induction 2 | |||
| Neutropenia duration (days) | |||
| Median (range) | 10.0 (0-38) | 14.0 (0-43) | P = .015 |
| Fever incidence | 48/60 (80%) | 50/67 (75%) | P = .47 |
| Intravenous anti-infectives incidence | 46/60 (77%) | 47/67 (70%) | P = .41 |
| Consolidation 1 | |||
| Neutropenia duration (days) | |||
| Median (range) | 4.0 (0-46) | 11.0 (0-22) | P = .0001 |
| Fever incidence | 80/162 (49%) | 99/157 (63%) | P = .014 |
| Intravenous anti-infectives incidence | 71/162 (44%) | 85/157 (54%) | P = .08 |
| Consolidation 2 (DAV 2 + 5 + 5) | |||
| Neutropenia duration (days) | |||
| Median (range) | 5.0 (0-18) | 10.0 (0-22) | P = .0001 |
| Fever incidence | 28/60 (47%) | 33/58 (57%) | P = .27 |
| Intravenous anti-infectives incidence | 22/60 (37%) | 30/58 (52%) | P = .07 |
| Consolidation 2 (HD-Ara-C/DNR) | |||
| Neutropenia duration (days) | |||
| Median (range) | 13.0 (6-26) | 18.5 (12-42) | P = .001 |
| Fever incidence | 20/21 (95%) | 24/26 (92%) | P = .68 |
| Intravenous anti-infectives incidence | 19/21 (90%) | 24/26 (92%) | P = .82 |
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Induction 2 | |||
| Neutropenia duration (days) | |||
| Median (range) | 10.0 (0-38) | 14.0 (0-43) | P = .015 |
| Fever incidence | 48/60 (80%) | 50/67 (75%) | P = .47 |
| Intravenous anti-infectives incidence | 46/60 (77%) | 47/67 (70%) | P = .41 |
| Consolidation 1 | |||
| Neutropenia duration (days) | |||
| Median (range) | 4.0 (0-46) | 11.0 (0-22) | P = .0001 |
| Fever incidence | 80/162 (49%) | 99/157 (63%) | P = .014 |
| Intravenous anti-infectives incidence | 71/162 (44%) | 85/157 (54%) | P = .08 |
| Consolidation 2 (DAV 2 + 5 + 5) | |||
| Neutropenia duration (days) | |||
| Median (range) | 5.0 (0-18) | 10.0 (0-22) | P = .0001 |
| Fever incidence | 28/60 (47%) | 33/58 (57%) | P = .27 |
| Intravenous anti-infectives incidence | 22/60 (37%) | 30/58 (52%) | P = .07 |
| Consolidation 2 (HD-Ara-C/DNR) | |||
| Neutropenia duration (days) | |||
| Median (range) | 13.0 (6-26) | 18.5 (12-42) | P = .001 |
| Fever incidence | 20/21 (95%) | 24/26 (92%) | P = .68 |
| Intravenous anti-infectives incidence | 19/21 (90%) | 24/26 (92%) | P = .82 |
Wilcoxon Rank Sum Test used for comparing durations and χ 2 test for comparing incidences.
Abbreviations: DAV: daunorubicin, cytosine-arabinoside, etoposide. HD-Ara-C/DNR: high-dose cytosine-arabinoside/daunorubicin.
Hematological recovery. The median ANC in induction course 1 is shown in Fig 4. The main differences in the neutrophil profiles occurred after day 18. The time to neutrophil recovery in induction 1, defined as the number of days from the first day of chemotherapy until the first of 3 days with an ANC ≥0.5 × 109/L, was reduced from a KM median of 25 days (95% CI, 24 to 27) in the placebo group to 20 days (95% CI, 19 to 20) in the filgrastim group (P = .0001). The presence or absence of neutropenia during days 1 to 5 of induction 1 was also found to influence neutrophil recovery. The reduction in the KM median in the filgrastim group was greater for patients with no neutropenia during days 1 to 5 (6 days) than for the patients with neutropenia (5 days). The median time to platelet recovery to 20 × 109/L independent of platelet transfusions was 21 days. This did not differ between treatment groups.
Fever and infections. Fever was defined as oral temperature ≥38°C, which was considered resolved at a temperature <37.5°C. The duration of IV anti-infective therapy was calculated as the number of days on which at least one nonprophylactic anti-infective was given. Each reported infection was coded before unblinding the treatment codes according to criteria defined by a consensus panel from the Immunocompromised Host Society.31 Three categories are defined: microbiologically defined infection, clinically defined infection, and unexplained fever. An additional category of probable transfusion reaction was added for this study. The incidence and durations of fever and IV anti-infective therapy and the incidence of the infection types during induction 1 were calculated from day 6, the earliest day for randomization.
The incidence of fever, IV anti-infective therapy, and infection types in induction course 1 were not affected by the use of filgrastim (Table 5). In a subgroup analysis, the incidence of IV antifungal therapy was reduced in the filgrastim group (P = .03). This difference was mainly caused by a reduction in the number of patients treated with amphotericin B (34% filgrastim, 43% placebo; P = .04).
Duration of Hospitalization (days)
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Induction therapy | |||
| Median | 23.0 | 29.0 | P = .00018-150 |
| Quartiles | 18.0-34.0 | 22.0-40.0 | |
| Range | 2-104 | 7-93 | |
| Number of patients | 259 | 262 | |
| Induction and consolidation | |||
| Median | 42.0 | 55.0 | P = .00018-150 |
| Quartiles | 36.0-51.0 | 45.0-65.0 | |
| Range | 15-140 | 23-114 | |
| Number of patients | 162 | 157 |
| . | Filgrastim . | Placebo . | |
|---|---|---|---|
| Induction therapy | |||
| Median | 23.0 | 29.0 | P = .00018-150 |
| Quartiles | 18.0-34.0 | 22.0-40.0 | |
| Range | 2-104 | 7-93 | |
| Number of patients | 259 | 262 | |
| Induction and consolidation | |||
| Median | 42.0 | 55.0 | P = .00018-150 |
| Quartiles | 36.0-51.0 | 45.0-65.0 | |
| Range | 15-140 | 23-114 | |
| Number of patients | 162 | 157 |
Durations counted from day 6 of induction 1.
Wilcoxon Rank Sum Test.
The durations of fever and IV anti-infective therapy in induction course 1 were significantly reduced in the filgrastim group compared with the placebo group (Table 6). Factors found to influence the duration of fever were study center, presence or absence of neutropenia or microbiologically defined infection between days 1 and 5 of the first induction chemotherapy, and treatment with filgrastim. Factors found to influence the duration of IV anti-infective therapy were study center, presence or absence of neutropenia or IV anti-infective therapy between days 1 and 5 of first induction chemotherapy, use of prophylactic IV anti-infectives, and treatment with filgrastim. After adjusting for these factors, the effect of treatment with filgrastim was still to reduce the duration of fever (P = .014) and anti-infective therapy (P = .001).
Similar reductions in durations of neutropenia and infection-related endpoints are seen for subsequent chemotherapy courses (Table 7).
Hospitalization. The duration of hospitalization during induction course 1 was significantly reduced from 25 days in the placebo group to 20 days in the filgrastim group (P = .0001). Similar effects were observed for the whole induction period including patients with one or two induction courses as well as for the induction and consolidation period (Table 8).
Adverse events. The nature and incidence of reported adverse events was similar in both treatment groups for all phases of the study. In induction course 1, the most frequently reported adverse events considered related to the study drug were rash (filgrastim 3%, placebo 2%) and musculo-skeletal pain (filgrastim 2%, placebo 1%). All other treatment-related events were reported at an incidence of 1% or less during induction course 1.
DISCUSSION
This study has shown that filgrastim does not worsen the outcome of the disease in patients with de novo AML. Patients receiving filgrastim achieved the same CR rate as those receiving placebo, and the remissions were of similar durability. The study showed that filgrastim is a safe and effective means of reducing the morbidity associated with treatment of AML.
Remission rates in recent studies of growth factors (rHuGM-CSF and rHuG-CSF ) given to patients with acute myeloid leukemias expressed as the odds ratio and the 95% confidence interval. The numbers in parentheses indicate the number of patients enrolled in the trial. The studies have been selected on the basis of appropriate data published so far to calculate the odds ratio. For references see text.
Remission rates in recent studies of growth factors (rHuGM-CSF and rHuG-CSF ) given to patients with acute myeloid leukemias expressed as the odds ratio and the 95% confidence interval. The numbers in parentheses indicate the number of patients enrolled in the trial. The studies have been selected on the basis of appropriate data published so far to calculate the odds ratio. For references see text.
Number of patients experiencing a documented infection per day (prevalence) during and after the first induction course (upper part) and the median absolute neutrophil count (×109/L) per day during and after the first induction course (lower part) for the filgrastim and the placebo group.
Number of patients experiencing a documented infection per day (prevalence) during and after the first induction course (upper part) and the median absolute neutrophil count (×109/L) per day during and after the first induction course (lower part) for the filgrastim and the placebo group.
The scope for a growth factor to improve CR rate, if it has no fundamental biological action on the disease itself, is entirely dependent on its ability to reduce the rate of neutropenia-related death. The majority of deaths associated with the treatment of AML occur early, within the first 28 days after induction treatment. In this study, 46 patients died during initial remission-induction treatment, of whom 38 died within the first 28 days. Although there were apparently fewer infection-related deaths in the filgrastim group (9 v 18), the overall death rate (21 filgrastim, 25 placebo) was not different. This reflects the prolonged period of neutropenia experienced by both groups of patients before neutrophil recovery began, during which time most of the deaths occurred. Factors known to influence the CR rate, such as age and cytogenetic status, remain the most important determinants of outcome and are not affected by the use of filgrastim.
A number of studies have now been published that address the influence of myeloid growth factors in AML.13-16 Figure 5 shows the odds ratios and 95% CI for several growth factor trials17-21,23-26,28 that have been selected on the basis of appropriate data for the calculation of the odds ratio. The differences in impact of growth factor on remission rate in these studies may be caused by differences in sample size, patient populations, timing of randomization, timing of the administration of growth factor, the growth factor used, and timing of remission assessment. This study using filgrastim with 521 randomized patients and the study by Stone et al19 using rHuGM-CSF with 388 randomized patients are the largest undertaken to date and show no impact of growth factor on CR rate. It is likely that neither rHuGM-CSF or rHuG-CSF influence CR rate, and differences seen in some smaller studies may represent random variation. This aspect is supported by the results of our interim analyses of the CR rate after every 60 randomized patients (data not shown), which displayed a considerable variation of the CR rate among the patient cohorts. As a consequence, adequately powered studies are necessary when addressing the influence of a growth factor on CR rate in this disease. The study by Dombret et al26 using lenograstim included 173 de novo AML patients and showed an apparently large difference in CR rate in favor of the growth factor–treated patients. This was not paralleled by improvements in early mortality, disease-free survival, or overall survival. The explanation for this result is not clear but may be a function of the methods of assessing remission status used in this study.13
Treatment with filgrastim significantly reduced the duration of neutropenia and associated clinical consequences in patients with AML. During induction therapy, patients in both treatment groups were profoundly neutropenic, for approximately 18 days, before neutrophil recovery began. During this time, the majority experienced fever and required IV anti-infectives and in-patient care. Treatment with filgrastim did not impact the incidence of this early morbidity. However, by reducing the duration of neutropenia, it significantly reduced the duration of all of these clinical events. Patients receiving filgrastim spent significantly less time in hospital recovering from induction and consolidation therapy. The duration of hospitalization was reduced by 5 days during the first induction course and a total of 13 days for patients undergoing induction and one course of consolidation. This effect was seen across all participating centers regardless of institutional policies for discharge.
The incidence of microbiologically documented infection was identical in both groups, because infections occurred early during treatment, before neutrophil recovery had begun. Determining the actual duration of a documented infection is difficult. In an attempt to investigate the potential relationship between the duration of neutropenia and infection, the percentage of patients reporting a documented infection on each day of induction therapy (prevalence) was plotted against the median ANC profile (Fig 6). This indicates that, as the ANC recovers, the prevalence of documented infection tends to fall in both treatment groups and that this occurs earlier in the filgrastim group.
In this population, a major consequence of prolonged neutropenia is fungal infection with organisms such as Aspergillus. The incidence of fungal infection is increasing in patients with hematologic malignancies.32-35 The persistence of fever for more than 48 hours after the start of broad-spectrum antibacterials usually necessitates the introduction of systemic antifungal therapy, such as amphotericin-B, which is associated with significant toxicity.36-38 In this study, filgrastim significantly reduced the number of patients requiring systemic antifungal therapy. This is an important clinical benefit, not previously documented in studies of growth factors in the oncology setting, and serves to reduce the burden of toxicity in these patients.
This study has shown that filgrastim is a safe and effective adjunct to the treatment of AML, significantly reducing neutropenia and associated morbidity without worsening disease outcome. Filgrastim is effective when used in conjunction with all courses of chemotherapy, including standard and high-dose consolidation and its routine use in reducing morbidity will need to be considered in the light of its pharmaco-economic impact.
Further improvements in the outlook for patients with AML may depend on the better definition of prognostic factors and the prognostic factor adjusted application of high-dose consolidation therapy. The use of additional growth factors, such as megakaryocyte growth and development factor,39,40 which stimulates the production and maturation of platelets,41-43 may further reduce treatment-associated morbidity. The application of hematopoietic growth factors in this setting may enable very intensive chemotherapy to be administered with acceptable toxicity and may allow the exploration of further intensive schedules with or without hematopoietic progenitor cell support.
APPENDIX
The following hospitals and investigators participated in the International AML study group: Derriford Hospital, Plymouth, UK (A. Prentice); Manchester Royal Infirmary, Manchester, UK (J.A. Liu Yin); Royal London Hospital, London, UK (A. Newland); Addenbrooke's Hospital, Cambridge, UK (R. Marcus); Musgrove Park Hospital, Taunton, UK (S. Johnson); Guys Hospital, London, UK (S. Schey); John Radcliffe Hospital, Oxford, UK (T. Littlewood, C. Bunch); University of Frankfurt, Frankfurt, Germany (D. Hoelzer, A. Ganser); University of Ulm, Ulm, Germany (G. Heil, H. Heimpel); University of Freiburg, Freiburg, Germany (R. Mertelsmann, A. Lindemann); Johannes-Guttenberg University, Mainz, Germany (C. Huber, K. Kolbe); Ospedali Riuniti, Bergamo, Italy (T. Barbui); Ospedale Regionale, Bolzano, Italy (P. Coser); Ospedale San Bortoli, Vicenza, Italy (R. Battista, F. Rodeghiero); Ospedale S. Eugenio, Roma, Italy (G. Papa, A. Venditti); University Eppendorf, Hamburg, Germany (D. Hossfeld); Stuyvenberg Ziekenhuis, Antwerpen, Belgium (P. Zachée); Ospedale Nuovo San Gerardo, Monza, Italy (G. Corneo, E. Pogliani); Hospital La Fe, Valencia, Spain (M. Sanz, G. Martı́n); Hospital de la Princesa, Madrid, Spain (J. Fernández-Rañada, J. Tomás); University of Vienna, Vienna, Austria (K. Lechner, K.Geissler); Belfast City Hospital, Belfast, UK (T.C.M. Morris); University of Gent, Gent, Belgium (L. Noens); Centre Hospitalier Sart Tilman, Liège, Belgium (G. Fillet); Instituto Portugues de Oncologia, Lisbon, Portugal (A. Parreira); University Hospital of Lund, Lund, Sweden (P.G. Nilsson); and the Australian Leukaemia Study Group: Peter McCallum Cancer Institute, Melbourne, Australia (J. Bishop); Royal Adelaide Hospital, Adelaide, Australia (C. Juttner, J. Ho); Royal Prince Alfred Hospital, Sydney, Australia (D. Joshua); Westmead Hospital, Sydney, Australia (K. Bradstock); Alfred Hospital, Melbourne, Australia (J. Szer); and Royal Melbourne Hospital, Melbourne, Australia (J. Szer).
Members of the Data Monitoring Committee include Prof D. Linch, University College London, London, UK; Dr D. Machin, MRC Cancer Trials Unit, Cambridge, UK; Dr N. Testa, Christie Hospital, Manchester, UK; and Dr P. Taylor, Rotherham District Hospital, Rotherham, UK.
Sponsored by Amgen Ltd, Cambridge, UK and F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Address reprint requests to Gerhard Heil, MD, PhD, Department of Hematology and Oncology, Hannover Medical School, Carl-Neuberg-Str 1, D-30623 Hannover, Germany.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal