Abstract
In experimental autoimmune encephalomyelitis (EAE) inflammatory cells cross the endothelial blood-brain barrier (BBB) and gain access to the central nervous system (CNS). Here we show that E- and P-selectin are not involved in the recruitment of inflammatory cells across the BBB. Neither expression of E- nor P-selectin is induced in BBB-forming endothelium at any time after initiation of EAE. Some of the inflammatory cells present in the CNS during EAE express ligands for E- or P-selectin. However, anti–E- and P-selectin antibodies influence neither immigration of inflammatory cells across the BBB nor the development of EAE. In general, suppression of E- and P-selectin expression on BBB endothelium is dependent on factors derived from the CNS microenvironment, eg, astrocytes. Our results suggest that during EAE suppression of E- and P-selectin expression on the BBB provides a CNS-specific mechanism to reduce leukocyte recruitment into the CNS.
MECHANISMS MEDIATING leukocyte recruitment into the central nervous system (CNS) during inflammation are still poorly understood. In the healthy CNS, highly specialized endothelial cells form the blood-brain barrier (BBB), a tightly interconnected cellular monolayer that strictly controls the exchange of solutes and other molecules between the blood and the neuropil. It has therefore been assumed that the BBB also forms an effective barrier for blood cells.1-3 However, in human diseases, such as multiple sclerosis, or in experimental models of inflammatory diseases of the CNS, such as experimental autoimmune encephalomyelitis (EAE), leukocytes do gain access to the CNS parenchyme.
EAE is mediated by activated autoaggressive CD4+ T cells. Autoaggressive T cells can either be activated in vivo by subcutaneous immunization of susceptible animals with spinal cord homogenate or components of the CNS myelin, such as myelin basic protein (MBP) or protein lipid protein (PLP) in complete Freund's adjuvant.4 Alternatively, EAE can be mediated by adoptive transfer of in vitro freshly activated MBP- or PLP-specific CD4+ T cells into naive syngeneic recipients.5 6 In both cases the peripherally activated autoantigen-specific CD4+ T cells have to cross the BBB to gain access to the CNS, recognize their antigen, and initiate the events leading to edema, inflammation, and destruction of myelin. Contact between the circulating autoaggressive T cells and the BBB endothelium can be assumed to be the first critical step instrumental for CNS invasion and thus development of EAE. The first wave of T-cell immigration must occur across the “normal” BBB, whereas later recruitment of inflammatory cells into the CNS is probably facilitated by prestimulation of the BBB through the previous passage of encephalitogenic T cells. Immigration of mononuclear cells into the CNS during EAE is not random at any time during the disease. Selectivity of CNS infiltration is characterized by the presence of CD4+ T cells and macrophages, whereas neutrophils, B cells, and CD8+ T cells are rarely detected.
It has become evident that cell adhesion molecules (CAMs) on the surface of leukocytes and endothelial cells are actively involved in the recruitment of specific leukocyte subsets into different tissues.7 Considering the structural uniqueness of the BBB endothelium, the question has been posed whether differentiation of the BBB endothelium includes the expression of BBB-specific CAMs. In fact, there is no evidence for BBB-specific CAMs to date. A series of studies performed by us and others have shown that ICAM-1 and VCAM-1 are upregulated on cerebral vessels during inflammatory conditions of the CNS, such as EAE.8-11 Upregulation of both CAMs precedes the perivascular infiltration and the onset of disease (Schulz and Engelhardt, unpublished observation, 1994),10,11 suggesting that their expression is a prerequisite for inflammatory cell entry into the CNS. Evidence for the functional importance of cerebral endothelial expression of ICAM-1 and VCAM-1 is provided by studies using a modified Stamper-Woodruff frozen section assay8 that show that lymphocytes bind to inflamed cerebral vessels with increased levels of ICAM-1 and VCAM-1 via interaction with their known ligands LFA-1/Mac-1 and α4-integrin, respectively, in vitro.8,12 There is accumulating evidence that, in vivo, α4-integrin/VCAM-1 interaction plays a privotal role in the recruitment of inflammatory cells across the BBB during EAE. Intravenous application of anti–α4-integrin antibodies reduces cellular infiltration of the CNS and inhibits the development of EAE.12,13 Furthermore, the ability of autoaggressive T-cell lines to cross the BBB seems to correlate with their expression levels of α4-integrin.14 The functional relevance of ICAM-1 induced on cerebral vessels during EAE for the recruitment of inflammatory cells across the BBB in vivo, however, is still a matter of debate.9,15 16
It is generally accepted that leukocyte extravasation is an active process requiring multiple steps.17,18 This multistep paradigm postulates that traffic signals for circulating leukocytes function in a sequence, where tethering of the leukocyte from the flowing blood to the vessel wall, subsequent rolling of the leukocyte along the vessels wall, activation-dependent firm adhesion of the leukocyte to the endothelium, and subsequent diapedesis can be distinguished as separate steps. Members of the selectin family have been shown to mediate the first step of leukocyte-endothelial interaction, namely the tethering of the leukocyte to the endothelial vessel wall and subsequent rolling of the leukocyte along the endothelium. The subsequent firm adhesion of leukocytes to the endothelial cell surface and migration across the vessel wall are regulated independent of the initial binding. These latter stages are mediated by functional activation of integrins on the leukocyte presumably by chemoattractants located in the vessel wall17,18 and their subsequent binding to their endothelial ligands, which are members of the Ig-superfamily. The sequence of molecular interactions, and thus the validity of the multistep paradigm, has been shown for the recruitment of neutrophils and macrophages in vitro and in vivo.17,18 There is mounting evidence that T-cell recruitment during inflammation is regulated in multiple steps by the same molecules.19
No Expression of E- and P-Selectin at the Protein and mRNA Level in Cerebral Endothelial Cells During EAE in the SJL/J Mouse
| Investigated Mice* . | Timepoint . | P-Selectin . | E-Selectin . |
|---|---|---|---|
| Untreated | ⊘ | ⊘ | |
| aEAE-⊘ | 4 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 10 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 24 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 2 days p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 4 days p.I. | ⊘ | ⊘ |
| aEAE-+++ | 14 days p.I. | ⊘ | ⊘ |
| aEAE-+++ | 14 days p.I. | ⊘ | ⊘ |
| aEAE-relapse ++ | 64 days p.I. | ⊘ | ⊘ |
| aEAE-relapse ++ | 72 days p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 85 days p.I. | ⊘ | ⊘ |
| Investigated Mice* . | Timepoint . | P-Selectin . | E-Selectin . |
|---|---|---|---|
| Untreated | ⊘ | ⊘ | |
| aEAE-⊘ | 4 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 10 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 24 hrs p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 2 days p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 4 days p.I. | ⊘ | ⊘ |
| aEAE-+++ | 14 days p.I. | ⊘ | ⊘ |
| aEAE-+++ | 14 days p.I. | ⊘ | ⊘ |
| aEAE-relapse ++ | 64 days p.I. | ⊘ | ⊘ |
| aEAE-relapse ++ | 72 days p.I. | ⊘ | ⊘ |
| aEAE-⊘ | 85 days p.I. | ⊘ | ⊘ |
Abbreviation: p.I., postimmunization.
n ≥ 5 for protein expression, n = 1 for mRNA expression.
Based on the multistep paradigm, ICAM-1 and VCAM-1 expressed on inflamed cerebral vessels are prime candidates for mediators of the secondary adhesive interactions of leukocytes with the BBB endothelium. However, the molecules mediating the initial contact of circulating leukocytes with the BBB endothelium are still unknown. E- and P-selectin have been shown to be critically involved in the tethering and rolling of leukocytes in vitro20,21 and in vivo.22 Additionally, both selectins have been shown to mediate rolling of T lymphocytes in vitro23,24 and to play a vital role in T-lymphocyte recruitment into inflamed skin.25 26 This study shows that unexpectedly, E- and P-selectin are not involved in the tethering and rolling of “CNS-seeking” inflammatory cells at the BBB endothelium during EAE in the SJL/J mouse.
In situ hybridization analysis of E-selectin and P-selectin mRNA expression in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. Neither E-selectin nor P-selectin mRNA can be detected. Vessels surrounded by inflammatory cuffs are shown, (a) and (c), dark field; (b) and (d), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
In situ hybridization analysis of E-selectin and P-selectin mRNA expression in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. Neither E-selectin nor P-selectin mRNA can be detected. Vessels surrounded by inflammatory cuffs are shown, (a) and (c), dark field; (b) and (d), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
Absence of E- and P-selectin on cerebral vessel endothelium during EAE in SJL/J mice. An inflammatory cuff in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. CD31/PECAM-1 is expressed by all cerebral endothelial cells. E- and P-selectin cannot be detected. ⊘; isotype matched control. Immunoperoxidase staining, hematoxylin counterstain; original magnification × 240.
Absence of E- and P-selectin on cerebral vessel endothelium during EAE in SJL/J mice. An inflammatory cuff in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. CD31/PECAM-1 is expressed by all cerebral endothelial cells. E- and P-selectin cannot be detected. ⊘; isotype matched control. Immunoperoxidase staining, hematoxylin counterstain; original magnification × 240.
MATERIALS AND METHODS
Mice.Female SJL/J mice were obtained from Bommice, Denmark between 3 to 4 weeks of age or from our own breeding facilities at the Max-Planck Institute for Physiological and Clinical Research, Bad Nauheim, Germany. Female Balb/C mice and C57B1/6 mice were obtained from our own breeding facility.
Monoclonal antibodies.Mec13.3 (anti-mouse PECAM-1/CD31) was a kind gift of Dr E. Dejana (Milano, Italy27 ); Hermes-1 (=9B5, anti-human CD44, used as an isotype-matched control) was provided by Dr E.C. Butcher (Stanford, CA28). The hybridomas PS/2 (anti-mouse α4-integrin), M1/9 (anti-mouse CD45), M1/70 (anti-mouse Mac-1), B220 (anti-mouse CD45R), and GK1.5 (anti-mouse CD4) were obtained from ATCC (Rockville, MD). RB40.34/4 (anti-mouse P-selectin) was raised in D.V.'s laboratory29; UZ 4 and UZ 7 (both anti-mouse E-selectin) were raised in R.H.'s laboratory.30 ER-TR2 (rat anti-mouse major histocompatibility complex [MHC] class II) was purchased from Dianova, Hamburg, Germany.
Induction of EAE.Active EAE (aEAE) was induced as described before8 31 by immunizing SJL/J mice with 100 μg of spinal cord homogenate (SCH) from syngeneic mice in complete Freund's Adjuvant (CFA; GIBCO Laboratories, Grand Island, NY), containing 60 μg/mL Mycobacterium tuberculosis H37Ra and 10 μg/mL Mycobacterium butyricum (Difco Laboratories, Detroit, MI) into hind footpads. 3 × 109 organisms of heat-killed Bordetella pertussis organisms (kindly provided by Dr Kolbe, Behring Werke, Marburg, Germany) were injected in 0.5 mL of phosphate-buffered saline (PBS) on days 1 and 3 postimmunization. Passively transferred EAE (tEAE) was either induced by intravenous (IV) injection of 3 × 106 freshly activated, encephalitogenic CD4+ T line cells SJL.PLP1 or SJL.MBP.1 or by injection of 5 × 107 freshly stimulated lymph node cells derived from primary cultures of draining lymph nodes of mice before immunized with SCH. Animals were checked daily for signs of clinical disease and killed as indicated. Clinical scoring was as follows: 0.5 = limp tail, 1 = hindleg weakness, 2 = hindleg paraplegia, 3 = hindleg paraplegia with incontinence, 4 = sudden death. In aEAE clinical disease occurred approximately 14 days postimmunization with SCH, whereas tEAE started at about day 7 postimmunization. As additional control, untreated littermates, which were kept in the same cages, were killed.
T-lymphocyte lines.The encephalitogenic T-lymphocyte lines SJL.PLP1 and SJL.MBP1 were established from draining lymph nodes of SJL/J mice immunized with syngeneic SCH using a modified protocol.32 SJL.PLP1 was raised in vitro against amino acids 139-153 of murine PLP, whereas SJL.MBP1 was raised against guinea pig MBP. The lines were propagated by periodically alternating antigen-depending activation episodes with T-cell growth factor–driven propagation phases. The lines contained CD4+ T cells only and recognized their antigen in the molecular context of MHC class II. Both lines were used for experiments starting after the third round of antigen-specific stimulation, when proliferation only occured in response to the specific antigen as measured by the incorporation of 3H-thymidine.
Treatment of EAE.For treatment of EAE, monoclonal antibodies (MoAbs) were injected IV. Endotoxin levels of MoAb preparations were routinely determined by Fresenius (Tanusstein, Germany) and were below detection levels (<0.6 international units [EU]/mL). In case of aEAE, mice were injected on days −1, 4, and 9 or on days −1, 4, 9, and 11 with the day of immunization considered to be day 0. Two hundred micrograms of MoAb were applied per injection and mouse. Another study was performed using a single IV injection of MoAb at day 9 (400 μg per mouse). In case of tEAE, MoAb was injected together with the encephalitogenic T cells IV at day 0 and again on day 4. Serum-detection levels of the MoAbs used in this study remained stable for 4 consecutive days as determined by fluorescence-activated cell sorter (FACS) analysis or anti-rat–Ig enzyme-linked immunosorbent assay (ELISA) when injected into untreated animals or into the tEAE model. In aEAE animals MoAb serum levels also remained stable for 4 consecutive days with exception to day 9 postimmunization, when levels of MoAb gradually dropped starting at day 2 after MoAb injection and could not be detected in the serum of injected animals at day 4 after MoAb injection. This was independent of whether it was a first or repeated injection of rat IgG. Biological activity of detectable serum levels of MoAbs was confirmed by the 4-day delay of clinical onset of EAE after a single injection of anti–α4-integrin MoAb PS/2. Injection of 9B5 (rat anti-human CD44) was used as a MoAb control. Injection of anti-CD4, anti-MHC class II, or anti–α4-integrin was used as a positive control.
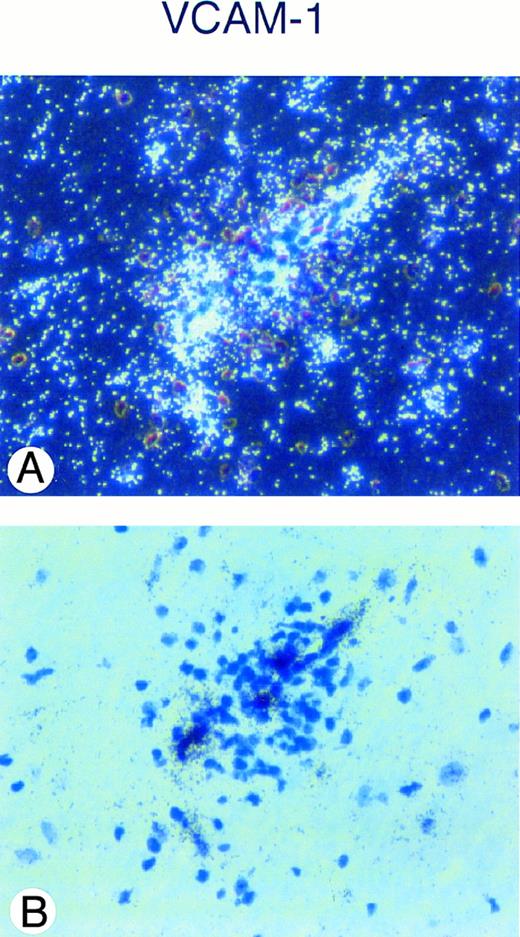
In situ hybridization analysis of VCAM-1 mRNA expression in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. A strong hybridization signal for VCAM-1 mRNA can be detected in a vessel surrounded by an inflammatory cuff (A), dark field; (B), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
In situ hybridization analysis of VCAM-1 mRNA expression in the spinal cord of a SJL/J mouse afflicted with acute EAE (day 14, clinical score +++) is shown. A strong hybridization signal for VCAM-1 mRNA can be detected in a vessel surrounded by an inflammatory cuff (A), dark field; (B), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
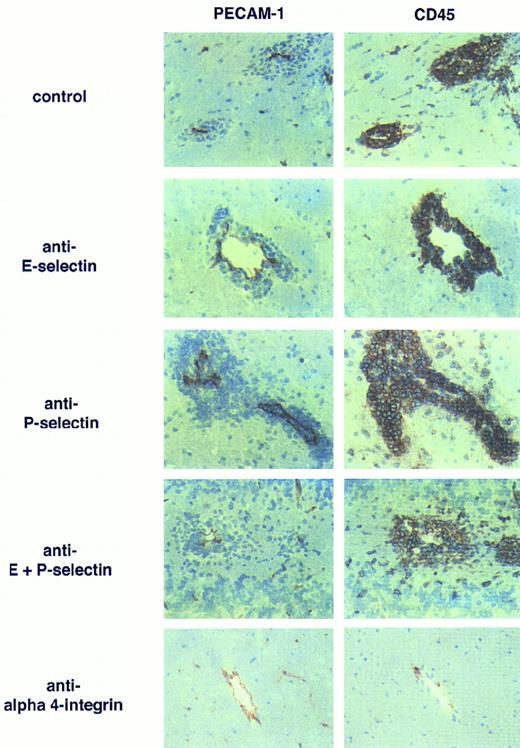
Anti–E- and anti–P-selectin antibodies do not influence the development of EAE. Inflammatory cuffs in the CNS of mice afflicted with EAE are shown. Left panel: staining of vessels with anti-PECAM-1/CD31; right panel: staining of leukocytes with anti-CD45. Inflammatory cuffs develop in rat IgG-treated (control) EAE animals but also in presence of anti–E- or anti–P-selectin or both MoAbs. In contrast, in animals treated with anti–α4-integrin, MoAb inflammatory cuffs cannot be detected. Single CD45+ cells adjacent to the vessels can also be seen in the healthy CNS and are most probably perivascular macrophages. Immunoperoxidase staining, hematoxylin counterstain. Original magnification × 200.
Anti–E- and anti–P-selectin antibodies do not influence the development of EAE. Inflammatory cuffs in the CNS of mice afflicted with EAE are shown. Left panel: staining of vessels with anti-PECAM-1/CD31; right panel: staining of leukocytes with anti-CD45. Inflammatory cuffs develop in rat IgG-treated (control) EAE animals but also in presence of anti–E- or anti–P-selectin or both MoAbs. In contrast, in animals treated with anti–α4-integrin, MoAb inflammatory cuffs cannot be detected. Single CD45+ cells adjacent to the vessels can also be seen in the healthy CNS and are most probably perivascular macrophages. Immunoperoxidase staining, hematoxylin counterstain. Original magnification × 200.
Expression of E- and P-selectin ligands on peripheral lymph node cells (PLN) and inflammatory cells isolated from the CNS of mice afflicted with EAE (IC-EAE) as determined by FACS analysis. Subpopulations of CD45+ IC-EAE and Thy 1.2+ IC-EAE T cells express E- and P-selectin ligands as determined via positive staining with selectin-Ig chimeras.
Expression of E- and P-selectin ligands on peripheral lymph node cells (PLN) and inflammatory cells isolated from the CNS of mice afflicted with EAE (IC-EAE) as determined by FACS analysis. Subpopulations of CD45+ IC-EAE and Thy 1.2+ IC-EAE T cells express E- and P-selectin ligands as determined via positive staining with selectin-Ig chimeras.
Expression of E- and P-selectin ligands on the encephalitogenic CD4+ T-cell line SJL.PLP1 as determined by FACS analysis. A subpopulation of the SJL.PLP1 expresses E- and P-selectin ligands as determined via positive staining with selectin-Ig chimeras.
Expression of E- and P-selectin ligands on the encephalitogenic CD4+ T-cell line SJL.PLP1 as determined by FACS analysis. A subpopulation of the SJL.PLP1 expresses E- and P-selectin ligands as determined via positive staining with selectin-Ig chimeras.
Development of aEAE in SJL/J Mice Treated With Repeated Injections of Anti–E-Selectin and/or Anti–P-Selectin MoAbs
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease* . | No. of Animals With Detectable Infiltrates in CNS† . |
|---|---|---|---|---|
| ⊘ | 15 | 11 | 13.8 ± 4.5 | 15 |
| PBS | 11 | 8 | 13.8 ± 1.9 | 11 |
| IgG | 5 | 4 | 14.0 ± 0.7 | 5 |
| RB40 (anti–P-selectin) | 7 | 5 | 15.3 ± 3.2 | 7 |
| UZ4 (anti–E-selectin) | 9 | 6 | 15.3 ± 5.3 | 9 |
| RB40 + UZ4 | 3 | 2 | 13.5 ± 0.5 | 3 |
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease* . | No. of Animals With Detectable Infiltrates in CNS† . |
|---|---|---|---|---|
| ⊘ | 15 | 11 | 13.8 ± 4.5 | 15 |
| PBS | 11 | 8 | 13.8 ± 1.9 | 11 |
| IgG | 5 | 4 | 14.0 ± 0.7 | 5 |
| RB40 (anti–P-selectin) | 7 | 5 | 15.3 ± 3.2 | 7 |
| UZ4 (anti–E-selectin) | 9 | 6 | 15.3 ± 5.3 | 9 |
| RB40 + UZ4 | 3 | 2 | 13.5 ± 0.5 | 3 |
Only animals developing clinical disease were taken into account.
Animals were sacrificed 4 days after onset of clinical disease for histopathological analysis of the CNS tissue, making statistical analysis of clinical severity of the disease at the same time impossible. Although not all animals developed a clinical disease, they all showed inflammatory cuff formation in their spinal cords and brains. No significant differences were observed in number and size of inflammatory cuffs when comparing the different treatment groups or clinically diseased versus nondiseased aEAE animals.
Development of aEAE in SJL/J Mice Treated With a Single Injection of Anti–E-Selectin and/or Anti–P Selectin MoAbs
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease3-150 . | Mean Severity of Clinical Disease at Day 14 p.I. . |
|---|---|---|---|---|
| PBS | 4 | 4 | 10.5 ± 0.5 | 3 ± 1 |
| PS/2 | 6 | 5 | 14 ± 0.53-151 | 0.5 |
| RB40 + UZ4 | 7 | 7 | 10.5 ± 0.5 | 3 ± 0.5 |
| RB40 | 2 | 2 | 11 ± 0 | 3.5 ± 0.5 |
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease3-150 . | Mean Severity of Clinical Disease at Day 14 p.I. . |
|---|---|---|---|---|
| PBS | 4 | 4 | 10.5 ± 0.5 | 3 ± 1 |
| PS/2 | 6 | 5 | 14 ± 0.53-151 | 0.5 |
| RB40 + UZ4 | 7 | 7 | 10.5 ± 0.5 | 3 ± 0.5 |
| RB40 | 2 | 2 | 11 ± 0 | 3.5 ± 0.5 |
400 μg MoAb were injected IV on day 9 postimmunization (p.I.).
Only animals developing clinical disease were taken into account.
Significant difference in mean day of onset of disease (Student t-test).
Induction of selectins in vivo.Balb/C, C57B1/6, and SJL/J mice were injected IV with 50 μg lipopolysaccharide (LPS; Sigma, Zeisenhofen, Germany) or 50 μg recombinant human tumor necrosis factor-α (TNF-α; gratefully provided by M. Robinson, Celltech, UK). Three or four hours later mice were perfused with 1% formaldehyde and tissue was removed and snap frozen for immunohistochemistry or in situ hybridization (see below).
Immunohistochemistry.Animals were anesthetized using Isoflurene anesthesia (Abbott, Wiesbaden, Germany) and were perfused with either PBS or 1% formaldehyde (PFA) in PBS through the left ventricle of the heart. Tissue was removed, embedded in Tissue-tec, (OCT; Miles Inc, Vogel, Giessen, Germany), and snap-frozen in a 2-methylbutane (Merck, Darmstadt, Germany) bath at −80°C. Cryostat sections (6 μm) were air-dried overnight, acetone fixed, and stained using a three-step immunoperoxidase technique. Sections were incubated sequentially with primary MoAbs, biotinylated secondary goat anti-rat IgG (Vector, Boehringer Ingelheim Bioproducts, Heidelberg, Germany), and horseradish peroxidase–conjugated Streptavidin (Vector) for 30 minutes each step in a humidified chamber, with PBS washes in between the single steps. Sections were developed with 0.07% amino-ethylcarbazol (AEC; Sigma) and 0.009% hydrogen peroxide in 0.01 mol/L acetate buffer (pH 5.2) for 10 minutes. Sections were counterstained with Hematoxylin-Eosin (Gill's formula, Merck), coverslipped with Aquatex (Merck), and immediately analyzed.
In situ hybridization.The techniques used for in situ hybridization were as described.31 Single-stranded 35S-labeled antisense or sense RNA probes were generated by in vitro transcription using T3 or T7 RNA polymerases as described by the manufacturer (Stratagene, Heidelberg, Germany) using 5′ fragments of cDNA clones of murine E-selectin and P-selectin in BlueskriptKS+. The mouse E-selectin probe used for in situ hybridization was generated from an EcoRI fragment that contained bases 1 to 1487 of the published mouse E-selectin cDNA (GenBank/EMBL Data Bank M 87862).33 The mouse P-selectin probe was generated from an EcoRI fragment that contained bases 1 to 1409 of the published cDNA sequence (GenBank/EMBL Data Bank M 87861).33 The mouse VCAM-1 probe was generated from a 700-bp EcoRI fragment that contained bases 1 to 573 of the published muVCAM-1 seven-domain cDNA (GenBank accession number M84487). Sense RNA probes were used as a control and did not show specific hybridization. After hybridization, slides were coated with photographic emulsion (Kodak NTB-2; Eastman Kodak, Rochester, NY) and exposed for 2 weeks. After fixation, sections were counterstained with Toluidin Blue, dehydrated, and mounted.
Isolation of inflammatory cells from CNS tissue.Isolation of inflammatory cells was performed as described before.34 Briefly, 15 brains and spinal cords of SJL/J mice afflicted with EAE were dissected and minced between slides in 40 mL Dulbecco's modified Eagle's medium (DMEM) supplemented with 25 mmol/L HEPES, pH 7.2. The tissues were enzymatically digested for 1 hour at 37°C with 1 mg of collagenase per mouse in a total volume of 40 mL. After filtration through nylon mesh (100 μm), cells were run over a fetal calf serum (FCS) gradient (4°C, 10 minutes, 300g) to remove myelin, washed two times in PBS plus 3% CS, then resuspended in 5 mL of isotonic Percoll 50% in DMEM (per 2 brains + spinal cords) overlayed with 8 mL of 30% Percoll. After a 30-minute centrifugation at 4°C and 1,000g, leukocytes were collected from the interface, washed twice in DMEM/HEPES, and used for analysis. The average number of inflammatory cells (IC) collected per preparation was about 5 × 106.
Flow cytometry.Cell populations (5 × 104 to 1 × 106 cells/sample) were incubated with the selectin-Ig chimeras (30 μg/mL),25,26 washed twice with FACS-buffer (PBS supplemented with 2 mmol/L Ca2+/Mg2+, 1% bovine serum albumin [BSA], and 0.1% NaN3 ), and incubated with a Phycoerythrin (PE)-conjugated goat anti-human IgG (10 μg/mL; Jackson/Dianova, Hamburg, Germany). Specificity controls ensuring the lectin-mediated binding of the selectin-Ig constructs used in these studies have been performed before25 26 and were confirmed by us by showing that positive staining using selectin-Ig chimeras could only be obtained in presence of divalent cations. MurineHT7-IgG (kindly provided by G. Fachinger, Bad Nauheim, Germany) was used as negative control IgG-fusion protein. After washing twice, the cells were blocked with 5% normal rat serum in FACS-buffer (5 minutes) and then incubated with FITC-conjugated goat anti-mouse MoAb. After washing twice cells were fixed in 1% formaldehyde. All antibody incubation periods were for 20 minutes at 4°C. Flow cytometry analysis was performed on a FACScan (Becton Dickinson, Heidelberg, Germany) using the Cellquest software. For analysis, light scatter gates were drawn to include either all live cells or lymphocytes only, as indicated in results. Percentages of positively stained cells were calculated for each experiment by subtracting the fluorescence-histogram obtained with the control-Ig chimera from the histogram obtained with the selectin chimeras.
Isolation and culture of primary mouse brain endothelial cells (MBEs).Cerebral capillaries from mice were isolated using a slightly modified procedure as described before.35 Briefly, cortices from 15 brains were dissected free of meninges and white matter in buffer A (153 mmol/L NaCl, 5.6 mmol/L KCl, 2.3 mmol/L CaCl2 , 15 mmol/L HEPES, pH 7.4, 10 mg/mL BSA). Using surgical blades, the tissue was minced, transferred to a 50-mL plastic tube, pelleted, and resuspended in 0.1% (wt/vol) collagenase (CLS II, ca. 200 U/mL; Biochrom, Berlin, Germany) in buffer A. Tissue was digested for 30 minutes at 37°C under shaking in a waterbath. The digest was pelleted, resuspended in 40 mL of 25% BSA in buffer A, and centrifuged at 1,000g for 20 minutes at 4°C. The resultant capillary pellet was digested again in 1 mL 0.1% collagenase/dispase (Boehringer Mannheim, Germany) containing DNAse (1 mg/mL) for 10 minutes at 37°C. Capillary fragments were purified by centrifugation in Percoll density gradients exactly as described.35 The fragments were then resuspended in 10 mL MBE medium: DMEM enriched with 10% calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% nonessential amino acids, sodium pyruvate (1 mmol/L), L-glutamine (2 mmol/L), 5 × 10−5 2-mercaptoethanol (all additives from or PAN-Systems, Aidenbach, Germany), and 1% (vol/vol) bovine retinal extract37 as a crude source of endothelial cell growth factors. Capillaries were plated onto 5 collagen-coated 35-mm petri dishes (Falcon, Becton Dickinson). The capillary fragments were allowed to attach for 45 minutes in an incubator (37°C, 7% CO2 ). Cells in the supernatant were removed and plated onto fresh collagen-coated 35-mm petri dishes. Primary cultures of MBEs were used for assays starting at culture-day 1.
Isolation and culture of astrocytes.Cortices from neonatal rats or mice were removed, prepared free of meninges, and cut with curved scissors until tissue was homogenized. The slurry was resuspended in 10 mL PBS, transferred into an Erlenmeyer flask and washed by carefully stirring for 10 minutes at 37°C using a magnetic stir bar. Tissue was allowed to settle for 5 minutes. The supernatant was decanted. The pellet was resuspended in 10 mL PBS supplemented with 0.25% Trypsin/1 mg/mL EDTA/1% DNAse and digested for 30 minutes at 37°C under constant stirring. The digest was allowed to settle for 5 minutes. The supernatant was collected and digestion of the pellet repeated for up to 2 times. The astrocyte containing supernatants was treated separately. The trypsin digestion was stopped by adding 10% (vol/vol) CS. Cells were filtered through a 180 μm nylon mesh (Verseidag, Krefeld, Germany), pelleted, resuspended in DMEM/10% FCS, and plated onto 100-mm petri dishes at a concentration of 2 × 107 cells. Cells were grown to confluence in DMEM/10% FCS.
Astrocyte-conditioned MBE medium.Confluent astrocyte cultures were used to obtain astrocyte-conditioned supernatant. The culture medium was replaced by DMEM containing all additives with the exception of serum. Serum was replaced either by 1% BSA or by 5% PAN-colostrum (PAN-Systems, Aidenbach, Germany). The astrocyte-conditioned serum-free supernatant was removed on the 5th day of culture. Astrocyte-conditioned MBE-medium (ACM) was prepared by adding 20% (vol/vol) of the serum-free astrocyte-supernatant to the regular MBE medium.
Immunofluorescence staining of primary MBEs.For surface staining of membrane molecules MBEs cultures were washed with DMEM/25 mmol/L HEPES/5% CS and stained for 20 minutes with primary MoAb (5 μg in 500 μL) at room temperature. After two washes with DMEM/25 mmol/L HEPES/5% CS, FITC goat anti-rat IgG was added (10 μg/mL) and incubated for 20 minutes at room temperature. Cells were washed and fixed with 1% paraformaldehyde in PBS for 5 minutes. Cultures were rehydrated for 5 minutes in PBS. Cells were permeabilized using precooled (−20°C) methanol for 5 minutes at room temperature. Cells were counterstained with rabbit anti-human von Willebrand factor (vWF) antiserum (Dako, Hamburg, Germany). After two washes in PBS, goat anti-rabbit rhodamin was added for 20 minutes. Cells were washed twice and embedded in glycerol:PBS (9:1) containing 2.5% DABCO (1.4 Diazobicyclo(2.2.2)octan, Aldrich, Steinheim, Germany). Immunofluorescence staining was analyzed immediately. Semiquantitative analysis of E- and P-selectin induction on MBEs was performed by two investigators, one of whom was “blinded” to the respective treatments.
RESULTS
Lack of E- and P-selectin expression during EAE.Constitutive expression of P- or E-selectin could not be detected in the CNS of healthy SJL/J, Balb/C, and C57B1/6 mice by performing immunohistochemistry on frozen sections of brains and spinal cords, respectively. It should be noted that stainings were always controlled within the same experiment by including tissue, where expression of E- and P-selectin has been shown on endothelial cells before (see below). Induction of E- and P-selectin expression on vessels in the spinal cord and brain of SJL/J mice was then investigated at the timepoints indicated in Table 1 after initiation of aEAE. We could never show any expression of E- or P-selectin on vessels in the brain or spinal cord of SJL/J mice (Table 1 and Fig 1). In contrast, ICAM-1 and VCAM-1 expression was readily upregulated on cerebral endothelium of mice immunized with SCH starting at day 2 after immunization (data not shown).8 Expression of E- or P-selectin could also not be detected on vessels in the brain and spinal cord of mice undergoing the first clinical EAE episode (Table 1). Also, there was no expression of E- and P-selectin in the brain and spinal cord of mice undergoing a clinical EAE relapse and thus having developed chronic EAE. Nor did we detect induction of E- and P-selectin on vessels in the CNS of mice at any time after injection of encephalitogenic T cells to passively transfer EAE (data not shown).
We considered the possibility that transcriptional regulation of E- and P-selectin genes was different in cerebral vessels. To find out whether there was any sign of elevation of transcription of E- and P-selectin, we performed in situ hybridizations on frozen sections of brains and spinal cords of mice after the initiation of aEAE at the timepoints indicated in Table 1. Again, within each experiment tissue was included where transcriptional induction of E- and P-selectin expression had been shown on endothelial cells before (see below).38 We did not detect any mRNA for E- or P-selectin in endothelium of the brain or spinal cord of healthy SJL/J mice or in the brains and spinal cords of mice at any timepoint after induction of EAE or during clinical EAE (Table 1 and Fig 2). In contrast to this, mRNA for VCAM-1 can easily be detected on cerebral vessels during EAE (Fig 3). Thus, there is no detectable expression of E- and P-selectin on endothelial cells within the CNS of healthy mice, and detectable induction of E- and P-selectin expression is lacking during the development and clinical course of EAE in the SJL/J mouse.
Expression of E- and P-selectin ligands on inflammatory cells recruited into the CNS of EAE animals.To determine if encephalitogenic T cells mediating tEAE and inflammatory cells present in the CNS during EAE also lack E- and P-selectin ligands, we performed FACS analysis on encephalitogenic T-cell lines and inflammatory cells isolated from the brains and spinal cords of mice afflicted with EAE using P-selectin– and E-selectin–Ig chimeras. In three independent experiments we found that 4% ± 0.3% and 37% ± 0.5% of CD45+ inflammatory cells isolated from the CNS of mice afflicted with EAE stained positive above background levels obtained with the control-Ig chimera using the E-selectin–Ig chimera and the P-selectin–Ig chimera, respectively (Fig 4). When investigating the Thy 1.2+ T-cell population recruited into the CNS we found staining for E-selectin ligands on 3.5% ± 0.2% and for P-selectin ligands on 35% ± 2% Thy1.2+ CNS-seeking T cells. Thus, compared with peripheral or mesenteric lymph node cells of the same animals, the inflammatory cells present in the CNS during EAE showed an enrichment for leukocytes expressing P-selectin ligands among both the CD45+ as well as among the Thy1.2+ population (Fig 4), also to a small degree there might be some enrichment for leukocytes expressing E-selectin ligands. Performing FACS analysis using the same selectin-Ig chimeras, we found that ligands for E- and P-selectin are also expressed on a subpopulation of T cells within the encephalitogenic T-cell lines SJL.MBP1 and SJL.PLP1. Up to 4% T cells of both T-cell lines express ligands for E-selectin and less than or equal to 25% ligands for P-selectin as detected with the selectin-Ig constructs (Fig 5). Therefore, lack of E- and P-selectin expression on BBB endothelium does not correspond to a lack of expression of selectin ligands on leukocytes that are able to cross the BBB.
Development of tEAE in SJL/J Mice Treated With Anti–E-Selectin and Anti–P-Selectin MoAbs
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease4-150 . | No. of Animals With Detectable Infiltrates in CNS4-151 . |
|---|---|---|---|---|
| ⊘ | 2 | 2 | 8 | 2 |
| IgG | 5 | 5 | 8 + 0.5 | 5 |
| anti-MHC II | 2 | 1 | 9 | 1 |
| anti-CD4 + anti-MHC II | 3 | 0 | no disease | 0 |
| PS/2 | 5 | 1 | 14 | 1 |
| RB40 + UZ4 | 5 | 5 | 7.5 + 0.5 | 5 |
| Treatment . | No. of Animals . | No. of Animals Developing Clinical Disease . | Mean Day of Onset of Clinical Disease4-150 . | No. of Animals With Detectable Infiltrates in CNS4-151 . |
|---|---|---|---|---|
| ⊘ | 2 | 2 | 8 | 2 |
| IgG | 5 | 5 | 8 + 0.5 | 5 |
| anti-MHC II | 2 | 1 | 9 | 1 |
| anti-CD4 + anti-MHC II | 3 | 0 | no disease | 0 |
| PS/2 | 5 | 1 | 14 | 1 |
| RB40 + UZ4 | 5 | 5 | 7.5 + 0.5 | 5 |
Only animals developing clinical disease were taken into account.
Animals were sacrificed 3 days after onset of clinical disease; animals in the treatment groups anti-CD4/MHC II and PS/2 were sacrificed at days 10 postimmunization (p.I.), 12 p.I., and 15 p.I. respectively; animals in the treatment group anti-MHC II were killed at days 10 and 15 p.I.
Antibodies directed against E- and P-selectin do not influence the development of EAE.To find out whether E- or P-selectin could still be involved in the recruitment of inflammatory cells into the CNS despite the lack of detectable E- and P-selectin expression on vessels within the CNS parenchyme, we investigated the effect of inhibitory MoAbs directed against E- and P-selectin on the development of EAE. Neither the single nor the repeated IV injection of MoAbs directed against either P-selectin (RB40.34) or E-selectin (UZ4) or the injection of a cocktail of both MoAbs interfered with the development of aEAE. Similarly, the same MoAbs did not influence the development of tEAE by injection of activated encephalitogenic T-cell lines SJL.MBP1 or SJL.PLP1 into naive SJL/J mice (Tables 2, 3, and 4). In contrast, in the very same experiments MoAb PS/2 directed against α4-integrin inhibited the development of tEAE (Table 4) or significantly delayed the onset of aEAE after a single injection on day 9 postimmunization (Table 3). Neither the onset of the disease nor the recruitment of inflammatory cells into the CNS of animals treated with anti–E-selectin or P-selectin MoAbs were altered when compared with sham-treated control animals (Tables 2, 3, and 4; Fig 6 [see page 4462]). In contrast, inflammatory cuffs could not be detected in the CNS of mice treated with MoAb directed against α4-integrin (Fig 6). Immunohistochemical analysis of frozen sections of the brains and spinal cords of mice treated with selectin antibodies in comparison with control EAE animals or sham-treated EAE animals did not reveal any difference in the amount, size, or composition of the inflammatory infiltrates, which consisted mainly of CD4+ T cells, Mac-1+ macrophages, some B220+ B cells, and rare CD8+ T cells. Thus, inhibition of the interaction of E- and P-selectin with their respective ligands in vivo did not alter the development of EAE.
Impaired inducibility of E- and P-selectin on BBB endothelium in vivo.To define whether BBB-forming endothelium generally lacks the capability to express E- and P-selectin, we compared the inducibility of E- and P-selectin on the BBB with their inducibility on vessels outside the CNS. Inducibility of E- and P-selectin in the EAE-susceptible SJL/J mouse was furthermore compared with their inducibility in Balb/c and C57B1/6 mice. Mice were injected IV with TNF-α (50 μg) or LPS (50 μg), both of which have previously been shown to induce E- and P-selectin transcription and protein expression in vivo. Mice were sacrificed 3 hours or 4 hours after injection of either LPS or TNF-α and expression of E- and P-selectin was investigated immunohistochemically. Positive staining for E-selectin could not be detected in untreated animals; however, some endothelial cells outside the CNS showed intracellular staining for P-selectin, thus detecting the stored P-selectin in the Weibel-Palade bodies. In cytokine-injected mice, both E- and P-selectin were easily detectable on vessels in kidney, skin, gut, immune organs, and tongue (Tables 5, and 6; Fig 7) but not on vessels within the CNS parenchyme (Table 3). We did not observe significant differences in the inducibility of E- and P-selectin when comparing the 3 different mouse strains, the 2 treatments, and the 2 timepoints, respectively.
Induction of E- and P-Selectin Protein in LPS- or TNF-α Treated Mice
| Organ . | E-Selectin . | P-Selectin . |
|---|---|---|
| Brain + spinal cord | ⊘ | ⊘ |
| Kidney | On glomerular capillaries | On large venules |
| Tongue | Many venules + some capillaries | On large venules |
| Gut | Venules + some capillaries | +/− on some venules |
| Skin | On Capillaries and some venules | +/− on some venules |
| Spleen | On arterioles in white pulp | ⊘ |
| Thymus | On venules | Trabecular venules |
| Lymph node | +/− on HEVs | +/− on HEVs |
| Organ . | E-Selectin . | P-Selectin . |
|---|---|---|
| Brain + spinal cord | ⊘ | ⊘ |
| Kidney | On glomerular capillaries | On large venules |
| Tongue | Many venules + some capillaries | On large venules |
| Gut | Venules + some capillaries | +/− on some venules |
| Skin | On Capillaries and some venules | +/− on some venules |
| Spleen | On arterioles in white pulp | ⊘ |
| Thymus | On venules | Trabecular venules |
| Lymph node | +/− on HEVs | +/− on HEVs |
In total 12 mice consisting of 4 mice each of the strains SJL/J, C57Bl/6, and Balb/C mice were investigated. From each mouse strain 4 animals were sacrificed, tissue was removed and snap frozen after the following 4 treatments: IV TNF-α (50 μg, 3 hours), IV TNF-α (50 μg, 4 hours), IV LPS (50 μg, 3 hours), and IV LPS (50 μg, 4 hours). We did not observe significant differences in the inducibility of E- and P-selectin comparing the different mouse strains, treatments, and timepoints investigated.
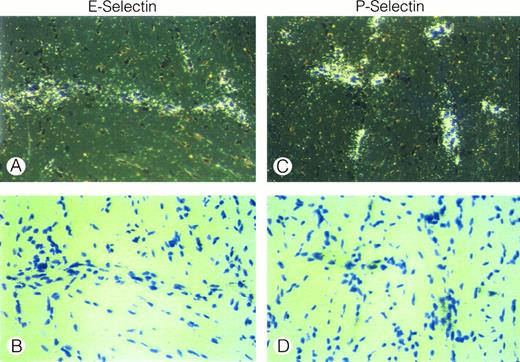
E- and P-selectin expression can be induced in peripheral vessels outside the CNS after injection of LPS or TNF-α. Immunoperoxidase staining for E- and P-selectin in the tongue of a SJL/J mouse 4 hours after injection of 50 μg LPS is shown. ⊘, isotype matched control. Immunoperoxidase staining, hematoxylin counterstain. Original magnification × 420.
E- and P-selectin expression can be induced in peripheral vessels outside the CNS after injection of LPS or TNF-α. Immunoperoxidase staining for E- and P-selectin in the tongue of a SJL/J mouse 4 hours after injection of 50 μg LPS is shown. ⊘, isotype matched control. Immunoperoxidase staining, hematoxylin counterstain. Original magnification × 420.
To define whether TNF-α and LPS fail to induce E- and P-selectin on the transcriptional level in BBB endothelium we then performed in situ hybridizations comparing hybridization on frozen sections of brain and spinal cord with hybridization on frozen sections of kidney and tongue of SJL/J mice injected with LPS or TNF-α 3 hours or 4 hours before. Sections of brain, kidney, and tongue of a LPS-injected Balb/C mouse were hybridized as a control. Whereas a strong hybridization signal for E- and P-selectin was observed on vessels of the kidney and tongue of all injected animals (Tables 5 and 6; Fig 8), we did not see positive hybridization for E- and P-selectin on vessels within the CNS parenchyme. We conclude that, in vivo, expression of E- and P-selectin cannot be induced in endothelial cells within the CNS parenchyme of mice.
Induction of E- and P-Selectin mRNA in LPS- or TNF-α Treated Mice
| Organ . | E-Selectin . | P-Selectin . |
|---|---|---|
| Brain + spinal cord | ⊘ | ⊘ |
| Kidney | On glomerular capillaries | On large venules |
| Tongue | Many venules and capillaries | Many venules and capillaries |
| Organ . | E-Selectin . | P-Selectin . |
|---|---|---|
| Brain + spinal cord | ⊘ | ⊘ |
| Kidney | On glomerular capillaries | On large venules |
| Tongue | Many venules and capillaries | Many venules and capillaries |
In total 4 SJL/J mice and 1 Balb/c mouse were investigated. Animals were sacrificed, tissue was removed and snap frozen after the following 4 treatments: IV TNF-α (50 μg, 3 hours), IV TNF-α (50 μg, 4 hours), IV LPS (50 μg, 3 hours), and IV LPS (50 μg, 4 hours). The Balb/c mouse was investigatd after 4 hours LPS. We did not observe significant differences in the inducibility of E- and P-selectin comparing the different mouse strains, treatments, and timepoints investigated.
E- and P-selectin gene expression is induced in peripheral vessels outside the CNS after injection of LPS or TNF-α. In situ hybridization analysis of E- and P-selectin mRNA expression in the tongue of a SJL/J mouse 4 hours after injection of 50 μg LPS is shown. (a), dark field; (b), bright field; (c), dark field; (d), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
E- and P-selectin gene expression is induced in peripheral vessels outside the CNS after injection of LPS or TNF-α. In situ hybridization analysis of E- and P-selectin mRNA expression in the tongue of a SJL/J mouse 4 hours after injection of 50 μg LPS is shown. (a), dark field; (b), bright field; (c), dark field; (d), bright field. Sections are counterstained with toluidine blue. Original magnification × 480.
Induction of E- and P-selectin on mouse brain endothelium in vitro is suppressed by astrocyte-derived factors.Primary cultures of MBEs were established to determine whether BBB-forming endothelial cells have completely lost the capacity to express E- and P-selectin during differentiation. Primary MBE cultures contained up to 40% nonendothelial cells, such as pericytes and rarely astrocytes. MBEs were identified by positive staining for vWF and PECAM-1. Primary MBEs did not express E- or P-selectin on their surface as determined by immunofluorescence staining of living cells. However, treatment of MBE cultures with LPS, TNF-α, or Il-1, interleukin-1 (IL-1) but not with interferon γ (IFN-γ), for 3 or 4 hours induced expression of E- and P-selectin on the surface of some but not all MBE cells in vitro (Fig 9, Table 7). More MBEs stained positive for E- and P-selectin expression after IL-1 and TNF-α treatment compared with LPS treatment, and the staining on single cells was brighter. Inducibility of E- and P-selectin on MBEs in vitro suggested that the CNS microenvironment might be responsible for the lack of inducibility of E- and P-selectin on cerebral endothelial cells in vivo. Thus, to mimic at least part of the CNS microenvironment in vitro, MBEs were cultured in ACM. Inducibility of E- and P-selectin after LPS, TNF-α, and IL-1 treatment on the surface of MBEs was then compared on MBEs grown in the presence or absence of ACM. When MBEs were cultured in ACM for at least 5 days, LPS-induced induction of both selectins was almost completely abolished compared with induction of both selectins on MBEs grown in absence of ACM (Fig 9). Incubation of MBEs in ACM for less than 5 days did not show a significant influence on the inducibility of E- and P-selectin expression on MBEs in vitro, and neither did growth of MBEs in fibroblast-conditioned medium (data not shown). Induction of E- and P-selectin on MBEs after treatment with IL-1 or TNF-α was suppressed by ACM in such a way that E- and P-selectin were induced on fewer cells and with a reduced staining intensity (Table 7). Only primary cultures of MBEs showed responsiveness to ACM as inducibility of E- and P-selectin was not influenced by ACM on passaged cultures of MBEs. Taken together, these data indicate that expression of both E- and P-selectin on cerebral endothelial cells is suppressed by soluble factors derived from astrocytes.
Induction of E- and P-selectin on primary MBEs in vitro. The same cells are shown from left to right: surface, green fluorescence membrane–staining of live MBEs; vWF, red fluorescence–staining of intracellular vWF in fixed MBEs. E- and P-selectin can be induced on the surface of some vWF-positive MBEs after treatment with LPS (10 μg/mL; 3 hours). No LPS mediated induction of E- and P-selectin when MBEs were cultured in astrocyte conditioned medium (+ ACM) for 5 days. Original magnification × 400.
Induction of E- and P-selectin on primary MBEs in vitro. The same cells are shown from left to right: surface, green fluorescence membrane–staining of live MBEs; vWF, red fluorescence–staining of intracellular vWF in fixed MBEs. E- and P-selectin can be induced on the surface of some vWF-positive MBEs after treatment with LPS (10 μg/mL; 3 hours). No LPS mediated induction of E- and P-selectin when MBEs were cultured in astrocyte conditioned medium (+ ACM) for 5 days. Original magnification × 400.
Inducibility of E- and P-Selectin on Primary MBEs Is Reduced by ACM
| Treatment . | E-Selectin . | E-Selectin + ACM . | P-Selectin . | P-Selectin + ACM . |
|---|---|---|---|---|
| LPS (n = 7) | ||||
| (10 μg/mL, 3 h) | + | ⊘ to +/− | +/− | ⊘ |
| IL-1 (n = 3) | ||||
| (5 ng/mL, 3 h) | ++ | + | + | +/− |
| TNF-α (n = 3) | ||||
| (5 nmol/L, 3 h) | + | +/− | +/− | +/− to ⊘ |
| Treatment . | E-Selectin . | E-Selectin + ACM . | P-Selectin . | P-Selectin + ACM . |
|---|---|---|---|---|
| LPS (n = 7) | ||||
| (10 μg/mL, 3 h) | + | ⊘ to +/− | +/− | ⊘ |
| IL-1 (n = 3) | ||||
| (5 ng/mL, 3 h) | ++ | + | + | +/− |
| TNF-α (n = 3) | ||||
| (5 nmol/L, 3 h) | + | +/− | +/− | +/− to ⊘ |
Semiquantitative analysis of surface immunofluorescence staining on vWF-positive MBEs. Staining for PECAM-1 as a positive control was considered +++ (see Fig 9): +++, strongly positive staining; ++, positive staining; +, low staining intensity; +/−, very low staining intensity; ⊘, no staining.
DISCUSSION
Our study provides the first evidence that E- and P-selectin are not involved in the immigration of encephalitogenic T cells across the “normal” BBB or the recruitment of inflammatory cells across the “stimulated” BBB at later stages during the development of EAE. There is no E- and P-selectin expression on the CNS microvasculature of mice afflicted with EAE, and MoAbs which inhibit the interaction of E- and P-selectin with their known ligands, do not influence the development of EAE. Furthermore, in contrast to vessels in other organs, E- and P-selectin expression cannot be induced by TNF-α or LPS on vessels within the CNS parenchyme in vivo. Lack of inducibility of E- and P-selectin expression is not caused by a general lack of brain endothelial cells to express E- and P-selectin but rather to a suppressive influence provided by the CNS microenvironment.
Our first observation was that there is no constitutive expression of E- and P-selectin in the microvasculature of the CNS in the investigated mouse strains SJL/J, Balb/C, and C56B1/6, which is consistent with previous findings by others.36,38 Whereas E-selectin has not been reported to be constitutively expressed, P-selectin is usually constitutively expressed by endothelial cells and stored in the endothelial cell–specific storage granules the Weibel-Palade bodies. Weibel-Palade bodies also contain vWF, which we and others showed immunohistologically in a subset of cerebral vessels in vivo and in primary cultures of MBEs in vitro.36 Lack of constitutive expression of P-selectin in the CNS microvasculature therefore is not due to a lack of Weibel-Palade bodies, but rather Weibel-Palade bodies of CNS endothelium are devoid of P-selectin. Contents of the Weibel-Palade bodies are translocated to the cell surface of the endothelium within minutes upon stimulation. A lack of stored P-selectin in Weibel-Palade bodies of the endothelium forming the BBB provides evidence that the BBB lacks a rapid pathway of leukocyte adhesion and thus protects the CNS against the rapid influx of potentially dangerous leukocytes across the stimulated BBB.
In vitro, the induction of E- and P-selectin on the surface of endothelial cells by proinflammatory cytokines precedes the induction of ICAM-1 and VCAM-1 expression.39 In EAE, ICAM-1 and VCAM-1 have been shown to be induced on cerebral endothelial cells already two days after induction of EAE (Schulz and Engelhardt, unpublished observations).8 11 Thus, we investigated the induction of E- and P-selectin expression on the protein and mRNA level at very early timepoints after induction of EAE. However, at no timepoint before or during clinical EAE could we detect any E- or P-selectin expression immunohistochemically or by in situ hybridization.
The lack of E- and P-selectin on BBB endothelium led us to the question whether inflammatory cells recruited into the CNS during EAE are also devoid of selectin ligands. It has been shown that inflammatory cells recruited into the CNS express a distinct phenotype34,40 and can be easily differentiated from inflammatory cells recruited into other tissues based on their expression of CAMs (Engelhardt et al, submitted). To our surprise, a minor subpopulation of CD45+ inflammatory cells present in the CNS during clinical EAE seem to express E-selectin ligands, and almost half of the inflammatory cell population expressed P-selectin ligands as detected by immunofluorescence staining using selectin-Ig chimeras. Focusing on the inflammatory Thy1.2+ T cells present in the CNS during EAE revealed an E- and P-selectin ligand-positive phenotype comparable with that recently described for TH1 cells homing to the inflamed skin in the mouse.25 However, whereas antibodies directed against E- and P-selectin inhibited the recruitment of TH1 cells into inflamed skin in vivo,25 the same antibodies did not influence recruitment of inflammatory cells into the CNS during EAE. These results underline the lack of an involvement of E- and P-selectin and their ligands in the recruitment of inflammatory cells across the BBB during EAE.
We then investigated whether as a consequence of BBB differentiation cerebral endothelial cells lose the ability to express E- and P-selectin. BBB endothelial cells differ from other endothelial cells as they have been shown to lack storage of P-selectin in their Weibel-Palade bodies.3 Besides the constitutive expression of P-selectin, both P- and E-selectin expression can be induced on the transcriptional level by proinflammatory cytokines such as TNF-α, IL-1, and LPS in vivo and in vitro.38 39 Consistent with these reports we could induce E- and P-selectin at the mRNA and protein level on the microvasculature of many organs but never on BBB-forming vessels within the CNS parenchyme.
Lack of E- and P-selectin inducibility on BBB-forming endothelial cells could be either an intrinsic characteristic of these highly specialized endothelial cells or brought about by suppressive influences provided by the CNS microenvironment. It is known that BBB characteristics of endothelial cells are strictly dependent on the presence of the CNS microenvironment.41 When cerebral endothelial cells are isolated and put into culture they rapidly lose part of their BBB characteristics,42 which can partially be restored in coculture with astrocytes or in presence of astrocyte conditioned medium.42 43 Treatment of primary cultures of MBEs with LPS, TNF-α, or IL-1 leads to induction of E- and P-selectin on the surface of some, but not on all, MBEs. Induction of E- and P-selectin was, however, suppressed by astrocyte-derived factors in vitro and therefore suggests that induction of endothelial selectins in vivo is actively suppressed by the CNS microenvironment.
Our findings suggest that leukocyte traffic across the highly differentiated vascular wall into the CNS is controlled by the suppression of E- and P-selectin to reduce the number and to control the subtype of infiltrating cells during inflammation. As the encephalitogenic T-cell lines SJL.PLP1 and SJL.MBP1 lack expression of L-selectin (Hoch and Engelhardt, unpublished observation) transmigration of those T cells across the normal BBB occurs in absence of any known selectin interaction. Lack of selectin expression therefore implies that other molecular interactions must exist that mediate the tethering of the circulating autoaggressive T lymphocyte to the BBB endothelium to slow them down for subsequent interactions with the BBB endothelium. Interestingly, α4-integrin has been shown to mediate interaction of T lymphocytes with both its endothelial ligands, VCAM-1 and MAdCAM-1, under physiological flow in vitro44,45 and to support leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo.46 We and others have shown that in vivo treatment of EAE with antibodies directed against α4-integrin inhibits the development of clinical EAE and more importantly of inflammatory infiltrates in the CNS.12,13 Whereas VCAM-1 is expressed on the microvasculature of the CNS during EAE,8,14 the other α4-integrin ligands, MAdCAM-147 and α4-integrin itself,48 have not been shown on the luminal surface of BBB-endothelium (Schulz and Engelhardt, unpublished observation).8 Therefore, the interaction of α4β1-integrin with VCAM-1 might play a decisive role in the interaction of BBB endothelium with circulating CNS-seeking leukocytes by mediating both their initial tethering and rolling plus their firm adhesion to the BBB endothelium.
In summary, this study shows that immigration of inflammatory cells into the CNS across the normal and the stimulated BBB does not involve E- and P-selectin. It is tempting to speculate that suppression of E- and P-selectin expression at the BBB prevents more destructive leukocytes, such as neutrophils,22 from entering the CNS, and thus ensures a reduction of tissue damage during inflammation.
ACKNOWLEDGMENT
We thank Michael Minne and Georg Breier for providing reagents for the in situ hybridizations. Gregor Fachinger is acknowledged for providing the HT7-Ig chimera. Werner Risau and Urban Deutsch are gratefully acknowledged for their critical discussion of the manuscript.
Supported by the DFG-grant En214/3-2 (M.S.), SFB 263 (R.H.), and SFB 293 (D.V.).
Address reprint requests to Britta Engelhardt, PhD, Max-Planck Institut für physiologische und klinische Forschung, W.G. Kerckhoff-Institut, Abt. Molekulare Zellbiologie, Parkstraβe 1, D-61231 Bad Nauheim, Germany.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal