Abstract
Thrombopoietin (Tpo) has proliferative and maturational effects on immature and more committed cells, respectively. We previously reported a role for Tpo as a survival factor in the factor-dependent human cell line M07e by demonstrating that Tpo suppresses apoptosis in the absence of induced proliferation. Wild-type p53 is a tumor suppressor gene that can play a vital role in mediating growth factor withdrawal-induced apoptosis in factor-dependent hematopoietic cells. Wild-type p53 can switch from a suppressor conformation, with an antiproliferative, pro-apoptotic phenotype, to a promoter conformation that has a diminished ability to mediate cell cycle arrest and apoptosis. In an effort to elucidate the mechanisms through which Tpo suppresses apoptosis, we investigated the effects of Tpo treatment on p53-mediated apoptosis in M07e cells. Tpo upregulated the expression of the promoter conformation of p53 in M07e cells coincident with a downregulation of Bax and Mdm2 protein levels. Protein levels of Bcl-2 and Bcl-xL did not significantly vary as a function of growth-factor stimulation. Conversely, the levels of suppressor conformation p53 were maximal when M07e was in a growth arrested state and decreased during factor stimulation. Furthermore, Tpo treatment induced an extranuclear buildup and greatly weakened the DNA binding capacity of p53. p53-specific antisense oligonucleotide treatment recapitulated the effects of Tpo treatment on the levels of Bax, Mdm-2, and Bcl-2. These results suggest that Tpo is suppressing growth factor withdrawal induced-apoptosis, at least in part, by downregulating the expression of pro-apoptotic Bax protein levels, through modulating the conformation of p53, which results in a functional inactivation of its pro-apoptotic abilities.
REGULATION OF hematopoietic cell numbers in vivo involves a delicate balance between extracellular signals that induce either the survival/proliferation or death of target cells.1 Superfluous or unwanted cells of the hematopoietic compartment undergo apoptosis in response to a variety of negative stimuli. Examples of these include soluble cytokines or cell surface biomolecules, which deliver death signals through ligand-receptor interaction, such as tumor necrosis factor-α (TNF-α) and Fas.2 Another process of this type results from the deprivation of factors that maintain the integrity of the cell, termed growth factor withdrawal-induced apoptosis.3 Survival-promoting cytokines, such as interleukin-3 (IL-3), granulocyte-macrophage colony stimulating factor (GM-CSF), thrombopoietin (Tpo), or steel factor (SLF) maintain cellular viability by abrogating the apoptotic process, which naturally ensues following growth factor withdrawal in vitro.4-8 Cells that lose their dependency on paracrine signaling for viability become orphaned from the homeostatic process and eventually may give rise to a pathologic state in vivo.9 The biochemical mechanisms underlying growth factor withdrawal-induced apoptosis are poorly understood, although a number of highly prolific areas of apoptosis research have emerged in the last few years.
One particular field of interest has centered around the DNA-binding tumor-suppressor protein p53, which has been established as a critical regulatory component of the apoptotic, cell cycle arrest and DNA damage response machinery.10-12 Wild-type p53 is a transcriptional activator of certain genes whose protein products are involved in these different processes, such as Bax, p21waf1/cip1, and Gadd45, respectively.13-15 Interestingly, wild-type p53 has been shown to be a potent molecular mediator of growth factor withdrawal-induced apoptosis in growth factor-dependent hematopoietic cells.16-18 In addition, the over-expression of wild-type p53 in p53-deficient leukemic cells induces apoptosis that is rescuable by hematopoietic cytokines such as SLF, IL-3, IL-6, and erythropoietin (Epo),10,19,20 while the introduction of dominant negative mutant p53 retards the apoptotic process following growth factor withdrawal, supporting the idea that p53 may have an important role in conferring growth factor dependency in hematopoietic cells.18
Wild-type p53 exists as a conformationally flexible phosphoprotein that can be immunostained with monoclonal antibodies that are sensitive to conformational changes.21-23 It has been reported that following growth factor stimulation, wild-type p53 can switch from a suppressor conformation,24 associated with an antiproliferative, pro-apoptotic phenotype, to a promoter conformation,24 that has a diminished ability to retard cellular proliferation and mediate apoptosis.22,25,26 The ability of p53 to be immunostained with mutant, or promoter conformation-specific antibodies does not reflect a germline alteration in the p53 gene and reinforces the notion that immunodetection of p53 with promoter conformation-specific antibodies should not be considered diagnostic of mutation.27
Another family of proteins associated with growth factor withdrawal-induced apoptosis has emerged, having structural, and sometimes functional homology with Bcl-2, a 26-kD protein that in the membrane bound form antagonizes the apoptotic process following growth factor withdrawal in most but not all cell systems,28 possibly in part by blocking the activation of ICE and ICE-like proteases.29 Some members of this family have antiapoptotic attributes, such as the product of the long splice variant of the bcl-x gene, Bcl-xL .30 p21 Bax, a pro-apoptotic protein, is also found associated with internal membrane structures and was the first member of this family reported to have activities antagonistic to Bcl-2.31 High Bax levels can titre out the antiapoptotic phenotype of Bcl-2. Within this system, the ratio of Bax to Bcl-2 dictates cell fate with regard to the commitment to apoptosis. A cell could suppress apoptosis by either elevating Bcl-2 levels, by diminishing the levels of Bax, or both. Interestingly, p53 can modulate the transcription of both bcl-2 and bax in vivo and in vitro.32
Tpo, also known as megakaryocyte growth and development factor (MGDF) or the c-mpl ligand, is a hematopoietic cytokine that acts in the induction of megakaryocyte maturation and platelet formation in vivo and in vitro.33,34 Tpo synergizes with other cytokines in the induction of progenitor cell proliferation within the myeloid compartment, and is a potent inducer of megakaryocyte, as well as other myeloid, progenitor cell expansion.8,33,35-37 Tpo is therefore believed to be a critical thrombopoietic and megakaryocytopoietic regulator in vivo. We recently reported that Tpo behaves as a survival factor for M07e by suppressing growth factor withdrawal-induced apoptosis in the absence of induced proliferation.8 M07e is a growth factor-dependent myeloid cell line derived from a patient with acute megakaryoblastic leukemia that expresses both early progenitor and platelet-specific cell surface markers.38 In an effort to elucidate potential mechanisms through which Tpo suppresses apoptosis, we found that Tpo up-regulates the promoter conformation of p53 in M07e cells. Coincident with this, we found that Tpo treatment of M07e markedly decreases the expression of Bax, providing a phenotypic advantage to the antiapoptotic members of the Bcl-2 family.
MATERIALS AND METHODS
Cytokines and reagents. Recombinant human (rhu) GM-CSF was a kind gift from Immunex Corp (Seattle, WA). rhuTpo was kindly provided by Zymogenetics, Seattle, WA. Concentrations used in these assays were 100 U/mL for GM-CSF and 50 ng/mL for Tpo. Dilipidated, deionized, and dialyzed bovine serum albumin (BSA), insulin, saturated human transferrin, low-density lipoprotein (LDL), paraformaldehyde, sucrose, trypan blue, HEPES, DTT, EDTA, and saponin were purchased from Sigma Chemical Co (St Louis, MO).
Cells. The human growth factor-dependent subline, M07e, was obtained from Genetics Institute (Boston, MA). The M07e subline, and its parental line M07, have been biologically characterized previously.38-40 The culture and factor-starvation conditions for M07e have also been described in detail.40 41 Serum-free media consisted of RPMI 1640 (Bio Whittaker) with 2% dilipidated, deionized, and dialyzed BSA, 10 μg/mL insulin, 200 μg/mL saturated human transferrin, 50 μmol/L β-mercaptoethanol, 40 μg/mL LDL.
Antisense oligonucleotides. The p53-specific 18-mer oligonucleotides were synthesized by an automated process (Dr Mark Marshall, Indiana University School of Medicine, Indianapolis) and purified. The sequences of the p53-specific oligonucleotides were: 5′-CGG CTC CTC CAT GGC AGT-3′ (antisense); 5′-ACT GCC ATG GAG GAG CCG-3′ (sense). The target region of p53 mRNA, the affinity for the target region, and the translation inhibition efficiency have been described previously.42 43 The oligonucleotides were resuspended at a final concentration of 5 μmol/L in serum-free assay medium. Cell viability was measured using the trypan blue exclusion test.
Flow cytometric analysis. Cells were collected at the indicated time points for flow cytometric analysis, washed, and simultaneously permeabilized and fixed in a 4% paraformaldehyde, 0.1% saponin 10 mmol/L HEPES solution for 15 minutes at 4°C. Cells were stained with 1 μg/mL of the primary antibodies for 20 minutes at 4°C, followed by incubation with the appropriate secondary fluorescently labeled antibody for 20 minutes. Cells were then washed and resuspended in 4% paraformaldehyde 10 mmol/L HEPES solution for flow cytometric analysis. This methodology has been demonstrated to provide good nuclear staining in our group.
Antibodies and Western blotting. Antibodies used in these studies include α-p53 (Pab 1620), an IgG2a murine monoclonal that recognizes the suppressor form of p53; α-p53 (Pab 240), an IgG1 murine monoclonal that recognizes the promoter form of p53 (both from Oncogene Science); α-p53 (clone DO-1), an IgG2a murine monoclonal that recognizes both suppressor and promoter forms of p53 (Santa Cruz Biotechnology, Inc); α-p53 (Clone Ab 7), a sheep polyclonal recognizing human and murine p53 (Oncogene Science); α-Bcl-2 (clone 100), an IgG1 murine monoclonal that recognizes human Bcl-2 (Santa Cruz Biotechnology, Inc); α-Bcl-xL (clone E-20), a goat polyclonal IgG recognizing Bcl-xL but not Bcl-xS (Santa Cruz Biotechnology, Inc); α-Mdm2 (clone N-20), a rabbit polyclonal IgG recognizing human Mdm2 (Santa Cruz Biotechnology, Inc); α-Bax (clone Ab-1), a rabbit polyclonal IgG recognizing human or murine Bax (Oncogene Science); and α-GAPDH (clone 6C5), a murine monoclonal recognizing gluteraldehyde-3-phosphate dehydrogenase (Biodesign Int). All secondary, HRP-labeled antibodies were purchased from Amersham Life Science. Samples analyzed by Western blot were adjusted for total protein content and equal protein amounts were loaded per well as previously described.44 Samples were electrophoresed and transferred to Immobilon-P membranes (Millipore), blocked with 2% BSA, probed, and developed with ECL Western blot detection reagent kit (Amersham Life Science).
Nuclei isolation. Nuclei were isolated by layering whole cell suspension over 10 mL of 1.6 mol/L sucrose and 3 mmol/L CaCl2 that was initially layered over 2.0 mol/L sucrose and 3 mmol/L CaCl2.45 Samples were centrifuged at 50,000g for 30 minutes after which the supernatant was removed and the pelleted nuclei resuspended in 0.25 mol/L sucrose and 3 mmol/L CaCl2 . For the comparison between nuclear to whole cell levels of p53, the number of nuclei and whole cells were adjusted equally to 5 × 107 per mL and mixed 1:1 with sodium dodecyl sulfate polyacrilamide gel electrophoresis (SDS-PAGE) sample buffer. Five × 105 of either nuclei or whole cells were loaded per lane and analyzed by Western blot analysis as described.
Electric mobility shift assay (EMSA). EMSA was carried out as described previously.46 47 Briefly, nuclei from M07e cells treated with either control media or cytokine were isolated as described and adjusted to contain equal amounts of p53 by Western blot analysis. Nuclear lysates were incubated with 10,000 cpm end-labeled double-stranded oligonucleotides containing normal p53 DNA target sequence (5′-TAC AGA ACA TGT CTA AGC ATG CTG GGG ACT-3′ ), plus 1 μg salmon sperm DNA, 5 mmol/L DTT, 1 mmol/L EDTA, 100 molar excess mutated DNA target sequence (5′-TAC AGA ATC GCT CTA AGC ATG CTG GGG ACT-3′ ) (Santa Cruz Biotechnology), or 100 molar excess cold competitor. Reaction samples were run on a 4% PAGE in TBE.
RESULTS
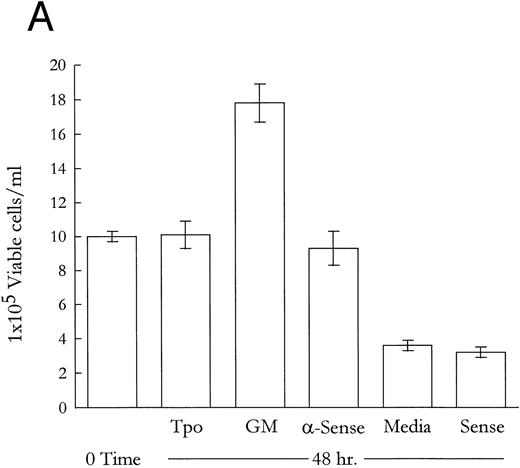
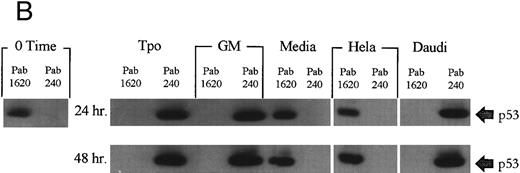
M07e expresses phenotypically wild-type p53 that is required for growth factor withdrawal-induced apoptosis. To confirm that the reported role of p53 in mediating growth factor withdrawal-induced apoptosis is functional in our system, M07e cells were treated with p53-specific antisense oligonucleotides. Figure 1A shows that after 48 hours, cells that were treated with antisense had a viability profile virtually indistinguishable from the Tpo-treated cultures. This suggested to us that Tpo-induced suppression of apoptosis may involve the functional inactivation of p53. In contrast, sense oligonucleotide or control serum-free media cultures underwent apoptosis and cell viability was approximately 25% of the initial innoculum by 48 hours. To confirm that the antisense oligonucleotides were blocking the translation of p53-specific mRNA, Western blot analysis revealed that cultures treated with antisense oligonucleotides had a barely detectable level of p53 protein in comparison with the other, noncytokine-treated cultures, such as sense oligonucleotide or control media treated cells, as shown in Fig 1B. GM-CSF was added in these studies as a proliferative control. Not shown, antisense treatment did not provide a proliferative advantage when coupled with Tpo or GM-CSF treatment. The high level of p53 expression in cytokine-stimulated cells could be due to the longer half life, or lower turnover rate, that the promoter conformation of p53 is reported to have.48 This set of data demonstrates that M07e contains phenotypically wild-type p53 that is required in this system for mediating growth factor withdrawal-induced apoptosis. With this information, we addressed the effects of Tpo treatment on p53 function in M07e cells.
Modulation of cell viability with a 48-hour treatment of M07e cells with p53-specific antisense oligonucleotides and Western blot analysis of the effects of p53 protein negation on the levels of protein products of p53-responsive genes in M07e cells. Following an 18-hour factor starvation period, 1 × 106 cells were treated for 48 hours with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), antisense oligonucleotide (5 μmol/L), control serum-free media, or sense oligonucleotide (5 μmol/L). (A) Cell viability was measured at 48 hours by trypan blue exclusion. Each data point was sampled in triplicate. (B) The levels of p53, Bax, Mdm-2, and Bcl-2 were analyzed by Western blot analysis as described in Materials and Methods. This figure represents data from one of three experiments which yielded similar results.
Modulation of cell viability with a 48-hour treatment of M07e cells with p53-specific antisense oligonucleotides and Western blot analysis of the effects of p53 protein negation on the levels of protein products of p53-responsive genes in M07e cells. Following an 18-hour factor starvation period, 1 × 106 cells were treated for 48 hours with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), antisense oligonucleotide (5 μmol/L), control serum-free media, or sense oligonucleotide (5 μmol/L). (A) Cell viability was measured at 48 hours by trypan blue exclusion. Each data point was sampled in triplicate. (B) The levels of p53, Bax, Mdm-2, and Bcl-2 were analyzed by Western blot analysis as described in Materials and Methods. This figure represents data from one of three experiments which yielded similar results.
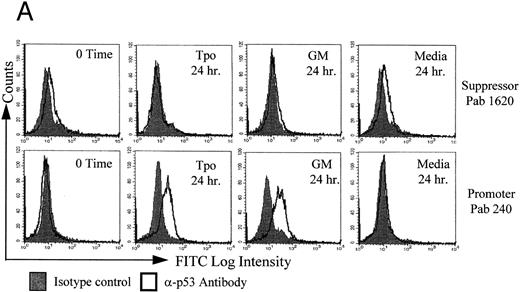
Tpo upregulates the promoter conformation of p53. Following an 18-hour factor starvation period to remove residual GM-CSF signals and phase the cells in G0/G1 , M07e cultures were stimulated for 24 hours with either Tpo, GM-CSF, or control serum free media. At time 0 and after 24 hours, M07e cells were analyzed by flow cytometry for the expression of immunologic variants of p53 using either suppressor-specific Pab 1620 or promoter-specific Pab 240, as shown in Fig 2A. At time 0, after confirming by cell cycle analysis that the cells were growth arrested and quiescent, no promoter species of p53 could be detected. However, the Tpo and GM-CSF treated cells showed a marked increase in p53 in the promoter conformation. In contrast, no upregulation of the promoter p53 isoform in control serum-free media-treated cells was detected. GM-CSF was added in this set of experiments as a proliferative control, to determine if the effect of cytokine signaling on p53 conformation was a function of growth stimulation in M07e. As shown in Fig 2B, we confirmed these results by immunoprecipitating p53 with either Pab 1620 or Pab 240, and then analyzed the immunoprecipitates by western blot using a sheep α-p53 antibody diluted 1:5000 that recognizes both conformations of p53, followed by a goat α-sheep that does not react with the immunoprecipitating murine IgG. The lack of Pab 1620+ p53, and high levels of Pab 240+ p53 at 48 hours in the cytokine-treated cultures indicates that the high levels of p53 shown in the cytokine-stimulated cultures in Fig 1B is indeed due to p53 being in the promoter conformation. Hela cell lysate, which contains wild-type p53, was used as a positive control for Pab 1620 and Daudi cell lysate, which contains mutant p53, as a positive control for Pab 240. The relatively lower level of p53 expression seen in factor-starved or control media cultures is most likely due to p53 being in the suppressor conformation. Growth-suppressive p53 has a very short half life.49 We have included the flow cytometric data in this report to demonstrate the ability to successfully detect p53 conformational changes using this technique in this cell line. Since we have shown previously and in this report (Fig 1A) that Tpo does not induce an appreciable amount of proliferation in M07e cells,8 this would indicate that the cytokine-induced change in conformation in p53 is independent of proliferation induction.
Tpo upregulates the promoter conformation of p53 in M07e cells. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 or 48 hours. (A) The cells were prepared for flow cytometric analysis for the detection of immunologic variants of p53. The individual cultures were split into four equal groups and treated with either suppressor conformation-specific IgG2a Pab 1620, an IgGa2 isotype control, promoter conformation-specific IgG1 Pab 240, or the IgG1 isotype control. These cultures were then all stained with anti-IgG FITC conjugated secondary antibody. (B) p53 was immunoprecipitated from cell lysates at the indicated time points with either Pab 1620 or Pab 240, transferred to membrane by SDS-PAGE, and probed for p53 with sheep α-p53. This figure represents flow data from four experiments and Western blot data from two experiments which yielded similar results.
Tpo upregulates the promoter conformation of p53 in M07e cells. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 or 48 hours. (A) The cells were prepared for flow cytometric analysis for the detection of immunologic variants of p53. The individual cultures were split into four equal groups and treated with either suppressor conformation-specific IgG2a Pab 1620, an IgGa2 isotype control, promoter conformation-specific IgG1 Pab 240, or the IgG1 isotype control. These cultures were then all stained with anti-IgG FITC conjugated secondary antibody. (B) p53 was immunoprecipitated from cell lysates at the indicated time points with either Pab 1620 or Pab 240, transferred to membrane by SDS-PAGE, and probed for p53 with sheep α-p53. This figure represents flow data from four experiments and Western blot data from two experiments which yielded similar results.
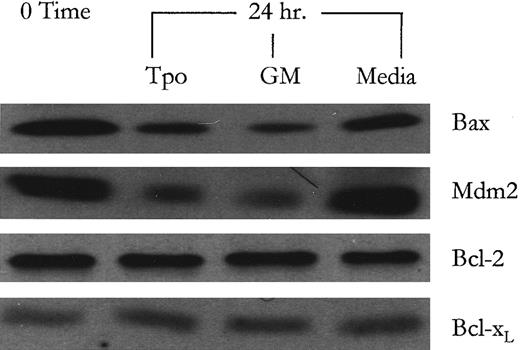
Analysis of protein products of p53-responsive genes following Tpo treatment. To provide a potential relationship between Tpo-induced conformational shifting of p53 and the suppression of apoptosis, the levels of protein products of p53-responsive genes involved in regulating apoptosis and in regulating p53 activity were examined. Western blot analysis showed that the protein levels of Bax and Mdm2, a protein which forms an autoregulatory negative feedback loop for p53,50 sharply decreased following Tpo treatment while remaining relatively elevated in the control serum-free media cultures, as shown in Fig 3. Conversely, Bcl-2 and Bcl-xL protein levels remained relatively constant suggesting that this cell line does not modulate the levels of these “pro-life” proteins to regulate its apoptotic machinery. Although it has not been shown that p53 can modulate the expression of Bcl-xL , we were interested to see if M07e modulated the levels of Bcl-xL , in lieu of Bcl-2. After p53-specific antisense oligonucleotide treatment, the Bax, Mdm2, and Bcl-2 protein levels were evaluated to determine if p53 negation recapitulated the effects of Tpo signaling on the modulation of these protein levels. The data show that when p53 protein levels are negated in the antisense oligonucleotide treated cultures (Fig 1B), the level of both Bax and Mdm2 proteins are decreased markedly. Again Bcl-2 protein levels remained relatively constant. These data suggest that the decreased levels of Bax and Mdm2 in the Tpo-treated cultures may be due to a Tpo-mediated inactivation of p53 function.
Western blot analysis of protein products of p53-responsive genes following Tpo and GM-CSF treatment. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 hours. Following this, protein levels were analyzed by Western blot analysis as described in Materials and Methods. This figure represents data from one of three experiments which yielded similar results.
Western blot analysis of protein products of p53-responsive genes following Tpo and GM-CSF treatment. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 hours. Following this, protein levels were analyzed by Western blot analysis as described in Materials and Methods. This figure represents data from one of three experiments which yielded similar results.
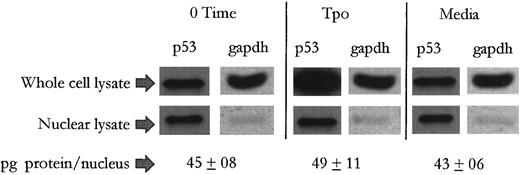
Tpo treatment induces an extranuclear buildup of and weakened DNA-binding capacity in p53 in M07e. We next wanted to determine if p53 in M07e cell treated with Tpo undergoes a change in its subcellular distribution, since p53 in the promoter conformation has been reported to be biased to the cytosol,51,52 by investigating whether the marked buildup of p53 protein levels in cytokine stimulated cells occurred in an extranuclear manner. As shown in Fig 4, the data reveals that following a 24-hour treatment with either Tpo or control serum-free media, nuclear protein levels of p53 changed little from 0 time. A cytosolic marker, glutaraldehyde-3-phosphate dehydrogenase (GAPDH) is virtually undetectable in the nuclear preps, but was readily detectable in the whole cell lysates. In addition, the protein content per nucleus was not significantly different between the treatment groups, suggesting that there was not an unequal leakage of nuclear proteins between treatment groups during the nuclear isolation procedure. In this study we compared equal numbers of nuclei with whole cells at a 1:1 ratio to show that on a per cell basis, the increase in overall p53 protein levels seen in Tpo-stimulated cultures was virtually all extranuclear. This simple method also circumvents errors that may arise when adjusting for yield between cytosolic and nuclear preparations from a given number of cells. This data would represent an inactivation of wild-type p53 function, as subcellular partitioning has been shown to be a mechanism through which the activities of p53 can be regulated.53
Tpo induces an extranuclear buildup of p53 in M07e cells. After an 18-hour factor starvation period, 5 × 106 cells were treated with either Tpo (50 ng/mL) or control serum-free media for 24 hours. Following this, the cell cultures were adjusted to contain 5 × 107 cells/mL and divided into two groups. Nuclei were isolated from one of those groups and then adjusted to contain 5 × 107 nuclei/mL. Thereafter, equal numbers of whole cells and nuclei from each treatment group were electrophoresed and analyzed by Western blot analysis. This figure represents data from one of three experiments which yielded similar results.
Tpo induces an extranuclear buildup of p53 in M07e cells. After an 18-hour factor starvation period, 5 × 106 cells were treated with either Tpo (50 ng/mL) or control serum-free media for 24 hours. Following this, the cell cultures were adjusted to contain 5 × 107 cells/mL and divided into two groups. Nuclei were isolated from one of those groups and then adjusted to contain 5 × 107 nuclei/mL. Thereafter, equal numbers of whole cells and nuclei from each treatment group were electrophoresed and analyzed by Western blot analysis. This figure represents data from one of three experiments which yielded similar results.
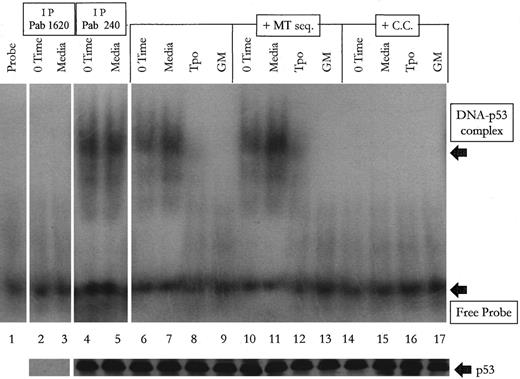
To determine if p53 in M07e cells undergo a change in its ability to bind DNA concurrent with its Tpo-induced change in conformation, nuclear lysates were incubated with p53-specific DNA consensus sequences and then analyzed by gel electrophoresis to detect shifting of the labeled DNA target sequence. As shown in Fig 5, after adjusting the lysates so that the p53 content was equal as determined by Western blot analysis (data not shown), we see that the labeled target is shifted in the time 0 and control serum-free media cultures, while cytokine treated cells had virtually no p53-DNA complex. This shifting could be competed out by molar excess of cold target (C.C.), but not by the mutated (MT) consensus sequences. The mutated consensus sequence appeared to enhance complex formation in the media and Tpo-treated lysates when compared with the media and Tpo-treated lysates with no mutated sequence. However, when analyzed densitometrically, the differences are not significant (P = .39 for the media-treated groups, P = .31 for the Tpo-treated groups). To confirm that the protein complexing with the labeled probe is p53, we first serially immunodepleted p53 from time 0 and control media-treated cultures with Pab 1620, and confirmed by western blot analysis that p53 was efficiently removed from these cell lysates (shown in the lower portion of Fig 5). We then incubated the labeled probe with these p53-deficient lysates and were unable to detect shifting of the target sequence as shown in lanes 2 and 3 in Fig 5. Pab 240 was unable to immunodeplete p53 from the 0 time and media lysates. We were also unable to supershift the p53-DNA complex in the 0 time and media groups (data not shown). This shows that the p53 that is complexing with DNA in the 0 time and control media groups is of the suppressor, and not the promoter, conformation. This set of data suggests that the treatment of M07e with Tpo results in the generation of p53, which has a decreased affinity for its DNA target sequences. This decreased affinity for DNA in Tpo-treated cultures would translate into a decreased p53-mediated transcription enhancement of Bax and Mdm2. The lower portion of Fig 5 shows by Western blot analysis that equal amounts of p53 were loaded per well. For each condition point an equal amount of adjusted lysate was either added to the EMSA reaction cocktail or added to SDS-PAGE buffer and analyzed by Western blot.
Tpo-treated M07e cells contain p53, which has a greatly diminished DNA-binding capacity. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 hours. Lysates were treated as described in Materials and Methods. Lane 1 contains probe with no lysate. Lanes 2 and 3 contain lysates that were first serially immunodepleted of p53 with Pab 1620 and then incubated with labeled probe. Lanes 4 and 5 contain lysates that were first incubated serially with Pab 240. Lanes 6 through 9 contain lysates with labeled target sequence only. Lanes 10 through 13 contain lysates with 100 molar excess of the mutated consensus DNA binding sequence (MT seq) plus the labeled probe. Lanes 14 through 17 contain lysates with 100 molar excess of cold competitor (C.C.) plus the labeled probe.
Tpo-treated M07e cells contain p53, which has a greatly diminished DNA-binding capacity. After an 18-hour factor starvation period, 1 × 106 cells were treated with either Tpo (50 ng/mL), GM-CSF (GM; 100 U/mL), or control serum-free media for 24 hours. Lysates were treated as described in Materials and Methods. Lane 1 contains probe with no lysate. Lanes 2 and 3 contain lysates that were first serially immunodepleted of p53 with Pab 1620 and then incubated with labeled probe. Lanes 4 and 5 contain lysates that were first incubated serially with Pab 240. Lanes 6 through 9 contain lysates with labeled target sequence only. Lanes 10 through 13 contain lysates with 100 molar excess of the mutated consensus DNA binding sequence (MT seq) plus the labeled probe. Lanes 14 through 17 contain lysates with 100 molar excess of cold competitor (C.C.) plus the labeled probe.
DISCUSSION
Hematopoietic cells possessing wild-type p53 die by apoptosis when deprived of growth factors in vitro.3 Cytokines that protect hematopoietic cells from apoptosis can induce a shift in p53 conformation.24-26 It seemed logical to ask what the downstream effects of that induced conformational shift were, and what molecular roles it played in the regulation of apoptosis. Cytokine-induced conformational switching of p53 has been reported before,22,25 26 but the cytokines used induced the proliferation of the cells being studied. In these studies, we have used Tpo as a survival factor to investigate the effects of conformational switching in p53 on the suppression of apoptosis, independent of significant proliferation induction.
Although direct sequencing of the p53 gene has not been performed on the M07e cell line, many lines of evidence suggest that the germline status of p53 in this cell line is wild-type. It is dependent on growth factors for viability, and it undergoes rapid growth arrest and apoptosis following ionizing radiation (data not shown), two processes dependent on wild-type p53. There is a lack of mutant, or promoter conformation p53 during the growth arrested state. A mutant allele of p53 would produce a protein detected constitutively by mutant-specific antibodies, irrespective of growth conditions. Lastly, the presence of promoter conformation p53 being found in wild-type p53 positive AML samples is common.26
It seemed likely that in order to suppress growth factor withdrawal-induced apoptosis, Tpo would in some way either functionally inactivate p53 so that it could not initiate apoptosis, or activate a cellular subsystem that would antagonize a p53-mediated, pro-apoptotic pathway. This latter scenario is seen in certain cellular systems that show upregulated Bcl-2 or Bcl-xL in response to survival factor signaling or after overexpression of these proteins through transfection studies.30,54 55 As we have seen in this report, Tpo signaling does not upregulate either Bcl-2 or Bcl-xL . Interestingly, growth factor withdrawal does not influence the levels of these proteins in this cell line either. Therefore, to modulate the ratio of Bax to Bcl-2, thereby influencing the cell's fate to live or die, survival factors in this cell line might diminish the levels of Bax, providing a phenotypic advantage to the antiapoptotic members of the Bcl-2 family. This is in fact what was observed following both Tpo and GM-CSF stimulation (Figs 1B and 3). We also show here that by limiting the expression, and hence activity, of p53, we could mimic the effects of Tpo and GM-CSF treatment on Bax and Mdm2 levels (Fig 1B). Again, Bcl-2 levels remained elevated. This was an interesting find, because wild-type p53 has been shown to transcriptionally repress bcl-2 expression, but does not appear to do so in M07e. p53 in M07e is capable of binding its target DNA sequence (Fig 5) and can markedly enhance the expression of Bax and Mdm2 (Fig 1B). Therefore, the inability of p53 in M07e to regulate Bcl-2 expression may arise from a defect in the p53 binding site upstream of the Bcl-2 gene. This defect, if it exists, could have been an oncogenic event contributing to the leukemic state from which the M07e cell line was derived.
How does the Tpo-induced switching in conformation of p53 result in an inactivation of its function? Nuclear localization is required for p53 to serve as a transcriptional modulator. In addition, others have shown that the promoter conformation is sequestered in the cytosol. As shown in Fig 4, the extranuclear content of p53 is markedly elevated in the Tpo-treated cells. However, the nuclear p53 content did not change in any appreciable amount from 0 time. p53 has been show to undergo subcellular redistribution in a cell cycle specific manner.56 We have shown previously that Tpo treatment in M07e cells does not allow progression into S phase and that the cells remain tightly in G1/G0 .8 Time 0 cells and control media-treated cells are also growth arrested in G1/G0 . Therefore, the constant level of nuclear p53 in the cytokine and noncytokine-treated cells may be due to a lack of progression through the cell cycle.
Another way that p53 activity can be modulated is by changing its DNA binding ability. We found that the DNA binding ability of p53 in Tpo-treated cells was dramatically lower than time 0 or control media cultures and was undetectable in the GM-CSF–treated cultures (Fig 5). Both of these inactivating events would greatly limit the ability of p53 to stimulate Bax expression. Therefore, it appears that Tpo inhibits p53-mediated apoptosis directly, by limiting its ability to activate downstream components of the pro-apoptotic machinery. However, Bax may not be the only pro-apoptotic component of p53-mediated apoptosis. By turning off Bax and providing a phenotypic advantage to Bcl-2, Tpo may be suppressing apoptosis by suppressing interleukin-1β converting enzyme (ICE) activation. ICE and ICE-like proteases are mammalian cysteine proteases that play a crucial role in mediating cell death in several systems, including neuronal cell death, in thymocytes, in Fas and TNF-α–induced apoptosis, and in response to inhibitors of protein and DNA synthesis in a monocytic tumor cell line.2,57 It is of interest to note here that during cytokine treatment of M07e, the functional inactivation of p53 would also translate into a decrease in other p53-mediated cellular subsystems, such as the p53-dependent induction of gadd45 or p21cip-1/waf-1 expression. Therefore, at least in M07e cells, p53-responsive genes would be regulated in a p53-independent manner during cytokine treatment. Reports have shown that both gadd45 and p21cip-1/waf-1 can be regulated in a p53-independent manner.58 59
The ability of Tpo to serve as a survival factor in vivo has some important considerations. First, Tpo's functional inactivation of p53 would allow the developing megakaryocyte to undergo endomitosis. Since p53 has been shown to arrest the cell and prevent DNA replication following the detection of DNA insults or amplification, and since p53-deficient cells are much more susceptible to aneuploidy,60 it would make sense that an endomitosis-inducing agent, such as Tpo, would have to functionally inactivate p53 or activate a p53-antagonizing subsystem in order to facilitate megakaryocyte maturation. Second, the endomitotic process is thought by some to be a form of abortive apoptosis. Therefore, Tpo may also serve to keep megakaryocytes alive during the endomitotic process that it initiated, by suppressing the proteolytic/nucleolytic mechanisms that would be normally activated by growth factor withdrawal-induced or perhaps abortive apoptosis. Another point to consider is the ability of Tpo to perhaps maintain stem cell survival during a quiescent state. These effects of Tpo on the conformation and functionality of p53, and its effect on the levels of pro- and antiapoptotic components downstream of p53 on primary normal myeloid progenitors and the maturing megakaryocyte are of future interest.
Supported by Public Health Service Grants RO1 HL 56416, RO1 HL 54037, and by a project in PO1 HL 53586 from the National Institutes of Health (NIH) to H.E.B. A.R. is supported by NIH training program T32 DK 07519 to H.E.B.
Address reprint requests to Hal E. Broxmeyer, PhD, Walther Oncology Center, Indiana University School of Medicine, 975 W Walnut St, Indianapolis, IN 46202-5121.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal