Abstract
B-cell commitment and early development from multipotent hematopoietic progenitor cells has until recently been considered to be dependent on direct interaction with stromal cells. We recently showed that the flt3 ligand (FL) has a unique ability to interact with interleukin-7 (IL-7) to directly and selectively promote B-cell development from murine bone marrow progenitor cells with a combined myeloid and lymphoid potential. Here we report that whereas IL-10 alone has no ability to stimulate growth of primitive (Lin−Sca-1+c-kit+) bone marrow progenitor cells, it potently enhances FL + IL-7–induced proliferation (sevenfold). This enhanced proliferation results from recruitment of progenitors unresponsive to FL + IL-7 alone, as well as from increased growth of individual clones, resulting in a 7,000-fold cellular expansion over 12 days. Single cell cultures and delayed addition studies suggested that the stimulatory effect of IL-10 was directly mediated on the progenitor cells. The cells generated in response to FL + IL-7 + IL-10 appeared to be almost exclusively proB cells, as shown by their expression of B220, CD24, CD43, and lack of expression of cμ, myeloid, erythroid, and T-cell surface antigens. Although IL-10 also enhanced kit ligand (KL) + IL-7–induced proliferation of Lin−Sca-1+c-kit+ progenitor cells, the resulting cells were predominantly myeloid progeny. Accordingly, FL + IL-7 + IL-10 was 100-fold more efficient in stimulating production of proB cells than KL + IL-7 + IL-10. In contrast to its ability to stimulate the earliest phase of proB cell formation and proliferation, IL-10 inhibited growth of proB cells generated in response to FL + IL-7. Analysis of CD19 expression on cells generated in FL + IL-7 + IL-10 showed that almost all cells generated under these conditions lacked expression of CD19, in contrast to cells generated in the absence of IL-10, which were predominantly CD19+. Replating of sorted CD19+ and CD19− proB cells in FL + IL-7 or FL + IL-7 + IL-10 showed that IL-10 efficiently blocked growth of CD19+, but not CD19− cells. Both CD19− and CD19+ cells expressed λ5 and VpreB , shown to be specific for B-cell progenitors. In addition, sorted CD19− cells generated CD19+ cells in response to FL + IL-7. Thus, IL-10 has a dual regulatory effect on early B-cell development from primitive murine bone marrow progenitor cells in that it enhances FL + IL-7–induced proB-cell formation and growth before acquisition of CD19 expression, whereas growth of CD19+ proB cells is inhibited.
INTERLEUKIN-10 (IL-10) is produced by many cell types (including T helper 2 cells, macrophages, T cells, and B cells) in response to activation of the immune system1-3 and is thought to play an important immunosuppressive role through its ability to block the synthesis of inflammatory cytokines and by inhibiting the activation of macrophages, T cells, and natural killer (NK) cells.1-3 In addition, IL-10 has been shown to stimulate the proliferation of murine thymocytes1,2,4,5 and to enhance the growth of mast cell and megakaryocyte progenitor cells.6-8 IL-10 also promotes IL-3–dependent growth and myeloid differentiation of primitive Lin−Thy-1+Sca-1+ progenitor cells.7
The role of different cytokines in regulating the earliest events in B-cell commitment and development remains largely unknown, although IL-7 has been found to be an essential stimulator of B lymphopoiesis.9,10 In addition, the ligand for c-kit (KL) has been shown to interact with IL-7 to stimulate growth of early B-cell progenitor cells,11-14 although a blocking antibody against c-kit rather enhances B lymphopoiesis while suppressing myelopoiesis.15 Recently, mice deficient in flt3, another tyrosine kinase receptor, have been shown to have a selective reduction in early B lymphopoiesis,16 and in line with this, the flt3 ligand (FL) can enhance stroma-independent growth of early B-cell progenitor cells in combination with IL-7.14,17,18 Furthermore, FL + IL-7 can selectively induce early B-cell development from primitive uncommitted murine bone marrow progenitor cells.19
IL-10 has been shown to provide a costimulation for the proliferation, differentiation, and isotype switching of mature human B cells1,3,20-22 and to enhance viability of murine B cells.23 Much less is known about the effects of IL-10 on the growth of B-cell progenitor cells, although IL-10 has been shown to enhance the growth of human fetal bone marrow B-cell precursors.24 In the murine system, one group reported IL-10 to inhibit the growth of preB progenitor cells,25 whereas others found enhanced preB progenitor cell clonal growth in response to IL-10 under seemingly similar conditions.26 Thus, the ability of IL-10 to affect the growth of the earliest stages of B-cell development remain largely unknown. However, in vivo administration of IL-10 was recently shown to accelerate preB cell, but not myeloid progenitor cell recovery, in myeloablated mice.26 Because such myeloablative treatment selectively depletes mature progenitor cells rather than primitive and quiescent stem cells,27 we speculated that IL-10 might act to directly promote early B-cell development, potentially from uncommitted bone marrow progenitor cells. A recently developed stroma-independent culture system, selectively promoting B-cell commitment and development,19 allowed us to address whether IL-10 could affect early B-cell development from progenitor cells not yet committed to either the myeloid or lymphoid cell lineages.
MATERIALS AND METHODS
Cytokines and antibodies. Recombinant human (rhu) FL was cloned and purified as previously described.28 Purified recombinant murine (rmu) IL-3 was from PeproTech Inc (Rocky Hill, NJ), purified rhuG-CSF was generously supplied by Dr Ian McNiece (Amgen Corp, Thousand Oaks, CA). Purified rhuIL-7 and recombinant murine mast cell growth factor (MGF; KL) was a gift from Immunex. rmu IL-10 and neutralizing rat antimouse IL-10 were purchased from Genzyme Inc (Minneapolis, MN), whereas a control antibody (rat IgG2a) was purchased from Pharmingen (San Diego, CA). All cytokines were used at predetermined optimal concentrations (unless otherwise stated): IL-3, 20 ng/mL; granulocyte colony-stimulating factor (G-CSF ), 50 ng/mL; FL, 50 ng/mL; IL-7, 100 ng/mL; IL-10, 20 ng/mL; and KL, 50 ng/mL.
Isolation of Lin−Sca1+c-kit+ bone marrow cells. Lineage depleted (Lin−) bone marrow cells were isolated from femurs and tibia of normal 8- to 14-week old female C57BL/6 mice according to a previously described protocol.29,30 Briefly, bone marrow cells were incubated in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) (PBSS) at 4°C for 30 minutes in a cocktail of predetermined optimal concentrations of antibodies against lineage-specific cell surface antigens; RA3-6B2 (anti-B220), RB6-8C5 (anti-GR-1), M1/70 (anti-Mac–1; CD11b), anti-CD4, anti-CD5, anti-CD8 (all from Pharmingen), and TER-119 (kindly provided by Dr Tatsuo Kina, Kyoto University, Kyoto, Japan). Cells (Lin+) were next incubated with sheep antirat IgG-conjugated immunomagnetic beads (Dynal, Oslo, Norway) at a ratio of 1:1 for 30 minutes at 4°C, and Lin+ cells were removed with a magnetic particle concentrator (Dynal). The procedure was repeated with the same (absolute) number of beads. The remaining Lin− cells were concentrated and incubated with saturating amounts of fluorescein isothiocyanate (FITC)-conjugated anti-Ly6A/E (Sca-1) and phycoerythrin (PE)-conjugated anti-c-kit or irrelevant isotype-matched control antibodies (all from Pharmingen). After washing and resuspending, Lin− Sca-1+c-kit+ cells (approximately 0.1% of nucleated bone marrow cells) were sorted on a Coulter Epics Elite cell sorter (Coulter Electronics, Hialeah, FL), as previously described.31-33 The purity of the cells was reproducibly greater than 95% as determined by reanalysis of sorted cells.
Single cell proliferation assay. Lin− Sca-1+c-kit+ cells were seeded in 60-well microtiter plates (Nunc, Kamstrup, Denmark) at a concentration of 1 cell per well in 20 μL Iscove's modified Dulbecco's medium (IMDM) (BioWhittaker, Walkersville, MD) supplemented with 20% FCS (BioWhittaker), antibiotics, L-glutamine (complete IMDM), and cytokines as indicated. Wells were scored for colony growth (>50 cells) and clusters (10 to 50 cells) after 10 to 12 days incubation at 37°C and 5% CO2 in air (without refeeding of the cultures). In some experiments, individual colonies were sampled, transferred to slides in a cytospin centrifuge, and examined morphologically following Giemsa staining or phenotyped by flow cytometry as described below.
Immunophenotyping by flow cytometry. Lin−Sca-1+c-kit+ cells were plated in complete IMDM and incubated for 10 to 12 days with optimal concentrations of cytokines at 37°C and 5% CO2 in air. Cell surface expression of GR-1, B220, IgM, CD19, CD4, CD24, CD43, and NK1.1 were determined by incubating cells for 30 minutes on ice with either PE- and FITC-conjugated monoclonal antibodies (MoAbs) against the different antigens or PE- and FITC-conjugated irrelevant isotype-matched controls (all from Pharmingen). Following washing and resuspension in PBS, cells were analyzed for antigen expression in a flow cytometer (FACSort, Becton Dickinson, San Jose, CA). At least 10,000 cells were analyzed for each antigen unless otherwise stated. The expression of cytoplasmic heavy chain (cμ) was determined after fixation of cells in ice-cold 70% ethanol for at least 15 minutes on ice as previously described.19 Briefly, cells were washed in PBSS, incubated with FITC-conjugated goat antimouse IgM, heavy chain specific (Southern Biotech, Birmingham, AL) for 30 minutes on ice and washed and resuspended in PBSS before being analyzed by flow cytometry.
[3H]TdR-incorporation of proB cells. Cells expanded in FL + IL-7 or FL + IL-7 + IL-10 for different periods of time were replated directly in round-bottom 96-well plates or washed twice and plated at a density of 105 cells per well in X-Vivo15 (BioWhittaker) supplemented with 1% detoxified bovine serum albumin (BSA) (Stem Cell Technologies, Vancouver, Canada) and cytokines as indicated. Following 24 hours incubation, each well was pulsed for 12 hours with 1 μCi [3H]TdR (Amersham, Arlington Heights, IL), harvested onto glass fiber filters, and counted in a β-counter.
Sorting and reculturing of CD19− and CD19+ cells generated in response to FL + IL-7. A total of 500 Lin− Sca-1+c-kit+ cells were expanded in FL + IL-7 for 12 days, and the resulting cells stained with a PE-conjugated antibody against CD19 (or an isotype-matched control antibody) and sorted into CD19+ and CD19− fractions (Coulter Epics Elite). To reduce the probability of contamination between the two populations, the sorting regions were clearly separated. The purity of the cells after sorting was greater than 95% for each subpopulation. After sorting, both CD19− and CD19+ cells were replated in fresh medium supplemented with FL + IL-7 or FL + IL-7 + IL-10, and cultured for various periods of time before viable cell numbers were determined by trypan blue exclusion and subsequently analyzed for expression of B220 and CD19 by flow cytometry.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of λ5 and VpreB expression. Semiquantitative RT-PCR was used to compare the levels of λ5 and VpreB expression of sorted CD19+ and CD19− cell populations. Briefly, Lin−Sca-1+c-kit+ cells were cultured at 500 cells/mL in the presence of FL and IL-7. At the end of the culture period, cells were stained with a PE-conjugated anti-CD19 antibody and sorted into CD19+ and CD19− subfractions as described above. Total RNA was isolated from both populations using Ultraspec RNA Isolation System (Biotex Laboratories, Inc, Houston, TX). cDNA was produced using SuperScript (Life Technologies, Täby, Sweden) and PCR was performed by using primers for either Hypoxanthine phosphoribosyltransferase (HPRT) (5′-GCTGGTGAAAAGGACCTCT-3′ and 5′-CACAGGACTAGAACACCTGC-3′), VpreB (5′-TGCTCATGCTGCTGGCCTAT-3′ and 5′-CTCCGGAGCCCCACGGCA-3′), or λ5 (5′-GAGATCTAGACTGCAAGTGAGGCTAGAG-3′ and 5′-CTTGGGCTGACCTAGGATTG-3′). HPRT was used as an internal control to verify that the starting material contained equal amounts of cDNA before the PCR amplification. After verifying that cDNA from the two populations (ie, from CD19+ and CD19− cells) had equal amounts of cDNA for HPRT, λ5 and VpreB , cDNAs were PCR amplified. The amplification was stopped at various time points to ensure that the amount of product generated was analyzed within the exponential phase and before the plateau phase was reached.
Myeloid potential of FL + IL-7 + IL-10–responsive Lin−Sca-1+c-kit+ progenitor cells. The potential of Lin−Sca-1+c-kit+ cells responding to FL + IL-7 + IL-10 to differentiate into myeloid cells was determined by seeding 180 cells at one cell per well in 10 μL complete IMDM with FL, IL-7, and IL-10 on day 0. After 4 and 10 days of incubation, responsive clones were identified (containing two or more cells) and 10 μL of complete IMDM containing a potent myeloid stimulus (KL + G-CSF + IL-3 at optimal concentrations) were added. Clones were examined after an additional 12 days of incubation for the development of granulocyte (G)- and/or macrophage (M)-containing colonies by microscopic evaluation of Giemsa-stained cytospin-preparations of individual clones.
PreB colony-forming cell (CFC) assay. A total of 1 × 105 unfractionated bone marrow cells from normal C57BL/6 mice were plated in methylcellulose (Methocult, Stem Cell Technologies) containing 5 ng/mL rhu IL-7. Cultures were incubated at 37°C in 5% CO2 in air for 7 days and colonies with greater than 30 cells were counted. A preB-cell phenotype was confirmed by picking colonies and analyzing the expression of B220, CD19, and cμ.
Statistical analysis. Levels of significance for comparisons between groups were determined by Student's t-test.
RESULTS
IL-10 directly stimulates growth of Lin−Sca1+c-kit+ progenitor cells. Lin−Sca1+c-kit+ cells, shown to be highly enriched in primitive hematopoietic progenitor cells with a combined myeloid and lymphoid potential,19,31-33 were cultured at one cell per well to exclude indirect effects from potentially contaminating accessory cells and examined for their growth response to IL-10 alone or in combination with IL-7 and the ligands for c-kit or flt3 (Table 1). Neither IL-7 nor IL-10, alone or in combination, induced any clonal growth of Lin−Sca-1+c-kit+ cells, whereas individually, FL and KL induced the formation of 11 and 17 clones (of 180 cells plated), respectively. IL-10 significantly enhanced the number of clones when combined with FL or KL (2.5- and 1.6-fold, respectively). As previously shown,19 IL-7 enhanced the number (threefold), as well as the size of FL-induced clones, but only slightly increased the number of KL-induced clones. Interestingly, the addition of IL-10 to FL + IL-7 further enhanced clonal formation by 44% (Table 1), and in particular, a profound effect was observed on the generation of the largest colonies (cells covering >50% of a well) in that nine such colonies were observed in the presence of IL-10, whereas none were seen in its absence. Neutralizing IL-10 with an antimouse IL-10 neutralizing antibody (see Materials and Methods) completely abolished the synergy with FL + IL-7 (Veiby and Jacobsen, 1996). IL-10 also potently enhanced the number (135%), as well as the size of KL + IL-7–responsive clones (Table 1).
IL-10 Enhances the Growth of Lin−Sca1+c-kit+ Bone Marrow Progenitor Cells
| . | Clone Size . | Total . | |||
|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . |
| Medium | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-7 + IL-10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| FL | 8 (1) | 3 (2) | 0 (0) | 0 (0) | 11 (4) |
| FL + IL-10 | 4 (2) | 16 (2) | 9 (2) | 0 (0) | 28 (3)* |
| KL | 5 (1) | 6 (2) | 6 (1) | 0 (0) | 17 (3) |
| KL + IL-10 | 6 (1) | 12 (2) | 10 (3) | 0 (0) | 27 (5)* |
| FL + IL-7 | 9 (1) | 14 (1) | 9 (2) | 0 (0) | 32 (2) |
| FL + IL-7 + IL-10 | 7 (3) | 17 (3) | 15 (3) | 9 (3) | 46 (3)* |
| KL + IL-7 | 5 (1) | 8 (3) | 7 (2) | 0 (1) | 20 (6) |
| KL + IL-7 + IL-10 | 6 (3) | 16 (3) | 20 (8) | 5 (3) | 47 (10)* |
| . | Clone Size . | Total . | |||
|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . |
| Medium | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IL-7 + IL-10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| FL | 8 (1) | 3 (2) | 0 (0) | 0 (0) | 11 (4) |
| FL + IL-10 | 4 (2) | 16 (2) | 9 (2) | 0 (0) | 28 (3)* |
| KL | 5 (1) | 6 (2) | 6 (1) | 0 (0) | 17 (3) |
| KL + IL-10 | 6 (1) | 12 (2) | 10 (3) | 0 (0) | 27 (5)* |
| FL + IL-7 | 9 (1) | 14 (1) | 9 (2) | 0 (0) | 32 (2) |
| FL + IL-7 + IL-10 | 7 (3) | 17 (3) | 15 (3) | 9 (3) | 46 (3)* |
| KL + IL-7 | 5 (1) | 8 (3) | 7 (2) | 0 (1) | 20 (6) |
| KL + IL-7 + IL-10 | 6 (3) | 16 (3) | 20 (8) | 5 (3) | 47 (10)* |
Lin−Sca1+c-kit+ cells were seeded at one cell per well in 20 μL complete IMDM with cytokines as indicated. After 10 days of incubation at 37°C and 5% CO2 in air, cultures were scored for clonal growth according to the following criteria: 1 = 10 to 50 cells, 2 = 50 cells − 10% of the well covered with cells, 3 = 10% to 50% covered, and 4 = 50% to 100% of the well covered with cells. Results are presented as the mean (SEM) from three independent experiments with 180 wells scored per group in each experiment.
P < .05 for total clone numbers when compared with cultures in the absence of IL-10.
If addition of IL-10 to FL + IL-7–stimulated Lin−Sca-1+c-kit+ cells was delayed for as little as 24 hours, no increase in the number of clones could be observed, although the size of the clones was enlarged (Table 2), implicating that the ability of IL-10 to recruit additional Lin−Sca-1+c-kit+ progenitors to proliferate requires its action at an early stage, but that IL-10 continues to stimulate enhanced proliferation of already recruited progenitors.
The Effect of Delayed Addition of IL-10 to FL + IL-7–Stimulated Lin−Sca-1+c-kit+ Progenitor Cells
| IL-10 . | Clone Size . | Total Clones . | |||
|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . |
| None | 10 (1) | 12 (1) | 9 (2) | 0 (1) | 32 (2) |
| 0 h | 8 (2) | 12 (3) | 15 (3)* | 16 (4)* | 49 (4)* |
| 24 h | 7 (2) | 7 (3)* | 12 (2) | 6 (3)* | 31 (3) |
| IL-10 . | Clone Size . | Total Clones . | |||
|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | . |
| None | 10 (1) | 12 (1) | 9 (2) | 0 (1) | 32 (2) |
| 0 h | 8 (2) | 12 (3) | 15 (3)* | 16 (4)* | 49 (4)* |
| 24 h | 7 (2) | 7 (3)* | 12 (2) | 6 (3)* | 31 (3) |
Lin−Sca-1+c-kit+ cells were plated at 1 cell per well in 20 μL complete IMDM supplemented with FL + IL-7 or FL + IL-7 + IL-10. IL-10 was added either at initiation of culture (0 h) or following 24 hours (24 h) of incubation. After 10 days incubation at 37°C and 5% CO2 in air, clones were identified in an inverted microscope and scored according to the criteria described in Table 1. Results represent the mean (SEM) of three independent experiments with 180 cells plated for each group in each experiment.
P < .05 when compared with cultures in the absence of IL-10.
IL-10 stimulates predominantly lymphoid development in combination with FL and myeloid differentiation in combination with KL. Individual clones were examined morphologically and phenotyped to determine which cell lineages were produced in response to IL-10 (Table 3). As previously described, all clones formed from FL + IL-7–responsive Lin−Sca-1+c-kit+ cells contained almost exclusively B220+ cells, whereas KL + IL-7 stimulated the formation of predominantly myeloid cells, as defined by their GR-1 expression and granulocyte/macrophage morphology.19 Ninety-five percent of the clones formed in response to FL + IL-7 + IL-10 consisted of B220+ cells (Table 3). Morphologically, these cells appeared as lymphoid cells and the clones were devoid of mature myeloid cells (Veiby and Jacobsen, unpublished observations). However, a low fraction (5%) of the clones generated in response to FL + IL-7 + IL-10 contained cells expressing both myeloid (GR1+) and lymphoid (B220+) cells (Table 3). The majority (>85%) of the cells in these mixed clones was, however, B220+. In striking contrast to the FL + IL-7 + IL-10–induced colonies, 73% of the clones formed in response to KL + IL-7 + IL-10 were mixed myeloid and lymphoid, 26% pure myeloid colonies, whereas none contained only B220+ cells (Table 3). In contrast to the mixed clones generated in FL + IL-7 + IL-10, the majority of the cells found in mixed clones recruited in response to KL + IL-7 + IL-10 was of myeloid origin (Veiby and Jacobsen, unpublished observation). Thus, IL-10 in combination with FL + IL-7 enhances formation of predominantly pure B220+ colonies from Lin−Sca-1+c-kit+ progenitor cells, whereas IL-10 when combined with KL + IL-7 results in an increase in colonies containing predominantly myeloid cells.
Phenotyping of Clones Formed From Lin−Sca-1+c-kit+ Progenitor Cells in Response to FL + IL-7 + IL-10 or KL + IL-7 + IL-10
| . | Phenotype, % of Total Colonies . | ||
|---|---|---|---|
| . | B220+ . | GR-1+ . | Mixed . |
| FL + IL-7 + IL-10 | 95 | 0 | 5 |
| KL + IL-7 + IL-10 | 0 | 26 | 73 |
| . | Phenotype, % of Total Colonies . | ||
|---|---|---|---|
| . | B220+ . | GR-1+ . | Mixed . |
| FL + IL-7 + IL-10 | 95 | 0 | 5 |
| KL + IL-7 + IL-10 | 0 | 26 | 73 |
Lin−Sca-1+c-kit+ cells were plated at a concentration of one cell per well and cultured in the presence of optimal concentrations of cytokines as indicated. Approximately 30 clones in each group were picked and analyzed for B220 and GR-1 expression by flow cytometry from a total of three experiments. A B220+ colony was scored as such when >95% of the cells in a single clone were positive for B220 and negative for GR-1 as compared with irrelevant isotype-matched control antibodies. Similarly, clones containing >95% GR-1+ cells were classified as GR-1+. If clones contained more than 5% of B220+, as well as GR-1+ cells, they were classified as mixed.
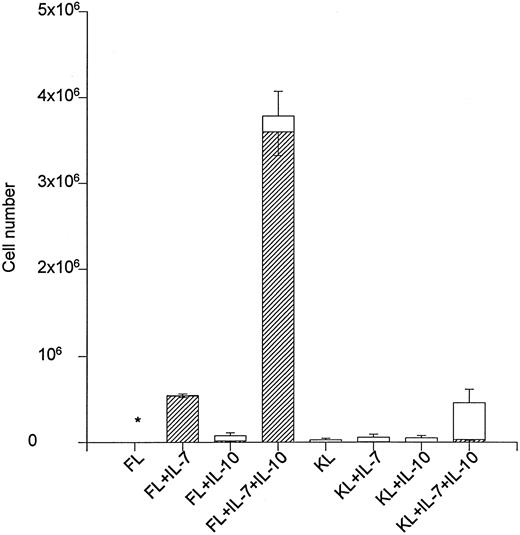
The ability of IL-10 to stimulate net cell expansion and in particular B220+ cell production in liquid cultures containing 500 Lin−Sca-1+c-kit+ cells was also investigated (Fig 1). FL + IL-7 expanded the cell number approximately 900-fold after 12 days of incubation, and all of these cells were B220+. The addition of IL-10 to FL + IL-7 resulted in a sevenfold expansion above that obtained in response to FL + IL-7, and 95% of these cells were B220+ (Fig 1). KL + IL-7 stimulated a 114-fold expansion, but less than 3% of these cells were B220+. KL + IL-7–stimulated cell production increased eightfold (P < .05) on stimulation with IL-10, but only 10% of these were B220+. Accordingly, cell expansion was similarly increased when IL-10 was combined with FL + IL-7 or KL + IL-7. However, whereas IL-10 in combination with FL +IL-7 selectively enhanced the production of B220+ cells, it predominantly increased the production of myeloid (GR-1+) cells in combination with KL + IL-7. In that regard, FL + IL-7 + IL-10 was 100-fold more potent in generating B220+ cells than KL + IL-7 + IL-10 (Fig 1).
The effect of IL-10 on FL + IL-7 and KL + IL-7–stimulated growth of Lin−Sca-1+c-kit+ progenitor cells. A total of 500 Lin−Sca-1+c-kit+ cells was cultured in complete IMDM and predetermined optimal concentrations of cytokines as indicated. After 12 days of incubation, total cell numbers were counted (□) and B220 expression (▨) determined by flow cytometry. The number of B220+ cells and total cell numbers represent the mean (SEM) of three independent experiments. * <2,000 cells. The number of B220+ cells produced in response to FL + IL-10, KL + IL-7 and KL + IL-10 was 15,667 ± 6,641, 3,972 ± 1,239 and <500, respectively.
The effect of IL-10 on FL + IL-7 and KL + IL-7–stimulated growth of Lin−Sca-1+c-kit+ progenitor cells. A total of 500 Lin−Sca-1+c-kit+ cells was cultured in complete IMDM and predetermined optimal concentrations of cytokines as indicated. After 12 days of incubation, total cell numbers were counted (□) and B220 expression (▨) determined by flow cytometry. The number of B220+ cells and total cell numbers represent the mean (SEM) of three independent experiments. * <2,000 cells. The number of B220+ cells produced in response to FL + IL-10, KL + IL-7 and KL + IL-10 was 15,667 ± 6,641, 3,972 ± 1,239 and <500, respectively.
Cells generated from Lin−Sca-1+c-kit+ cells in response to FL + IL-7 or FL + IL-7 + IL-10 are proB cells. The high expression of B220 combined with the lack of GR-1 expression and morphologic appearance compatible with lymphocytes suggested that cells produced in response to FL + IL-7 + IL-10 were predominantly of the B-cell lineage. Because B-cell progenitors differentiate through distinct stages that can be classified based on their expression of CD24, CD43, cμ, and sIgM,34 cells grown for 10 days in the presence of FL + IL-7 or FL + IL-7 + IL-10 were characterized with regard to their expression of these antigens. All cells produced in response to FL + IL-7 expressed high levels of B220, CD43, and CD24 , but not GR-1 (Fig 2, left panel). Almost all of the cells formed in FL + IL-7 + IL-10–containing cultures also expressed B220, CD43, and CD24 (Fig 2, right panel), although at consistently lower levels than in the absence of IL-10. Furthermore, in agreement with the finding of a low number of mixed myeloid/lymphoid colonies in response to FL + IL-7 + IL-10, a small population of GR-1+ cells was observed, as well. However, cells generated in response to FL + IL-7, as well FL + IL-7 + IL-10, were negative for cell surface antigens specific for erythroid (TER119) and T cells (CD4, CD8; Veiby and Jacobsen, unpublished observations). Cells generated in response to FL + IL-7 or FL + IL-7 + IL-10 were not preB cells, as they did not express cμ (Veiby and Jacobsen, unpublished observations).
Phenotyping of cells generated in response to FL + IL-7 or FL + IL-7 + IL-10. A total of 500 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in the presence of optimal concentrations of FL + IL-7 (left panels) or FL + IL-7 + IL-10 (right panels). After 12 days of incubation, cells were harvested and examined for expression of B220, CD43, CD24, and GR-1, as described in the Materials and Methods. Staining with the antigen-specific antibodies (black) was compared with that of irrelevant isotype-matched control antibodies (gray). Data are from one (of four) representative experiments. For all panels, the x-axis represents relative logarithmic fluorescence intensity and the y-axis represents relative cell number.
Phenotyping of cells generated in response to FL + IL-7 or FL + IL-7 + IL-10. A total of 500 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in the presence of optimal concentrations of FL + IL-7 (left panels) or FL + IL-7 + IL-10 (right panels). After 12 days of incubation, cells were harvested and examined for expression of B220, CD43, CD24, and GR-1, as described in the Materials and Methods. Staining with the antigen-specific antibodies (black) was compared with that of irrelevant isotype-matched control antibodies (gray). Data are from one (of four) representative experiments. For all panels, the x-axis represents relative logarithmic fluorescence intensity and the y-axis represents relative cell number.
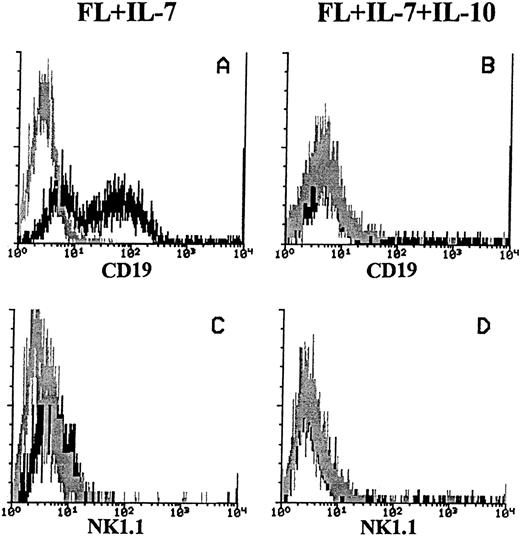
We also determined the cell surface expression of CD19 on cells generated in response to FL + IL-7 (in the absence or presence of IL-10), as it is a B-cell specific antigen, whereas B220 is not.35-37 In response to FL + IL-7, both CD19+ and CD19− cells were generated. In contrast, B220+ cells produced in FL + IL-7 + IL-10–containing cultures expressed little or no detectable levels of CD19 (Fig 3A and B, respectively). Because it recently has been shown that a population of NK cells express B220, but not CD19,35 we also analyzed cells generated in response to FL + IL-7 or FL + IL-7 + IL-10 for the expression of NK1.1, previously shown to be widely expressed on murine NK-cells.38 However, both the CD19+ cells generated in response to FL + IL-7, as well as the CD19− cells formed in FL + IL-7 + IL-10–stimulated cultures, did not express NK1.1 (Fig 3C and D, respectively).
Expression of CD19 and NK1.1 on cells produced in response to FL + IL-7 or FL + IL-7 + IL-10. A total of 1,000 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in FL + IL-7 (left panels) or FL + IL-7 + IL-10 (right panels). After 10 days of incubation at 37°C in 5% CO2 , cells were analyzed for expression of CD19 (A + B) and NK1.1 (C + D) as described in the Materials and Methods. The histograms represent data from one of three independent experiments with similar results. Gray lines show irrelevant control antibody staining, whereas black lines represent the binding of specific antibodies. For all panels, the x-axis represents relative logarithmic fluorescence intensity, and the y-axis represents relative cell number.
Expression of CD19 and NK1.1 on cells produced in response to FL + IL-7 or FL + IL-7 + IL-10. A total of 1,000 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in FL + IL-7 (left panels) or FL + IL-7 + IL-10 (right panels). After 10 days of incubation at 37°C in 5% CO2 , cells were analyzed for expression of CD19 (A + B) and NK1.1 (C + D) as described in the Materials and Methods. The histograms represent data from one of three independent experiments with similar results. Gray lines show irrelevant control antibody staining, whereas black lines represent the binding of specific antibodies. For all panels, the x-axis represents relative logarithmic fluorescence intensity, and the y-axis represents relative cell number.
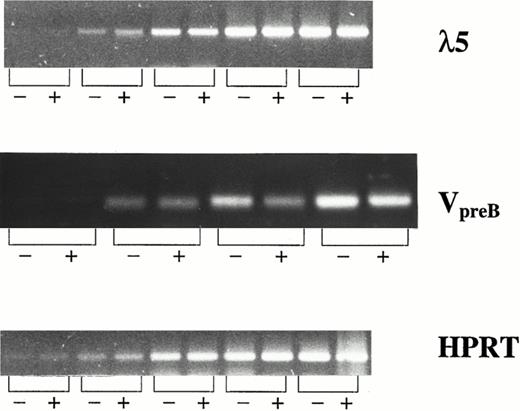
λ5 and VpreB , which constitute the surrogate light chain, are specifically expressed in B-cell progenitor cells and not in mature B cells or cells of other lineages.39-42 Thus, CD19− and CD19+ cells were sorted from FL + IL-7–stimulated cultures and investigated for expression of λ5 and VpreB by RT-PCR (Fig 4). Interestingly, CD19− and CD19+ cells expressed λ5 and VpreB at comparable levels, further supporting that both populations represent committed B-cell progenitors.
λ5 and VpreB expression in CD19− and CD19+ cells generated in response to FL + IL-7. mRNA expression of λ5, VpreB , and HPRT (control) was investigated by RT-PCR on CD19− (−) and CD19+ (+) cells isolated by fluorescence-activated cell sorting (FACS) as described in the Materials and Methods. For all three genes, the PCR amplification was stopped at intervals of five cycles (starting at 15 cycles). The PCR products generated by using primers for λ5, VpreB , and HPRT, resulted in products of 371, 343, and 248 bp, respectively.
λ5 and VpreB expression in CD19− and CD19+ cells generated in response to FL + IL-7. mRNA expression of λ5, VpreB , and HPRT (control) was investigated by RT-PCR on CD19− (−) and CD19+ (+) cells isolated by fluorescence-activated cell sorting (FACS) as described in the Materials and Methods. For all three genes, the PCR amplification was stopped at intervals of five cycles (starting at 15 cycles). The PCR products generated by using primers for λ5, VpreB , and HPRT, resulted in products of 371, 343, and 248 bp, respectively.
Lin−Sca-1+c-kit+ progenitor cells responsive to IL-10 are uncommitted progenitor cells with both myeloid and lymphoid potential. We have previously demonstrated that FL + IL-7–responsive Lin−Sca-1+ progenitor cells represent predominantly uncommitted progenitor cells in that they also can form myeloid progeny.19 To exclude the possibility that clones formed in response to FL + IL-7 + IL-10 (which ultimately would develop into B220+ colonies; Table 3), represented predominantly progenitor cells already committed to the B-cell lineage, FL + IL-7 + IL-10–induced clones were restimulated with a combination of cytokines (KL + IL-3 + G-CSF ) promoting myeloid differentiation. After 4 days incubation, 80% of the clones formed in response to FL + IL-7 + IL-10 maintained a potential to develop into large colonies consisting of mature myeloid cells (Table 4), demonstrating the combined myeloid and lymphoid potential of FL + IL-7 + IL-10–responsive Lin−Sca-1+c-kit+ progenitor cells. After 10 days incubation in FL + IL-7 + IL-10, only a low fraction (7%) of the FL + IL-7 + IL-10–responsive progenitors had the potential to produce myeloid progeny, suggesting that most FL + IL-7 + IL-10–responsive progenitors had committed to the B-cell lineage (Table 4).
Myeloid Potential of FL + IL-7 + IL-10–Responsive Lin−Sca-1+c-kit+ Progenitor Cells
| . | No. of Clones . | % Clones With Myeloid Potential . |
|---|---|---|
| Day 4 | 39 (1) | 80 (15) |
| Day 10 | 42 (4) | 7 (1) |
| . | No. of Clones . | % Clones With Myeloid Potential . |
|---|---|---|
| Day 4 | 39 (1) | 80 (15) |
| Day 10 | 42 (4) | 7 (1) |
A total of 180 Lin−Sca-1+c-kit+ cells were seeded at a concentration of 1 cell per well in complete IMDM and supplemented with FL + IL-7 + IL-10 at optimal concentrations. After 4 and 10 days of incubation, proliferating clones were identified (>2 cells) and KL + IL-3 + G-CSF added to stimulate myeloid differentiation. After an additional 10 days of incubation, clones were examined morphologically for myeloid cell production following Giemsa staining. Data represent the mean percentage (SD) of the fraction of clones with myeloid potential from three separate experiments. In comparison, 6 (2)% of the colonies formed from Lin−Sca-1+c-kit+ cells cultured throughout the incubation period with FL + IL-7 + IL-10 contained myeloid cells.
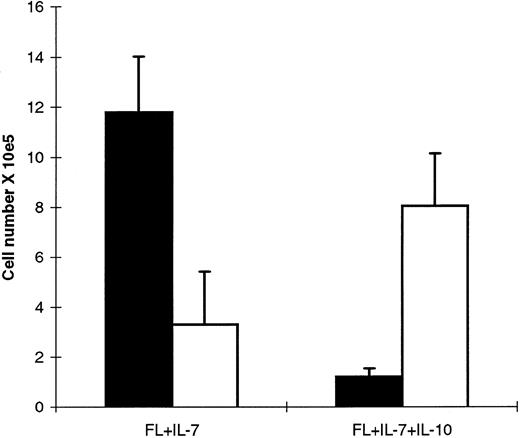
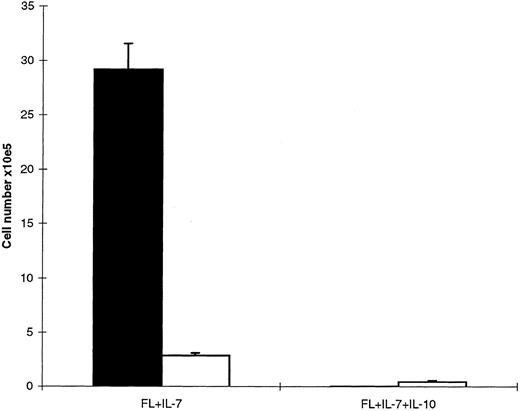
IL-10 inhibits growth of CD19+, but not CD19− proB cells. Although IL-10 selectively promoted FL + IL-7–stimulated formation of proB cells from Lin−Sca-1+kit+ progenitor cells (as measured after 12 days of incubation; Fig 1), a careful and daily assessment of the cultures indicated that after approximately 10 days of incubation, FL + IL-7 + IL-10–containing cultures (unlike FL + IL-7–stimulated cells ) ceased to expand, and rather a progressive reduction in cell numbers and viability was observed after 12 days of culture (Veiby and Jacobsen, unpublished observation, 1996). In agreement with this, IL-10 potently inhibited FL + IL-7–stimulated proliferation of proB cells obtained after 12 days incubation of Lin−Sca-1+c-kit+ progenitors in FL + IL-7 (Fig 5). To more specifically define the switch from a growth stimulatory to inhibitory effect of IL-10 on proB-cell growth, we assessed the effects of adding a neutralizing anti-IL–10 antibody to FL + IL-7 + IL-10–stimulated cultures of Lin−Sca-1+c-kit+ cells after 7 or 10 days incubation (Fig 6). If IL-10 was neutralized after 7 days incubation, a reduction in [3H]-TdR incorporation over the next 36 hours was observed, suggesting that IL-10 at this time still had a stimulatory effect on proB-cell proliferation (Fig 6A; P < .05). In contrast, neutralization of IL-10 after 10 days of incubation significantly enhanced proliferation (Fig 6B, P < .05), implicating that IL-10 at this stage rather inhibited growth. Similar experiments examining the effect of IL-10 on FL + IL-7–pretreated cultures showed stimulatory effect on cultures stimulated with FL + IL-7 for 7 days, but an inhibitory effect after 10 days (Veiby and Jacobsen, unpublished observations, 1996). It is noteworthy that cells stimulated for 7 days with FL + IL-7 were B220+CD19−, whereas after 10 days, cells were predominantly B220+CD19+ (Veiby and Jacobsen, unpublished observations, 1996).
IL-10 inhibits FL + IL-7–stimulated proB cell proliferation. A total of 500 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in the presence of FL + IL-7. After 12 days incubation, 1 × 105 of the generated B220+ cells were replated in triplicates in complete IMDM in medium alone, FL + IL-7, or FL + IL-7 + IL-10, as indicated. Cells were incubated for 48 hours and pulsed with 1 μCi [3H]-TdR during the last 12 hours of incubation. Samples were harvested and counted in a beta-counter as described in the Materials and Methods. Results represent the mean cpm (SEM) from three independent experiments.
IL-10 inhibits FL + IL-7–stimulated proB cell proliferation. A total of 500 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in the presence of FL + IL-7. After 12 days incubation, 1 × 105 of the generated B220+ cells were replated in triplicates in complete IMDM in medium alone, FL + IL-7, or FL + IL-7 + IL-10, as indicated. Cells were incubated for 48 hours and pulsed with 1 μCi [3H]-TdR during the last 12 hours of incubation. Samples were harvested and counted in a beta-counter as described in the Materials and Methods. Results represent the mean cpm (SEM) from three independent experiments.
IL-10 bidirectionally modulates growth and proB cell formation from Lin−Sca-1+c-kit+ progenitor cells. A total of 1,000 Lin−Sca-1+c-kit+ cells was plated in complete IMDM in the presence of FL + IL-7 + IL-10. After 7 (A) and 10 (B) days incubation, 10% of the resulting cells were replated per well in round bottomed 96-well plates in FL + IL-7 + IL-10 in the presence of a neutralizing IL-10 antibody (anti-IL–10) or an isotype-matched control antibody (ctr ab). After 24 hours of incubation, wells were pulsed with 1 μCi [3H]-TdR for an additional 12 hours incubation, at which time samples were harvested and counted in a beta-counter as described in the Materials and Methods. The results represent the mean cpm (SEM) from three independent experiments. In each experiment, each group was performed in triplicate.
IL-10 bidirectionally modulates growth and proB cell formation from Lin−Sca-1+c-kit+ progenitor cells. A total of 1,000 Lin−Sca-1+c-kit+ cells was plated in complete IMDM in the presence of FL + IL-7 + IL-10. After 7 (A) and 10 (B) days incubation, 10% of the resulting cells were replated per well in round bottomed 96-well plates in FL + IL-7 + IL-10 in the presence of a neutralizing IL-10 antibody (anti-IL–10) or an isotype-matched control antibody (ctr ab). After 24 hours of incubation, wells were pulsed with 1 μCi [3H]-TdR for an additional 12 hours incubation, at which time samples were harvested and counted in a beta-counter as described in the Materials and Methods. The results represent the mean cpm (SEM) from three independent experiments. In each experiment, each group was performed in triplicate.
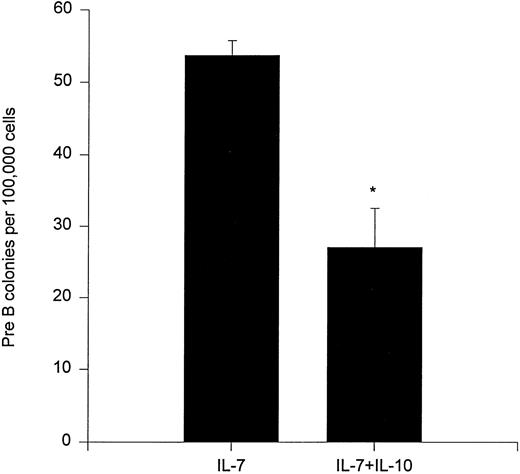
We also investigated whether IL-10 could affect the clonogenic growth of bone marrow preB progenitor cells. In agreement with a previously published report,25 IL-10 inhibited formation of preB cell colonies (Fig 7).
Effect of IL-10 on preB cell colony formation. A total of 100,000 unfractionated bone marrow cells were plated in 0.9% methylcellulose in the presence of IL-7 and/or IL-10. After 7 days of incubation at 37°C, 5% CO2 in air, preB cell colonies were counted. Results represent the mean ± SEM of three independent experiments with duplicate dishes for each group in each experiment. * P < .05, as compared with IL-7 alone.
Effect of IL-10 on preB cell colony formation. A total of 100,000 unfractionated bone marrow cells were plated in 0.9% methylcellulose in the presence of IL-7 and/or IL-10. After 7 days of incubation at 37°C, 5% CO2 in air, preB cell colonies were counted. Results represent the mean ± SEM of three independent experiments with duplicate dishes for each group in each experiment. * P < .05, as compared with IL-7 alone.
The finding that IL-10 stimulated growth of FL + IL-7–expanded cells before aquisition of CD19 expression, but inhibited growth of cells after appearance of CD19 expression, suggested that IL-10 might differentially affect growth of CD19− and CD19+ proB cells. Accordingly, the lack of CD19 expression on cells generated in response to FL + IL-7 + IL-10 could be a consequence of IL-10 preferentially inhibiting FL + IL-7–stimulated generation of CD19+ cells from CD19− proB cells. To address this, Lin−Sca-1+c-kit+ cells expanded in FL + IL-7 for 12 days were sorted into B220+CD19− and B220+CD19+ subpopulations and investigated for responsiveness to FL + IL-7, in the absence and presence of IL-10. CD19− cells recultured for 10 days expanded efficiently in FL + IL-7, as well as FL + IL-7 + IL-10 (68-fold and 41-fold, respectively; Fig 8). Most cells generated from CD19− cells recultured in FL + IL-7 became CD19+, whereas most cells recultured in FL + IL-7 + IL-10 remained CD19− (Fig 8). Thus, a slightly higher number of CD19− cells were generated in IL-10–supplemented cultures. Similarly, after 5 days of incubation, CD19− cells had expanded equally well in FL + IL-7 and FL + IL-7 + IL-10, and a fraction of cells expanded in FL + IL-7 (23% to 41%) had already acquired CD19-expression (Veiby and Jacobsen, unpublished observation, 1997). Thus, CD19− proB cells develop into CD19+ proB cells when cultured in FL + IL-7. Whereas these CD19− cells expand efficiently in the presence of IL-10, they do not acquire CD19 expression.
FL + IL-7–stimulated CD19− cells expand in the absence and presence of IL-10. A total of 500 Lin−Sca-1+c-kit+ cells was cultured in the presence of FL + IL-7 for 12 days. The resulting cells were counted and CD19− cells sorted as described in the Materials and Methods. Subsequently, 23,000 CD19− cells (mean of five experiments) were cultured in FL + IL-7 or FL + IL-7 + IL-10. Based on the total number of cells generated and analysis of CD19 expression, the number of CD19+ (▪) and CD19− (□) cells was calculated. Data represent absolute cell numbers (mean ± SEM) of five separate experiments.
FL + IL-7–stimulated CD19− cells expand in the absence and presence of IL-10. A total of 500 Lin−Sca-1+c-kit+ cells was cultured in the presence of FL + IL-7 for 12 days. The resulting cells were counted and CD19− cells sorted as described in the Materials and Methods. Subsequently, 23,000 CD19− cells (mean of five experiments) were cultured in FL + IL-7 or FL + IL-7 + IL-10. Based on the total number of cells generated and analysis of CD19 expression, the number of CD19+ (▪) and CD19− (□) cells was calculated. Data represent absolute cell numbers (mean ± SEM) of five separate experiments.
Next, CD19+ cells were compared for their growth responsiveness to FL + IL-7 and FL + IL-7 + IL-10, and a distinctly different pattern was observed than for CD19− cells. CD19+ cells cultured in FL + IL-7 expanded 146-fold after 10 days of incubation and contained almost exclusively CD19+ cells (Fig 9). In contrast, only a low number of cells were observed in cultures supplemented with IL-10, and these cells were CD19− (Fig 9). We speculated that this limited growth in response to FL + IL-7 + IL-10 after 10 days of incubation could result from expansion of contaminating CD19− cells in the starting population, as the purity of the sorted CD19+ cells was limited to 95% to 99% (see Materials and Methods). In agreement with this, CD19+ cells cultured in FL + IL-7 + IL-10 declined by 90% following 3 days of incubation (too few cells for analysis of CD19 expression), at which time, cells cultured in FL + IL-7 had expanded sixfold (Fig 10). Thus, FL + IL-7 stimulates the formation of CD19− and CD19+ proB cells from Lin−Sca-1+c-kit+ progenitor cells, of which CD19− cells expand efficiently in the presence of IL-10, whereas FL + IL-7–stimulated growth of CD19+ cells is efficiently blocked by IL-10.
IL-10 blocks FL + IL-7–stimulated growth of CD19+ proB cells. An average of 22,000 sorted CD19+ cells (mean of four experiments) generated from Lin−Sca-1+c-kit+ cells in response to FL + IL-7 (as in Fig 8), were replated in FL + IL-7 or FL + IL-7 + IL-10 as indicated. After 10 days of incubation, viable cells were counted and CD19 expression determined by flow cytometry. (▪), CD19+ cells; (□), CD19− cells. Data represent absolute cell numbers (mean ± SEM) of four separate experiments.
IL-10 blocks FL + IL-7–stimulated growth of CD19+ proB cells. An average of 22,000 sorted CD19+ cells (mean of four experiments) generated from Lin−Sca-1+c-kit+ cells in response to FL + IL-7 (as in Fig 8), were replated in FL + IL-7 or FL + IL-7 + IL-10 as indicated. After 10 days of incubation, viable cells were counted and CD19 expression determined by flow cytometry. (▪), CD19+ cells; (□), CD19− cells. Data represent absolute cell numbers (mean ± SEM) of four separate experiments.
Growth of CD19+ cells after short-term (3 days) incubation in FL + IL-7 or FL + IL-7 + IL-10. Sorted CD19+ cells were cultured in FL + IL-7 in the presence or absence of IL-10 as indicated. After 3 days of incubation, the number of viable cells was establsihed and CD19 expression examined by flow cytometry. Data represent absolute cell numbers (mean ± SEM) of four separate experiments.
Growth of CD19+ cells after short-term (3 days) incubation in FL + IL-7 or FL + IL-7 + IL-10. Sorted CD19+ cells were cultured in FL + IL-7 in the presence or absence of IL-10 as indicated. After 3 days of incubation, the number of viable cells was establsihed and CD19 expression examined by flow cytometry. Data represent absolute cell numbers (mean ± SEM) of four separate experiments.
DISCUSSION
Whereas a number of cytokines have been shown to stimulate the growth and multilineage myeloid differentiation of candidate bone marrow stem cell populations in the absence of stroma,27,43 the earliest phases of B-cell development in the bone marrow have been considered to be stroma cell-dependent.44-46 We recently showed that IL-7 in combination with FL can selectively promote early B-cell development from Lin−Sca-1+c-kit+ progenitor cells with a combined myeloid and lymphoid potential.19 While constituting only 0.1% of the nucleated bone marrow cells, Lin−Sca-1+c-kit+ cells contain all long-term repopulating pluripotent stem cells, is depleted in lineage committed progenitor cells, contain predominantly multipotent progenitor cells capable of growing under defined in vitro conditions, and thus represents an excellent population for studies of lineage commitment and differentiation from uncommitted progenitor cells.29-33
Because optimal myeloid clonogenic growth of Lin−Sca-1+c-kit+ progenitor cells requires the combined action of at least three to four cytokines,27,33,43 we argued that other known (or not yet identified) cytokines might act to further enhance the ability of FL + IL-7 to promote B-cell commitment and development from uncommitted progenitor cells. For several reasons, IL-10 appeared to be a candidate that might affect the earliest stages of B-cell development. First, IL-10 has been shown to be a multifunctional regulator of B-lymphocyte functions, in that it can promote growth at different maturation stages, viability, differentiation, and isotype switching.1-3 In addition, IL-10 has been shown to enhance multilineage myeloid/erythroid growth of primitive murine bone marrow progenitor cells in combination with IL-3,7 suggesting that the receptor for IL-10 might be expressed on very primitive hematopoietic progenitor cells. Although the increased cell production from the multipotent progenitor cells in this previous study did not include lymphocytes, this could result from a lack of an appropriate and essential signal for B-cell development.
In the present studies, we show for the first time that IL-10 in combination with FL and IL-7 promotes development of proB cells from primitive (Lin−Sca-1+c-kit+) and uncommitted bone marrow progenitor cells, thus defining a new stage at which IL-10 might act to promote B lymphopoiesis. The ability of IL-10 to enhance proB-cell development appears to be directly mediated on the primitive progenitors because the stimulatory effect of IL-10 was observed when Lin−Sca-1+c-kit+ cells were cultured individually and because as little as 24 hours delayed addition of IL-10 to such cultures abrogated the ability of IL-10 to enhance the cloning frequency of Lin−Sca-1+c-kit+ progenitor cells in response to FL + IL-7. This is noteworthy because it rules out that the stimulatory effect of IL-10 might result from its ability to affect cytokine synthesis and/or release from potentially contaminating accessory cells. In addition, it suggests that although early B-cell development in response to IL-10 might be promoted by cell to cell contact, such a community effect is not a requisite.
IL-10–induced enhancement of proB-cell formation in response to FL + IL-7 resulted from an increased number of Lin−Sca-1+c-kit+ progenitor cells being recruited to proliferate, as well as enhanced proliferation of individual progenitors, observed as an increase in colony numbers and size, respectively. When acting alone, IL-10 had no ability to stimulate growth of Lin−Sca-1+c-kit+ progenitor cells. Thus, as is also the case for other effects of IL-10 on growth, differentiation, and isotype switching of mature B cells,1-3 the ability of IL-10 to enhance early B-cell development requires coactivation signals. In fact, IL-10 in combination with IL-7, the primary regulator of murine B-cell development,9 10 also failed to induce any growth of Lin−Sca-1+c-kit+ progenitor cells. Thus, the ability of IL-10 to potently and selectively promote B-cell development from uncommitted Lin−Sca-1+c-kit+ progenitor cells requires simultaneous activation by IL-7 and FL.
KL, although somewhat disputed, has also been implicated in early B-cell development in combination with IL-7.11-14 However, in a recent study, we showed that FL is far superior to KL in promoting growth and in particular proB-cell formation from Lin−Sca-1+c-kit+ progenitor cells.19 In the present studies, KL + IL-7–induced growth was enhanced eightfold by IL-10, and although 90% of the resulting cells were myeloid, a 27-fold increase in absolute numbers of B220+ cells was observed, as well. However, when directly compared, FL + IL-7 + IL-10 stimulated the production of 100-fold more B220+ cells than KL + IL-7 + IL-10, despite all responsive progenitors expressing high levels of c-kit. Thus, IL-10 can enhance IL-7–dependent early B-cell development in combination with KL, but with much lower potency and selectivity than when combined with FL.
It might be important that whereas a large number of cytokines have been shown to enhance growth and selective myeloid differentiation from primitive hematopoietic progenitor cells,27,43 few identified cytokines have been found to selectively stimulate B-cell development from uncommitted progenitor cells. Thus, among identified cytokines, IL-10 together with FL and IL-7 might have a unique ability to potently and selectively promote early B-cell development from uncommitted murine bone marrow progenitor cells. This might simply reflect that other not yet identified cytokines might be involved in stimulating early B-cell development, or that there is less redundancy in early B-lymphoid development than what might be the case for myelopoiesis,43 or that direct cell to cell interactions might play a more critical role in early B lymphopoiesis. Whereas both IL-7 and FL appear to be essential for optimal B-cell development,9,10,16 no defects in B-cell development were observed in IL-10–deficient mice,47 suggesting that IL-10 is not essential for steady-state B-cell development. Although this might suggest that the actions of IL-10 in murine B-cell development are redundant, it remains possible, as has been shown for other cytokines,43 that an important effect of IL-10 in this process might only be uncovered in response to a challenge requiring increased cellular turnover.
Particularly noteworthy and of potential biologic significance was the observation that the B220+ cells generated in response to FL + IL-7 + IL-10 (unlike those formed in response to FL + IL-7 alone) did not express CD19. It has recently been shown that a subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains NK cell precursors.35 However, cells generated in response to FL + IL-7 + IL-10 did not express the NK cell antigen NK.1.1, but expressed CD24 and CD43 and not cμ, placing them among proB cells according to criteria defined by Hardy et al.34 In addition, they were negative for erythroid, myeloid, and T-cell antigens. Finally, the generated CD19− cells express components of the surrogate light chain, λ5 and VpreB , which are specific for B-cell progenitors and essential for normal B-cell development.39-42
Acquisition of CD19 expression is thought to occur early after B-cell commitment.35-37 However, the present studies suggest that stimulation of Lin−Sca-1+c-kit+ bone marrow progenitor cells with FL + IL-7 initially results in the generation of B220+CD19− proB cells, which subsequently develop into B220+CD19+ proB cells. This is supported by the finding that FL + IL-7 efficiently stimulated generation of CD19+ proB cells from purified CD19− cells. It is noteworthy that FL + IL-7–stimulated cultures contain both CD19+ and CD19− cells after 12 days of culture, and that both subpopulations are maintained/expanded over more prolonged culture (Veiby and Jacobsen, unpublished observation, 1996). Thus, it appears as if CD19− proB cells are maintained in FL + IL-7–supplemented cultures, despite also developing into CD19+ proB cells. Whether or not FL and/or IL-7 are required for the maintainence of such early CD19− B-cell prognitors is being addressed in ongoing studies.
IL-10 enhanced FL + IL-7–stimulated B220+ cell proliferation after 7 days of incubation, at which time cells formed in response to FL + IL-7 did not yet express CD19. In contrast, the ability of IL-10 to potently inhibit proB-cell proliferation after 10 days of incubation coincided with the proB cells produced in response to FL + IL-7 becoming CD19+. In agreement with this, the CD19− and CD19+ proB cells generated in response to FL + IL-7 differed in their responsiveness to IL-10, in that FL + IL-7–stimulated CD19− proB cells expanded efficiently in the presence of IL-10, whereas FL + IL-7–stimulated growth of CD19+ proB cells was blocked in response to IL-10. Thus, IL-10 might act to maintain/expand a pool of early (CD19−) B-cell progenitor cells, while inhibiting their development into CD19+ proB cells. In agreement with others,25 we also found that preB cell (also CD19+) colony formation from unfractionated bone marrow was inhibited by IL-10. Although, such a bidirectional (stimulatory and inhibitory) effect on different stages of B-cell development was somewhat surprising, it supports a number of previous studies showing apparently contrasting effects of IL-10 on growth and viability at different B-cell maturation stages.3 20 The mechanism for IL-10–induced growth suppression of CD19+ proB cells in the present studies remains unclear, and we found no evidence supporting that IL-10 (alone) induces apoptosis of (FL + IL-7–derived) CD19+ proB cells (Veiby and Jacobsen, unpublished observations, 1996).
Also other recent studies support the existence of primitive CD19− B-cell progenitors in murine bone marrow.42,48 Li et al48 showed the presence of B220+CD19− cells in unmanipulated bone marrow, which as the present CD19− proB cells expressed B-cell specific genes and, which in agreement with the present studies, appeared to represent earlier proB cells than those expressing CD19. Similarly, our own studies of transgenic mice expressing human CD25 under the control of the λ5 upstream regulatory region, showed the existence of B220+CD19−λ5+ proB cells, which in the presence of IL-7 and stroma, developed into B220+CD19+λ5+ proB cells.42 Accordingly, the present in vitro FL + IL-7–supplemented culture system appears to mimic the earliest stages of normal B-cell development, underscoring its potential use in future studies of the molecular mechanisms underlying B-cell commitment and early development.
Interestingly, Li et al48 identified two distinct subpopulations of CD19− proB cells based on distinct patterns of expression of λ5 and other early B lineage-specific genes. Additional studies will be required to determine whether λ5− and potentially other subsets of early proB cells are generated from Lin−Sca-1+c-kit+ progenitors in response to FL + IL-7.
In conclusion, IL-10 potently and directly promotes the earliest stage of B-cell development from uncommitted murine bone marrow progenitor cells in combination with FL and IL-7, whereas it inhibits growth of CD19+ proB cells implicating a dual role of IL-10 in early B-cell development.
ACKNOWLEDGMENT
We thank Per Anders Bertilsson and Joe Trask for expert assistance in flow cytometric cell sorting.
Supported by grants from the Swedish Medical Research Council, Kungliga Fysiografiska Society, and Österlunds, Kocks, Crafoords and Zoegas Foundations. A.M. is a recipient of a Fellowship from Pharmacia Upjohn.
Address reprint requests to Sten E.W. Jacobsen, MD, Stem Cell Laboratory, Department of Internal Medicine, University Hospital of Lund, 221 85 Lund, Sweden.


![Fig. 5. IL-10 inhibits FL + IL-7–stimulated proB cell proliferation. A total of 500 Lin−Sca-1+c-kit+ cells was seeded in complete IMDM in the presence of FL + IL-7. After 12 days incubation, 1 × 105 of the generated B220+ cells were replated in triplicates in complete IMDM in medium alone, FL + IL-7, or FL + IL-7 + IL-10, as indicated. Cells were incubated for 48 hours and pulsed with 1 μCi [3H]-TdR during the last 12 hours of incubation. Samples were harvested and counted in a beta-counter as described in the Materials and Methods. Results represent the mean cpm (SEM) from three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4321/3/m_bl_0029f5.jpeg?Expires=1765136367&Signature=unQviVRcgM~BoHwi2keRgCGgT~GoMnsYsDd~-wHgUix2qbNeoLho1W1GQdzcBa5Xg~zSZ3DqORG4s10lpq010oNEd236FkOqVJzXYzjCgaJQAfVzE~-BQ8U25jAvP9CFPDBGf-J2mxGLl5qoPy1fObOH4fJpIhCUcxf5HNzIB~4TZLdi4YsGquCqVEqORpVsyjMoxCbJFi8rtPFCi0LhhlpX~2Wv4-~Xd8GNQwbh8TYZIjsruwI9ozg0sW5A8TtVFPuyEtQyBbyNQu6CgX0iUxjqYw4MTkMuuGvZZPsctRXhLpZHKHP8a~jBlixC1szvKssma1G7pelHciHvEDsVWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. IL-10 bidirectionally modulates growth and proB cell formation from Lin−Sca-1+c-kit+ progenitor cells. A total of 1,000 Lin−Sca-1+c-kit+ cells was plated in complete IMDM in the presence of FL + IL-7 + IL-10. After 7 (A) and 10 (B) days incubation, 10% of the resulting cells were replated per well in round bottomed 96-well plates in FL + IL-7 + IL-10 in the presence of a neutralizing IL-10 antibody (anti-IL–10) or an isotype-matched control antibody (ctr ab). After 24 hours of incubation, wells were pulsed with 1 μCi [3H]-TdR for an additional 12 hours incubation, at which time samples were harvested and counted in a beta-counter as described in the Materials and Methods. The results represent the mean cpm (SEM) from three independent experiments. In each experiment, each group was performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4321/3/m_bl_0029f6a.jpeg?Expires=1765136367&Signature=pjNfrZVCjtCb7VhZl7dOcAB6OJ1JKAIZ~h7s-RjHrML~xxGYdnUdrkmw~9LA-lwVFH~zSseiPeYHD4ZF2B9NosLMszNj395f8c0ehgmBKSZG9ngdFkd6xXf7hw7XcPbv1eo9FZUkRbb52ywOpKcsSuKoBveaeOyL0nDB8XDZzvPubysqiK9F2E45HrZw3yz7JPsR6Mn5B65j9V324fnrTk6gr~7R1ZQr7HQEL3FzpKPEk0wpl828r3wpQJYAXmUSa3SZPDgcTIYbMRier7VbNarQA5clnG2KiLs~rx0~OV0S7rQQLCWN3zz4TJlZ6xaURHfUSjNdwD31V96fCWhDhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. IL-10 bidirectionally modulates growth and proB cell formation from Lin−Sca-1+c-kit+ progenitor cells. A total of 1,000 Lin−Sca-1+c-kit+ cells was plated in complete IMDM in the presence of FL + IL-7 + IL-10. After 7 (A) and 10 (B) days incubation, 10% of the resulting cells were replated per well in round bottomed 96-well plates in FL + IL-7 + IL-10 in the presence of a neutralizing IL-10 antibody (anti-IL–10) or an isotype-matched control antibody (ctr ab). After 24 hours of incubation, wells were pulsed with 1 μCi [3H]-TdR for an additional 12 hours incubation, at which time samples were harvested and counted in a beta-counter as described in the Materials and Methods. The results represent the mean cpm (SEM) from three independent experiments. In each experiment, each group was performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4321/3/m_bl_0029f6b.jpeg?Expires=1765136367&Signature=4fGCqBbifzhDPjSxUFCsDegbbkSMxzjL5infB18vub9guSOJZzefWkD8hiz2AypqJwhA6vvtzcyCoV7Yg6~Yyl5v73A7jDN1eXpepld1wndliz8OCfswD1ZJQkP8-lsfHeUUdYfQLEB-QQB9SWQE79YdaxRaGMz7FvJqX51rjKqbxTvHLI0yO~G21a90PTO5NcZlXUx3jVNNajK~s0H0EU~yogGgNgRxjVi0r4WeavEWeSmtXC8ievOU0-e9yKnn5WLLs9D2xBn6ta~MGaabMcWgRzb7-h3mCGnJ8igaQzNQS6yN~czkcSH~s~YSP~6nu6umFNl9XbdgjMpOrpCG0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal