Abstract
High-dose chemotherapy with hematopoietic progenitor cell support is administered increasingly to selected categories of patients with high-risk malignancies. Bone marrow and/or peripheral blood progenitor cells (PBPCs) are commonly cryopreserved with the cryoprotectant dimethyl sulfoxide (DMSO), which can cause a variety of systemic side effects when the graft is thawed and infused. The progenitor cells thought to be responsible for hematopoietic recovery express the CD34 antigen and constitute 1% to 3% of the marrow cells and 0.5% of the PBPC fraction. Transplantation of a CD34+ graft would markedly reduce the volume and thus the amount of DMSO required, thereby decreasing the infusion-related toxicities. In this study, 89 high-risk breast cancer patients received high-dose therapy and were randomized to receive an autologous CD34+ marrow graft (Arm A) versus a standard buffy coat fraction (Arm B). After marrow infusion, significant increases in diastolic and systolic blood pressure, as well as significant decreases in heart rate, were documented in Arm B compared to Arm A patients (P < .001). None of the patients in Arm A experienced any clinically serious adverse events associated with the marrow infusion compared to 6% of the Arm B patients. The median time to neutrophil engraftment was 13 days for Arm A and 11 days for Arm B patients (P = .218). The median time to platelet engraftment was 27 days for Arm A and 20 days for Arm B patients (0.051). There were no other significant differences between the two arms of the study with respect to thrombocytopenia-related complications or immune function reconstitution. Additionally, patients on Arm A who received ≥1.2 × 106 CD34+ cells/kg had no delay in platelet recovery (22 days), compared to patients on Arm B, who also received greater than 1.2 × 106 CD34+ cells/kg (20 days) (P = .604). In conclusion, this prospective randomized study demonstrates that breast cancer patients who receive high-dose therapy with autologous CD34+ marrow support have reduced marrow infusion-related toxicity, comparable time to neutrophil engraftment and immune function recovery posttransplant, and for those who receive <1.2 × 106 CD34+ cells/kg, comparable time to platelet engraftment compared to women who receive buffy coat fractions of marrow.
HIGH-DOSE chemotherapy with autologous hematopoietic progenitor cell support is administered increasingly to selected categories of patients with high-risk hematologic malignancies1 and solid tumors.2,3 The stem and progenitor cells required for hematopoietic recovery express the CD34 antigen, a 115-kD glycoprotein on the surface of 1% to 3% of human bone marrow (BM) cells and approximately 0.5% of peripheral blood progenitor cells (PBPCs).4 In animal models5 and preliminary human studies,6 transplantation of the CD34+ fraction resulted in hematopoietic recovery rates comparable to those of unseparated marrow or PBPC fractions. Typically, BM and/or PBPCs are collected from the patient and cryopreserved in liquid nitrogen with the cryoprotectant dimethyl sulfoxide (DMSO), which protects the integrity of the hematopoietic cells during the freezing procedure.7 Following high-dose therapy administration, the frozen marrow is thawed and immediately infused. The DMSO contained in the thawed graft can cause a variety of systemic side effects including nausea, vomiting,8 diarrhea9; severe hemolysis mimicking a transfusion reaction10; anaphylactic reactions manifested by rashes, flushing, and occasionally bronchospasm11,12; renal failure13; diastolic and systolic hypertension14; bradycardia; heart block15-17; and rarely pulmonary edema or cardiac arrest.18,19 An additional concern with autologous hematopoietic cell transplantation is the potential infusion of clonogenic tumor cells contained in the hematopoietic cell graft, which could lead to relapse of disease.20
Transplantation of enriched CD34+ cells could have several potential benefits. Isolating the normal progenitor cells from the bulk harvest markedly reduces the volume of the graft. The volume reduction would result in a decrease in the amount of DMSO required, thereby decreasing the infusion-related toxicities described above. Furthermore, the CD34 antigen is generally not expressed by epithelial tumors, including breast cancer,4 thus those types of tumor cells should be depleted by selection for normal CD34+ progenitors. The primary objective of the prospective randomized clinical trial was to compare the toxicity of the transplant infusion when using CD34+ marrow cells isolated with the CEPRATE SC stem cell concentration system (CellPro Inc, Bothell, WA) to standard unpurified BM buffy coat preparations. Additionally, we wanted to determine whether the CD34-selection procedure resulted in an autograft with comparable rate of hematopoietic recovery compared to a standard buffy coat preparation. Since the eligibility criteria included stage II, III, and IV patients and required that patients have no histologic evidence of breast cancer in the marrow, a beneficial “purging” effect resulting from the CD34-selection procedure was not evaluated in this study. Other published data have reported tumor reduction following CD34-selection procedure.6 21
MATERIALS AND METHODS
Study design. Women with stage II/III breast cancer involving ≥10 axillary lymph nodes or stage IV disease, which was stable or responding to induction therapy were enrolled on this study at one of five Bone Marrow Transplant Centers: University of Colorado (CU), University of Texas-San Antonio (UTSA), University of Texas-MD Anderson Cancer Center (MDACC), Emory University, and the University of Pittsburgh. Pretransplant evaluation included extent of disease staging with computerized axial tomography scans of the head, chest, abdomen, and pelvis; radionuclide bone scans; and bilateral BM biopsies. Eligibility criteria required that marrow biopsies obtained within 4 weeks of study entry have no histologic evidence of breast cancer on routine examination (hematoxylin and eosin staining). Patients were required to have acceptable cardiac, pulmonary, and renal function. Prior chemotherapy exposure was limited to two or fewer regimens, including adjuvant treatment.
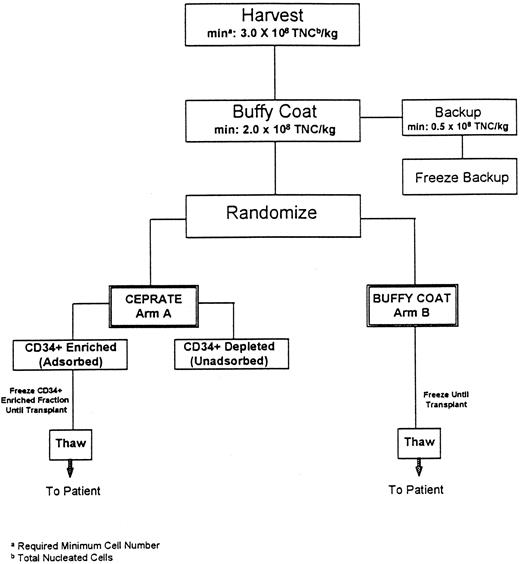
Study schema. As shown in the Fig 1 schema, BM was procured from all patients who were then prospectively randomized to have their marrow either CD34-selected (Arm A-CD34+) or processed using standard methods (Arm B-Buffy Coat) and cryopreserved. Patients then received the high-dose chemotherapy regimen determined by the enrolling transplant program on three or four consecutive days, day −7 through day −3. Each transplant center used a single high-dose chemotherapy regimen for all patients (CU: cyclophosphamide [CPA]/cisplatin/BCNU; UTSA: CPA/thiotepa; MDACC: CPA/thiotepa/BCNU; Emory: CPA/carboplatin/thiotepa; and Pittsburgh: CPA/carboplatin/VP16). Forty-eight hours after chemotherapy cessation, the patient's marrow was thawed and infused. Randomization was performed at each site independently so that the two arms of the study would be balanced with respect to the number of patients who received each of the high-dose chemotherapy regimens administered. Each center employed its own standardized supportive care protocols for patients on both arms of the study.
Flow diagram of autologous marrow processing procedure. To remain on the study, at each step outlined, patients were required to have a minimum (min) of 3.0 × 108 total nucleated cells per kilogram patients' weight (TNC/kg) at marrow harvest, and 2.0 × 108 TNC/kg in the buffy coat fraction with 0.5 × 108 TNC/kg as a backup fraction.
Flow diagram of autologous marrow processing procedure. To remain on the study, at each step outlined, patients were required to have a minimum (min) of 3.0 × 108 total nucleated cells per kilogram patients' weight (TNC/kg) at marrow harvest, and 2.0 × 108 TNC/kg in the buffy coat fraction with 0.5 × 108 TNC/kg as a backup fraction.
Study endpoints. There were two primary study endpoints. The first was successful engraftment, defined as neutrophil recovery on or before day +20 following marrow infusion. Neutrophil recovery was defined as the first day of a sustained absolute neutrophil count (ANC) ≥0.5 × 109/L. The second primary endpoint, cardiovascular toxicity following the marrow infusion, was defined as an increase in diastolic or systolic blood pressure, and/or a decrease in heart rate when compared to preinfusion levels. There were multiple secondary endpoints, including time to neutrophil engraftment as defined above, and time to platelet engraftment, defined as an unsupported platelet count of ≥20 × 109/L, number of bleeding episodes, platelet and red blood cell transfusions, infections, days hospitalized, and serious adverse events, as well as the extent of immune reconstitution posttransplant and the rates of progression-free and overall survival.
BM collection and processing methods. As outlined in Fig 1, the protocol required a minimum target number of marrow cells in the harvested fraction (≥3 × 108 total nucleated cells/kg) and the buffy coat fraction (≥2 × 108 total nucleated cells/kg), for the patient to remain on study. The BM was obtained from the posterior iliac crests of all patients under general anesthesia and a buffy coat concentrate was produced using a COBE 2991 Blood Cell Processor as previously described (COBE Laboratories, Lakewood, CO).6 For all patients, a fraction of backup buffy coat containing 0.5 × 108 nucleated cells/kg was cryopreserved, using a rate-controlled method, and stored at −196°C6 to be used should engraftment failure occur. For patients assigned to Arm B (Buffy Coat), the remaining buffy coat was cryopreserved as the primary graft using the same method. For Arm A patients, the remaining buffy coat fraction was CD34 selected with the CEPRATE System and cryopreserved as described below. Viability of the thawed cells was calculated at all of the sites using a Trypan Blue Dye exclusion assay (Sigma Inc, St Louis, MO).
CD34-selection procedure. The buffy coat marrow fraction was incubated with 20 μg/mL of the biotinylated-12.8 anti-CD34 monoclonal antibody in 150 mL of RPMI media containing 0.1% human serum albumin (HSA) 25% for 25 minutes at room temperature. The treated cells were washed twice with phosphate-buffered saline (PBS; GIBCO, Grand Island, NY) on the COBE 2991 Processor to remove any unbound antibody. The washed cells, in a volume of 300 mL of PBS, were then applied to a sterile column of avidin-linked polyacrylamide beads (CellPro Inc). After washing with PBS, the CD34+ cells were removed from the beads by gentle mechanical agitation and eluted with 90 mL of PBS into a collection bag containing 10 U/mL heparin and 4 mL of 25% HSA. After centrifugation for 10 minutes at 500g, the selected cells were resuspended in 76.5% medium 199 (M-199) (GIBCO), 7.5% DMSO (Research Industries Corp, Salt Lake City, UT), and 16% HSA to a final volume of 4.5 mL. A small aliquot was removed for analysis and the remaining cells were then cryopreserved in one (if ≤140 × 106 cells) 5 mL vial, or two (if >140 × 106 cells) 5 mL vials (Nalgene, Redmond, WA), using a rate-controlled method, and stored at −196°C.
Phenotypic analysis of marrow fractions. A small sample of each marrow graft was placed in a 1.0 mL cryotube with RPMI-1640 (GIBCO) containing 7% fetal bovine serum (FBS; Hyclone, Logan, UT) and shipped overnight to a central laboratory (Cytometry Associates, San Diego, CA) for flow cytometric analysis. Red cells were lysed with ammonium chloride and the remaining cells resuspended in a buffer containing PBS (GIBCO) with 1% bovine serum albumin (BSA; Sigma) and sodium azide (Sigma). Two μg/mL propidium iodide (Sigma), was used to detect nonviable cells; 5 × 105 cells per sample were stained with the phycoerythrin-conjugated anti-CD34 antibody, HPCA-2 (Becton Dickinson, San Jose, CA). Flow cytometric analysis was performed using a FACScan (Becton Dickinson); 100,000 events were counted for the buffy coat fractions and 20,000 events for the CD34-selected fractions.
Analysis of colony-forming units granulocyte-macrophage (CFU-GM). The tissue culture media employed contained a final concentration of 5.2% methylcellulose (Terry Fox Laboratories, Vancouver, BC, Canada), 30% FBS (HyClone), 14% alpha minimal essential media (GIBCO), 10% BSA (Boehringer-Mannheim, Indianapolis, IN), 1% beta-mercaptoethanol (Bio-Rad, Richmond, CA), and 1% methylprednisolone (Upjohn, Kalamazoo, MI). Additionally 5 U/mL of erythropoietin (Amgen Inc, Thousand Oaks, CA) and 10 ng/mL of IL-3 (Amgen) were added directly to each plate. Seeding densities of 2.5 × 104 cells/mL for buffy coat fractions and 1 × 103 cells/mL were employed with the CD34+ cell fractions. All samples were plated in quadruplicate in 35 × 10 mm gridded tissue culture plates (Nunc, Napperville, IL), and maintained at 37°C, 5% CO2 , and 95% humidity. After 14 days in culture, the plates were evaluated with an inverted microscope for myeloid progenitor cell growth by enumerating the number of colony-forming units in culture (CFC), which included CFU-GM, CFU-granulocyte erythroid-monocyte-macrophage, and burst forming unit-erythroid colonies using an inverted phase microscope.22
Statistical analysis. An “intent to treat” primary analysis of engraftment was performed that included all eligible and randomized patients. Patients who did not engraft on or before day +20 following the marrow infusion were considered engraftment failures. Patients who died of toxicity after marrow infusion but before engraftment were also considered engraftment failures. Estimates of the probability of progression-free survival and survival from the date of infusion were determined according to the Kaplan-Meier method23; comparisons of the treatment arms for the continuous data were based on either the Wald chi-square statistic (Cox proportional hazards model), Wilcoxon rank sum test, or t-test as appropriate.24 Comparisons for the categorical data were based on the chi square test or Fisher's exact test.
Evaluation of immune reconstitution. Immunophenotypic analysis of peripheral blood for the presence of natural killer (NK), T, and B cells was performed, as was an evaluation of serum Ig levels on available patients at day +100, 6 months, and 1 year posttransplant. Cell-mediated and humoral immune function were also evaluated posttransplant in available patients. Determination of cell-mediated immunity was accomplished using lymphocyte proliferation in response to mitogens and specific antigens. Humoral immunity to cytomegalovirus (CMV), herpes simplex virus (HSV), and varicella zoster virus (VZV) antigens was determined.
RESULTS
Study enrollment. Between July 1992 and July 1993, 95 breast cancer patients were enrolled on the study. Three of the 95 registered patients were randomized, but not infused (1 died 9 days after harvest, 1 refused transplant, and 1 had progressive disease noted after harvest and was therefore not eligible to proceed to transplant on this study). Three additional patients were randomized and infused, but subsequently ruled ineligible by an independent data monitoring committee. Thus, 89 patients were evaluable for marrow processing infusional toxicity and engraftment, 42 randomized to Arm A (CD34+) and 47 to Arm B (Buffy Coat).
Demographic data.As summarized in Table 1, the average patient was 43 years old and weighed 68.5 kg. One-third of the patients had stage II or III breast cancer involving 10 or more axillary lymph nodes, and the remaining two-thirds had stage IV disease. The patients in arms A and B were comparable with respect to age, weight, breast cancer stage and hormonal status, time from initial diagnosis to transplant, number of prior chemotherapy regimens and cycles, as well as whether or not they received prior radiation therapy.
Breast Cancer Patient Demographic Data
| Randomized Patients N = 89 . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P . |
|---|---|---|---|
| . | (n = 42) . | (n = 47) . | . |
| Age (yr) | |||
| Mean | 44 | 42 | .119 |
| Range | (32-58) | (30-55) | |
| Weight (kg) | |||
| Mean | 67 | 70 | .379 |
| Range | (34-97) | (50-122) | |
| Stage II/III | 17 (40%) | 17 (36%) | .710 |
| Stage IV | 25 (60%) | 30 (64%) | .827 |
| ER status | .380 | ||
| Positive (%) | 19 (45%) | 27 (57%) | |
| Negative (%) | 16 (38%) | 16 (34%) | |
| Unknown (%) | 7 (17%) | 4 (9%) | |
| Yr from initial diagnosis to BMT | 2.6 (0.2-16.7) | 2.1 (0.1-7.8) | .470 |
| No. of previous chemotherapy regimens | .751 | ||
| 1 | 23 (55%) | 28 (60%) | |
| 2 | 18 (43%) | 17 (36%) | |
| 3 | 1 (2%) | 2 (4%) | |
| No. previous chemotherapy cycles | 8 (3-18) | 7 (2-13) | .246 |
| No. patients who received prior RT | 9 (21%) | 11 (23%) | 1.00 |
| Randomized Patients N = 89 . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P . |
|---|---|---|---|
| . | (n = 42) . | (n = 47) . | . |
| Age (yr) | |||
| Mean | 44 | 42 | .119 |
| Range | (32-58) | (30-55) | |
| Weight (kg) | |||
| Mean | 67 | 70 | .379 |
| Range | (34-97) | (50-122) | |
| Stage II/III | 17 (40%) | 17 (36%) | .710 |
| Stage IV | 25 (60%) | 30 (64%) | .827 |
| ER status | .380 | ||
| Positive (%) | 19 (45%) | 27 (57%) | |
| Negative (%) | 16 (38%) | 16 (34%) | |
| Unknown (%) | 7 (17%) | 4 (9%) | |
| Yr from initial diagnosis to BMT | 2.6 (0.2-16.7) | 2.1 (0.1-7.8) | .470 |
| No. of previous chemotherapy regimens | .751 | ||
| 1 | 23 (55%) | 28 (60%) | |
| 2 | 18 (43%) | 17 (36%) | |
| 3 | 1 (2%) | 2 (4%) | |
| No. previous chemotherapy cycles | 8 (3-18) | 7 (2-13) | .246 |
| No. patients who received prior RT | 9 (21%) | 11 (23%) | 1.00 |
Abbreviations: ER, estrogen receptor; BMT, bone marrow transplant; RT, radiation therapy.
BM processing results. The total number of nucleated marrow cells and CD34+ cells per kilogram patient weight in the harvests and buffy coats were comparable in both arms of the study as shown in Table 2. There was a trend toward the total number of myeloid progenitors, measured as CFU-GM per kg patient weight, to be higher in the buffy coat fraction of the Arm B patients (Buffy Coat) when compared to the buffy coat fraction of Arm A patients (CD34+) (P = .065). For Arm A (CD34+) patients, the starting buffy coat contained a median of 1.7% (range 0.4 to 3.5) CD34+ cells. After the positive-selection procedure, 77.8% (range 15.9 to 92.4) of the cells were CD34+, representing a 40-fold enrichment over the starting fraction of CD34+ cells. After high-dose therapy administration, patients in Arm A (CD34+) received median 1.2 × 106 CD34+ cells/kg, while those in Arm B (Buffy Coat) received 3.7 × 106 CD34+ cells/kg (P < .001), showing that there was a significant difference in the median number of CD34+ cells infused. The number of CD34+ cells infused per kg was determined using the precryopreservation flow cytometric analysis of the marrow. The median viability of the thawed CD34+ marrow cells measured by Trypan Blue exclusion assay was 96% compared to 73% for the buffy coat fractions of marrow.
BM Processing Data
| Parameter Median Range . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P . |
|---|---|---|---|
| N = 89 . | N = 42 . | N = 47 . | . |
| Total no. nucleated cells in harvest (×108/kg) | 4.3 (3.5-8.7) | 4.4 (3.0-9.5) | .326 |
| Total no. nucleated cells in buffy coat (×108/kg) | 3.2 (0.3-5.8) | 3.2 (1.7-5.5) | .690 |
| Total no. nucleated cells in start (×108/kg) | 2.7 (0.2-5.3) | 2.6 (1.2-5.0) | .508 |
| Total no. CD34+ cells in start (×106/kg) | 4.4 (0.5-10.5) | 3.7 (0.6-23.3) | .561 |
| Total no. CFU-GM* in start (×104/kg) | 8.0 (0.9-31.0) | 10.1 (0.2-72.0) | .252 |
| Total no. nucleated cells infused (×106/kg) | 1.7 (0.8-4.5) | 261.8 (116.8-499.3) | <.001 |
| Total no. CD34+ cells infused (×106/kg) | 1.2 (0.4-3.0) | 3.7 (0.6-23.3) | <.001 |
| Total no. CFU-GM* infused (×104/kg) | 3.8 (0.4-21.1) | 10.1 (0.2-72.0) | <.001 |
| Parameter Median Range . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P . |
|---|---|---|---|
| N = 89 . | N = 42 . | N = 47 . | . |
| Total no. nucleated cells in harvest (×108/kg) | 4.3 (3.5-8.7) | 4.4 (3.0-9.5) | .326 |
| Total no. nucleated cells in buffy coat (×108/kg) | 3.2 (0.3-5.8) | 3.2 (1.7-5.5) | .690 |
| Total no. nucleated cells in start (×108/kg) | 2.7 (0.2-5.3) | 2.6 (1.2-5.0) | .508 |
| Total no. CD34+ cells in start (×106/kg) | 4.4 (0.5-10.5) | 3.7 (0.6-23.3) | .561 |
| Total no. CFU-GM* in start (×104/kg) | 8.0 (0.9-31.0) | 10.1 (0.2-72.0) | .252 |
| Total no. nucleated cells infused (×106/kg) | 1.7 (0.8-4.5) | 261.8 (116.8-499.3) | <.001 |
| Total no. CD34+ cells infused (×106/kg) | 1.2 (0.4-3.0) | 3.7 (0.6-23.3) | <.001 |
| Total no. CFU-GM* infused (×104/kg) | 3.8 (0.4-21.1) | 10.1 (0.2-72.0) | <.001 |
Abbreviation: CFU-GM, colony-forming unit–granulocyte-macrophage.
Included all myeloid progenitor cell colonies.
Infusional toxicity results. After marrow infusion, significant increases in diastolic and systolic blood pressure, as well as significant decreases in heart rate, were documented in Arm B (Buffy Coat) patients compared to those in Arm A (CD34+) (P < .001), as shown in Table 3. None of the patients in Arm A experienced any clinically serious adverse events associated with the infusion of the marrow. There were three (3/47 = 6%) serious clinical events associated with the infusion of the marrow in the Arm B patients.
Comparison of Hemodynamic Endpoints After Marrow Infusion
| . | Arm A (CD34+) . | Arm B (Buffy Coat) . | |
|---|---|---|---|
| . | (n = 42) . | (n = 47) . | |
| Infusional Toxicities . | Mean ± SD . | Mean ± SD . | P Value . |
| Maximum increase in systolic blood pressure (mm Hg) | 9 ± 11 | 22 ± 14 | P < .001 |
| Maximum increase in diastolic blood pressure (mm Hg) | 7 ± 7 | 16 ± 10 | P < .001 |
| Maximum decrease in heart rate (beats/min) | 9 ± 5 | 22 ± 11 | P < .001 |
| No. serious (grade IV) adverse events due to marrow infusion | 0 | 3 | P = .244 |
| . | Arm A (CD34+) . | Arm B (Buffy Coat) . | |
|---|---|---|---|
| . | (n = 42) . | (n = 47) . | |
| Infusional Toxicities . | Mean ± SD . | Mean ± SD . | P Value . |
| Maximum increase in systolic blood pressure (mm Hg) | 9 ± 11 | 22 ± 14 | P < .001 |
| Maximum increase in diastolic blood pressure (mm Hg) | 7 ± 7 | 16 ± 10 | P < .001 |
| Maximum decrease in heart rate (beats/min) | 9 ± 5 | 22 ± 11 | P < .001 |
| No. serious (grade IV) adverse events due to marrow infusion | 0 | 3 | P = .244 |
Fifteen minutes after the start of the marrow infusion, the first patient developed a severe cough concomitant with an 8% decrease in oxygen saturation to 86%. The cough became intractable and the patient required emergency endotracheal intubation and mechanical ventilatory support. The patient continued to require mechanical ventilatory support throughout her hospital course and died with an autopsy showing diffuse pulmonary congestion. It should be noted that this patient had experienced chemotherapy-related pulmonary toxicity requiring supplemental oxygen (50% O2 ) by face mask before transplant; however, the temporal association of the marrow infusion with the rapid deterioration of her pulmonary status suggests that the infusion played a significant role in the patient's development of acute respiratory failure.
The second patient developed acute allergic toxicity that began 15 minutes after the start of marrow infusion. The reaction consisted of the development of Grade 3 respiratory toxicity including acute bronchospasm resulting in a significant decrease in her oxygen saturation. Concurrently, the patient had the onset of Grade 4 nausea, vomiting, and abdominal cramping. The marrow infusion rate was reduced and the symptoms gradually resolved. This patient also required antihypertensive medication for significant hypertension associated with a severe headache.
After marrow infusion, the third patient developed acute renal insufficiency with hemoglobinuria, hematuria, and an increase in serum creatinine from 1.0 mg/dL pretransplant to 2.8 mg/dL on day +4. At 5 months posttransplant, the creatinine remained elevated.
Half as many Grade 3 or 4 adverse events occurred in the CEPRATE Arm A (16 events) when compared to Arm B (33 events). In addition, less than half as many medical interventions were required due to all adverse events occurring on day 0 or 1 after marrow infusion in Arm A (21 interventions) when compared to Arm B (43 interventions).
Engraftment and supportive care results. Table 4 summarizes the engraftment and supportive care data for patients on Arm A and B of the study. Successful neutrophil engraftment by day 20 postinfusion was documented in 38 of the 42 (91%) for Arm A (CD34+) patients, compared to 42 of 47 (88%) for Arm B (Buffy Coat) patients. The median time to neutrophil engraftment was 13 days for Arm A (CD34+), and 11 days for the Arm B (Buffy Coat) (P = .218). The median time to platelet engraftment was 27 days for the CD34-selected Arm and 20 days for the Buffy Coat Arm (P = .051). For Arm A (CD34+) patients, the platelet recovery rate was related to the number of CD34+ cells/kg the patient received. A multivariate analysis identified four risk factors that might have contributed to a delay in platelet engraftment (before radiotherapy, lower platelet count at the time of BM harvest, TNC at harvest, and number of CD34+ cells/kg infused). As shown in Table 5, the median time to platelet engraftment for Arm A patients receiving less than 1.2 × 106 CD34+ cells/kg was 31 days compared to 22 days for those receiving ≥ 1.2 × 106 CD34+ cells (P = .010). For the Arm A (CD34+) patients who received ≥ 1.2 × 106 CD34+ cells/kg, there were no significant differences in platelet recovery rates compared to those of the Arm B (Buffy Coat) patients (P = .604). Irrespective of the number of CD34+ cells/kg infused, there was no significant difference between the treatment arms in any clinically relevant parameter that might be associated with delayed platelet engraftment including number of red blood cell transfusions, platelet transfusions, or bleeding episodes. Additionally, there was no difference in any of the other secondary endpoints summarized in Table 4.
Engraftment and Supportive Care Data
| Endpoint . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P Value . | ||
|---|---|---|---|---|---|
| . | (n = 42) . | (n = 47) . | . | ||
| . | Median . | Range . | Median . | Range . | . |
| % of Patients engrafted by day 20 | 91 | N/A | 88 | N/A | N/A |
| Days to ANC ≥500/mm3 | 13 | 9-33 | 11 | 8-48 | .218 |
| Days to platelet engraftment | 27 | 11-68 | 20 | 8-61 | .051 |
| Units of RBCs transfused | 8 | 2-30 | 8 | 2-32 | .60 |
| Units of platelets transfused | 62 | 12-264 | 52 | 8-570 | .47 |
| No. of days hospitalized | 18 | 10-40 | 17 | 10-50 | .55 |
| % of Patients with at least one infection | 52 | 47 | .6 | ||
| % of Patients with any bleeding episodes | 19 | 26 | .46 | ||
| % of Patients with a grade III or IV bleeding episode | 2 | 4 | 1.00 | ||
| Endpoint . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P Value . | ||
|---|---|---|---|---|---|
| . | (n = 42) . | (n = 47) . | . | ||
| . | Median . | Range . | Median . | Range . | . |
| % of Patients engrafted by day 20 | 91 | N/A | 88 | N/A | N/A |
| Days to ANC ≥500/mm3 | 13 | 9-33 | 11 | 8-48 | .218 |
| Days to platelet engraftment | 27 | 11-68 | 20 | 8-61 | .051 |
| Units of RBCs transfused | 8 | 2-30 | 8 | 2-32 | .60 |
| Units of platelets transfused | 62 | 12-264 | 52 | 8-570 | .47 |
| No. of days hospitalized | 18 | 10-40 | 17 | 10-50 | .55 |
| % of Patients with at least one infection | 52 | 47 | .6 | ||
| % of Patients with any bleeding episodes | 19 | 26 | .46 | ||
| % of Patients with a grade III or IV bleeding episode | 2 | 4 | 1.00 | ||
Abbreviation: RBC, red blood cell count.
Days to Platelet Engraftment Defined by No. CD34+ Cells/kg Infused
| Strata . | All Patients . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P Value . |
|---|---|---|---|---|
| CD34/kg <1.2 × 106 | 32 Days (n = 22) | 31 Days (20 Pts) | NA | NE |
| CD34/kg ≥1.2 × 106 | 21 Days | 22 Days (22 Pts) | 20 Days (44 Pts) | .604 |
| P value comparing strata | .002 | .02 | NE |
| Strata . | All Patients . | Arm A (CD34+) . | Arm B (Buffy Coat) . | P Value . |
|---|---|---|---|---|
| CD34/kg <1.2 × 106 | 32 Days (n = 22) | 31 Days (20 Pts) | NA | NE |
| CD34/kg ≥1.2 × 106 | 21 Days | 22 Days (22 Pts) | 20 Days (44 Pts) | .604 |
| P value comparing strata | .002 | .02 | NE |
Abbreviations: NE, not evaluable; Pts, patients; NA, not applicable.
Immune reconstitution data. As shown in Table 6, there were no significant differences in either cell-mediated immunity or humoral immunity between patients on the two arms of the study. The majority of patients responded to phytohemagglutinin (PHA) and concanavalin A (ConA) while response to pokeweed mitogen (PWM) was reduced, as was the delayed hypersensitivity response to candida, tetanus, and purified protein derivative (PPD). For both arms, the number of NK and suppressor cells were normal by day +100, and the number of CD4+ T cells was normal by 1 year posttransplant. The number of circulatory CD19+B cells was normal by 6 months posttransplant. Additionally, patients in both arms produced normal levels of IgM, IgA, and IgG by 6 months posttransplant and the majority had IgG antibodies to CMV, HSV, and VSV. There were no unusual infections suggestive of immune dysfunction reported beyond day 100 for patients in either arm of the study.
Immune Function Data
| Cell-Mediated Immunity . | ||||
|---|---|---|---|---|
| Immunity . | Class of Test . | Test . | CEPRATE Arm A . | Control Arm B . |
| Cell-mediated immunity | Response to mitogens6-152 | PHA | 16/16 | 19/20 |
| CON A | 13/16 | 15/20 | ||
| PWM | 7/16 | 10/20 | ||
| Delayed hypersensitivity skin tests | Candida | 2/17 | None | |
| Tetanus | 3/17 | 2/17 | ||
| PPD | None | None | ||
| Immunophenotypes | ||||
| n = 45 at day 100 | ||||
| n = 12 at 6 mo. | ||||
| n = 21 at 12 mo. | NK cells (CD16+56+) | Normal no. by day 100 posttransplant | Normal no. by day 100 posttransplant | |
| Suppressor/cytoxic T cells (CD8+) | ||||
| Helper/inducer | Normal no. by 1 yr but not 100 days and 6 mo. posttransplant | Normal no. by 1 yr but not 100 days and 6 mo. posttransplant | ||
| T cells (CD4+) | ||||
| B cells (CD19+) | Normal at 6 & 12 mo. posttransplant | Normal at 6 & 12 mo. posttransplant | ||
| Helper/suppressor cells (CD4+/8+) | Reduced day 100, 6 & 12 mo. posttransplant | Reduced day 100, 6 & 12 mo. posttransplant | ||
| Humoral immunity | ||||
| Serum Igs in mg/dL | IgM and IgA | Normal by 6 mo. posttransplant | Normal by 6 mo. posttransplant | |
| IgG at 6 mo. post-TX6-150 | median = 783 | median = 863 | ||
| IgG at 12 mo. post-TX6-150 | median = 852 | median = 1,210 | ||
| Antibodies | CMV-IgG6-151 | 14/17 | 13/15 | |
| HSV-IgG6-151 | 11/13 | 11/14 | ||
| VZV-IgG6-151 | All | All | ||
| Cell-Mediated Immunity . | ||||
|---|---|---|---|---|
| Immunity . | Class of Test . | Test . | CEPRATE Arm A . | Control Arm B . |
| Cell-mediated immunity | Response to mitogens6-152 | PHA | 16/16 | 19/20 |
| CON A | 13/16 | 15/20 | ||
| PWM | 7/16 | 10/20 | ||
| Delayed hypersensitivity skin tests | Candida | 2/17 | None | |
| Tetanus | 3/17 | 2/17 | ||
| PPD | None | None | ||
| Immunophenotypes | ||||
| n = 45 at day 100 | ||||
| n = 12 at 6 mo. | ||||
| n = 21 at 12 mo. | NK cells (CD16+56+) | Normal no. by day 100 posttransplant | Normal no. by day 100 posttransplant | |
| Suppressor/cytoxic T cells (CD8+) | ||||
| Helper/inducer | Normal no. by 1 yr but not 100 days and 6 mo. posttransplant | Normal no. by 1 yr but not 100 days and 6 mo. posttransplant | ||
| T cells (CD4+) | ||||
| B cells (CD19+) | Normal at 6 & 12 mo. posttransplant | Normal at 6 & 12 mo. posttransplant | ||
| Helper/suppressor cells (CD4+/8+) | Reduced day 100, 6 & 12 mo. posttransplant | Reduced day 100, 6 & 12 mo. posttransplant | ||
| Humoral immunity | ||||
| Serum Igs in mg/dL | IgM and IgA | Normal by 6 mo. posttransplant | Normal by 6 mo. posttransplant | |
| IgG at 6 mo. post-TX6-150 | median = 783 | median = 863 | ||
| IgG at 12 mo. post-TX6-150 | median = 852 | median = 1,210 | ||
| Antibodies | CMV-IgG6-151 | 14/17 | 13/15 | |
| HSV-IgG6-151 | 11/13 | 11/14 | ||
| VZV-IgG6-151 | All | All | ||
Abbreviation: PPMV, cytomegalovirus.
Normal values = 800 to 1,800 mg/dL.
Only tested in patients who were reactive at baseline; median time for patients was at 13 months posttransplant.
Number of patients responding/number tested.
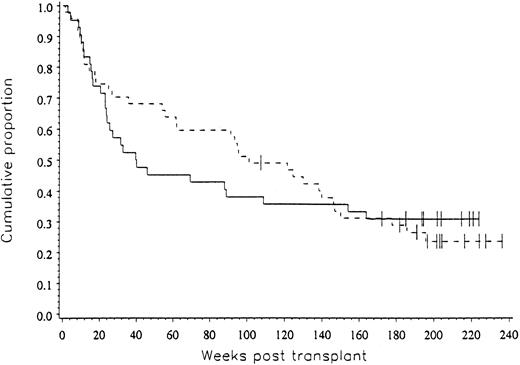
Clinical outcome data. The probability of progression-free survival at three years was 31% for patients in both arms of the study (P = .492), as shown in Fig 2.
The progression-free survival for patients on both arms of the study was 31% (P = .492).
The progression-free survival for patients on both arms of the study was 31% (P = .492).
DISCUSSION
This prospectively randomized clinical study showed that CD34+ cells enriched with the CEPRATE system restore hematopoiesis in breast cancer patients who received high-dose chemotherapy. The rate of neutrophil recovery was similar to that of the control patients who received unseparated buffy coat. Furthermore, there was a significant reduction in infusion-related hemodynamic side effects in patients who received CD34-selected marrow. The only serious or life-threatening events associated with the marrow infusion occurred in patients who received buffy coat. Additionally, there were no statistically significant differences between the treatment arms with respect to long-term hematopoietic engraftment, immune reconstitution, and progression-free survival.
The median time to platelet recovery was 27 days for patients on the CD34+ Arm and 20 days for the those on the Buffy Coat Arm (P = .051). There were no other significant differences between the two arms of the study with respect to clinical sequelae, such as the numbers of bleeding episodes or platelet and/or red blood cell transfusions that might be associated with delayed platelet engraftment. Patients on Arm A who received ≥1.2 × 106 CD34+ cells/kg had no delay in platelet recovery (22 days), compared to patients on Arm B, who also received greater than 1.2 × 106 CD34+ cells/kg (20 days) (P = .604). These results suggest that infusing a minimum CD34+ cell dose will markedly reduce the risk of delayed platelet engraftment.
Positive selection of CD34+ cells produced a 40- to 50-fold enrichment of the normal CD34+ hematopoietic progenitors with a median purity of 78%. In this study, the overall yield of CD34+ cells was 27%. This yield is lower than the median yields reported by other investigators which range from 33% to 73% for selection of BM25 and peripheral blood.25-28 Differences in yields may vary with the disease or the amount of treatment the patient has received.
In summary, this study showed a reduction in infusional toxicity with clinically comparable short and long-term hematopoietic reconstitution when breast cancer patients treated with high-dose chemotherapy received CD34-selected marrow compared to buffy coat fractions. When fewer than 1.2 × 106 CD34+ cells/kg of body weight were infused, there was a significant (1 week) delay in platelet recovery. No delay occurred in patients receiving ≥1.2 × 106 CD34+ cells/kg. Of note, none of the participating centers included radiation therapy in their preparative regimens, thus the efficacy of CD34+ cells in that setting remains to be determined. Many clinical studies are in progress in a variety of clinical settings which will determine whether the CD34-selection procedure improves the clinical outcome of hematopoietic cell transplant patients.6,12 29-33
Supported in part by Grant No. RO1 CA61508 from the National Institutes of Health/National Cancer Institute (Bethesda, MD).
Address reprint requests to Elizabeth J. Shpall, MD, University of Colorado, Bone Marrow Transplant Program, Box B190, 4200 E 9th Ave, Denver, CO 80262.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal