Abstract
We have recently demonstrated the presence of Kaposi's sarcoma–associated herpesvirus (KSHV) in cultured bone marrow (BM) stromal dendritic cells from all patients with myeloma studied. To show that these findings were not an artifact of tissue culture, we performed in situ hybridization (ISH) and polymerase chain reaction (PCR) to detect KSHV in BM core biopsies. Using ISH to open reading frame-72 (ORF 72), we localized KSHV to BM dendritic cells in 17 of 20 patients with myeloma, 2 patients with plasmacytosis associated with the acquired immunodeficiency syndrome, and 1 case of aplastic anemia. In contrast, BM from normal subjects (n = 4) and patients with lymphoma and leukemia (n = 21) did not contain KSHV. PCR amplification with KSHV primers demonstrated product in fresh BM biopsy samples from 6 of 7 myeloma patients, whereas three normal marrows contained no amplified product. These findings suggest that KSHV, possibly through alterations in the BM microenvironment and production of viral interleukin-6 (vIL-6), may stimulate and maintain abnormal plasma cell proliferation in myeloma and related disorders.
SINCE THE DISCOVERY of Kaposi's sarcoma–associated herpesvirus (KSHV),1 this virus has been shown to be associated with human disease including Kaposi's sarcoma, systemic Castleman's disease, and primary effusion lymphoma (PEL).2-11 In Kaposi's sarcoma and PEL, KSHV has been localized to the malignant cells.8,11,12 The exact role the virus plays in these diseases, as well as the range of cells infected with the virus, remains to be elucidated. However, a causative role for KSHV is suggested by serologic data, which showed that seroconversion to positivity for antibodies against KSHV-related nuclear antigens occurs before the clinical appearance of Kaposi's sarcoma.13
The cytokine interleukin-6 (IL-6) is an important growth factor for myeloma, and a biologically active homolog to human IL-6 termed vIL-6 has been identified in the KSHV genome.14 Bone marrow (BM) stromal cells contribute to the BM microenvironment by direct cell contact, and these cells produce cytokines including IL-6 which play a major role in the paracrine stimulation of tumor cell growth in patients with myeloma.15-19 We previously have detected KSHV DNA as well as vIL-6 RNA transcripts in cultured BM dendritic cells from all myeloma patients studied.20 In that report, the dendritic cells were purified by tissue culture techniques. The possibility exists that the identification of KSHV in BM dendritic cells of myeloma patients was an artifact of cell culture. In addition, the frequency of virally infected cells in fresh BM could not be determined on these cultured cells. Thus, we used ISH and PCR to determine the frequency of patients with myeloma and plasmacytosis associated with the acquired immunodeficiency syndrome containing KSHV-infected BM cells in fresh core biopsies.
MATERIALS AND METHODS
BM biopsy samples from 20 cases of multiple myeloma were obtained from the Hematopathology Division of UCLA Center for the Health Sciences. The frequency of plasma cells in the marrow samples varied from less than 5% in 9 patients in remission, to 100%. In addition, BM biopsy samples from 2 patients with acquired immunodeficiency syndrome (AIDS) and plasmacytosis, 4 cases of aplastic anemia, and 21 patients with lymphoma and leukemia were studied. The patients studied with lymphoma and leukemia included 6 individuals with large cell lymphoma, 5 with small cleaved cell lymphoma, 1 with immunoblastic lymphoma, 1 with lymphoblastic lymphoma, 1 with Burkitt's lymphoma, 3 with marginal zone lymphoma, 1 with small lymphocytic lymphoma/leukemia, 2 with Hodgkin's disease, and 1 with acute myelogenous leukemia. Seven normal BMs from patients without neoplastic disease were also studied. Samples were obtained after informed consent and in compliance with the Human Subjects Protection Committee.
Preparation of probes.Biotinylated probes to ORF-72 were prepared with the following sequences: Sense probe to open reading frame-72 (ORF 72) 5′ to 3′ GAGAACCTGACAGAGCACCC; anti-sense probe to ORF 72 CGCCCTAAACAAAATCACAA. Positive control probe to determine suitability of fixed samples for ISH consisted of a commercial biotinylated probe to total genomic DNA of cultured human cells (DAKO Corp, Carpinteria, CA). Two negative control probes (plasmid DNA) were prepared from the plasmid vector pUC18 (DAKO) and PCR 2.1 (Invitrogen, San Diego, CA). Additional probes made against sequences of human papillomavirus E6-S DNA were also used as negative controls (primers TAGAATGTGTGTACTGCAAG and TGATTACAGCTGGGTTTCTC).
ISH for KSHV.BM biopsy samples were fixed in formalin or B5, deparaffinized, and immersed in 3% hydrogen peroxide in methanol for 10 minutes. ISH was performed using modifications of methods previously described.20,21 Proteinase K solution (5 μg/mL) (GIBCO, Grand Island, NY) was applied to each section, and sections were incubated in a 37°C water bath humidity chamber for 10 minutes and washed with distilled water. KSHV probe (50 μg/μL) was diluted to 1:300, and 60 μL was applied to each slide and covered with a coverslip. Slides were placed on a metal plate in a water bath (surface temperature of the plate 95°C) for 5 minutes and then in 37°C water bath humidity chamber overnight (approximately 16 hours). The slides were then washed in 0.05 mol/L Tris-buffered saline (TBS), pH 7.4, followed by 0.2% sodium dodecyl sulfate (SDS). The DNA following hybridization wash was exposed to a 20-minute incubation at 37°C in 50% (vol/vol) deionized formamide (Sigma, St Louis, MO), containing 2X standard saline citrate (SSC). Slides were washed in TBS and rinsed with TBS-Tween (20 μL Tween-20 per liter of TBS) (TBST) for 3 minutes. To detect the ISH signal, we used tryamide signal amplification indirect for ISH (TSA-indirect kit; NEN Life Sciences, Boston, MA). Blocking step consisted of incubation of slides with TNB blocking buffer for 30 minutes. Slides were then incubated with streptavidin-conjugated horseradish peroxidase (streptavidin-HRP) 1:100 for 30 minutes followed by biotinyl tyramide solution 1:50 for 10 minutes, and secondary incubation with streptavidin-HRP 1:100 for 30 minutes. Reaction product was localized with the diaminobenzidine reaction and counterstained with Mayer's hematoxylin (DAKO). The probe was diluted in 10% (vol/vol) 20× SSC, 10% (vol/vol) Denhardt's solution, 2.5% (vol/vol) salmon testes DNA (10 mg/mL) (Sigma D-9156), 5% (vol/vol) yeast tRNA (10 mg/mL) (Sigma R-8508), and 50% (vol/vol) deioinized formamide (Sigma), 22.5% sterile water, with 10% (wt/vol) dextran sulfate (Sigma). Positive controls consisted of KS-1 cells productively infected with KSHV which showed strong nuclear and cytoplasmic localization of the ORF 72 probe.8 20 Negative controls consisted of cultured HL60 cells that did not contain virus. Both positive and negative control cultures were fixed in 10% neutral buffered formalin and embedded in paraffin. Slides were interpreted by two of the investigators (J.W.S. and K.H.) without knowledge of the histologic diagnosis or treatment status of the patients.
ISH for KSHV
| Diagnosis . | Cases KSHV+/Cases Studied . |
|---|---|
| AIDS with plasmacytosis | 2/2 |
| Multiple myeloma | 17/20 |
| Untreated | 5/5* |
| Treated | 12/16* |
| Conventional | 5/6 |
| Transplant | 7/10* |
| Aplastic anemia | 1/4 |
| Lymphoma | 0/20 |
| Acute myelogenous leukemia | 0/1 |
| Normal | 0/4 |
| Diagnosis . | Cases KSHV+/Cases Studied . |
|---|---|
| AIDS with plasmacytosis | 2/2 |
| Multiple myeloma | 17/20 |
| Untreated | 5/5* |
| Treated | 12/16* |
| Conventional | 5/6 |
| Transplant | 7/10* |
| Aplastic anemia | 1/4 |
| Lymphoma | 0/20 |
| Acute myelogenous leukemia | 0/1 |
| Normal | 0/4 |
One patient was studied at diagnosis and after transplant.
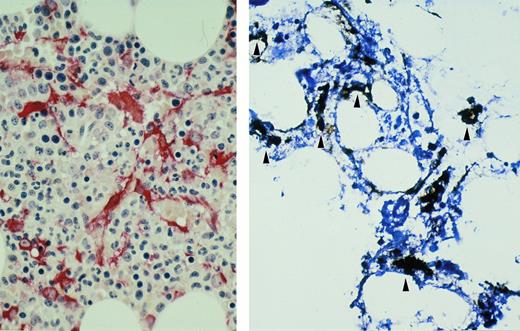
BM biopsy sample from patient with myeloma with in situ hybridization for KSHV ORF 72. Biotin-labeled probe is localized to BM stromal cells (arrowheads, brown) (left). Immunohistochemical stain for fascin (red) shows a similar distribution of BM dendritic cells within the stroma (right). Hematoxylin counterstain; original magnification × 400.
BM biopsy sample from patient with myeloma with in situ hybridization for KSHV ORF 72. Biotin-labeled probe is localized to BM stromal cells (arrowheads, brown) (left). Immunohistochemical stain for fascin (red) shows a similar distribution of BM dendritic cells within the stroma (right). Hematoxylin counterstain; original magnification × 400.
Polymerase chain reaction (PCR) of BM biopsy specimens.For PCR studies, fresh BM core biopsy samples were available from 7 patients with myeloma (5 also studied by ISH) and 3 normal subjects. A mortar and pestle were autoclaved and then treated with DNA Away (Molecular Bio-Products, San Diego, CA) to remove any contaminating DNA or deoxyribonuclease. The BM core biopsy specimens were macerated, and DNA was extracted using the Easy DNA kit (Invitrogen) per the manufacturer's instructions. PCR on DNA from BM core biopsy samples was then performed with KS 330233-specific primers as previously described.20
Immunohistochemical staining for fascin.Immunohistochemical staining for fascin, a dendritic cell marker, was performed using a five-step alkaline phosphatase anti-alkaline phosphatase technique as previously described.22
RESULTS
ISH for KSHV ORF 72.ISH was performed on BM from 20 patients with multiple myeloma and 17 demonstrated viral positive cells by ISH (Table 1). All 5 patients studied at diagnosis demonstrated viral positive cells. In the 6 patients analyzed while on conventional therapy, 5 showed the presence of virus and these same patients were either in relapse or showed no response to chemotherapy. The patient without KSHV was in nearly a complete remission after VAD chemotherapy. Seven of 10 patients studied after undergoing myeloablative chemotherapy with autologous stem cell support (1 month to 24 months after transplant) showed the presence of KSHV. One transplanted patient who showed virus in his BM at diagnosis did not demonstrate virus after transplant. Two other patients (12 and 24 months posttransplant) were also viral negative by ISH. Two patients with acquired immunodeficiency syndrome (AIDS)-associated plasmacytosis were also positive for KSHV in a similar distribution to patients with myeloma. One case of aplastic anemia also showed rare cells containing KSHV. Signal was detected in nuclei and cytoplasm of stromal-type cells which comprised approximately 2% to 10% of the nucleated cells in the BM (Fig 1). Other marrow elements including myeloid cells, erythroid cells, lymphoid cells, megakaryocytes, and plasma cells did not show the presence of virus. By contrast, BM samples from 4 normal subjects and 21 samples from patients with leukemia and lymphoma did not contain any viral staining. In all samples strong staining was present in nuclei of BM cells using control probe to human DNA. Negative control probes revealed no staining in all cases.
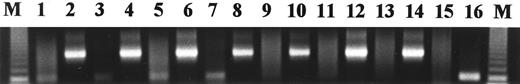
Agarose gel of representative PCR results on fresh BM core biopsy samples. KS330233 is amplified from the DNA from patients with myeloma (lanes 1, 3, 5, and 7), but not from normal individuals (lanes 9, 11, and 13). Even-numbered lanes represent the β-actin controls (650 bp product) for each of the preceding odd-numbered lanes, respectively. Placental DNA is the negative control (lane 15), and DNA from the KSHV-infected cell line KS-1 is the positive control (lane 16). M, 123-bp molecular-weight marker.
Agarose gel of representative PCR results on fresh BM core biopsy samples. KS330233 is amplified from the DNA from patients with myeloma (lanes 1, 3, 5, and 7), but not from normal individuals (lanes 9, 11, and 13). Even-numbered lanes represent the β-actin controls (650 bp product) for each of the preceding odd-numbered lanes, respectively. Placental DNA is the negative control (lane 15), and DNA from the KSHV-infected cell line KS-1 is the positive control (lane 16). M, 123-bp molecular-weight marker.
In previous studies, our laboratory determined that the cells infected with KSHV after long-term culture of BM from myeloma patients expressed dendritic cell markers (CD83 and fascin).20 To characterize the cells positive by ISH for KSHV in fresh BM biopsy samples, parallel sections were stained with antibodies to fascin, an antigen highly restricted to dendritic cells.22 On serial sections, the distribution of KSHV+ cells by ISH corresponded to the distribution of fascin+ cells, a finding which suggested that KSHV was contained in the dendritic cells (Fig 1). Moreover, these dendritic cells also showed long foot processes allowing each one to directly contact many cells in the BM compartment. Our attempts at double immunostaining for fascin and KSHV on the same sections resulted in loss of fascin staining, probably because the fascin reaction product was unstable through the ISH procedure.
PCR for KSHV.PCR-amplified product was detectable using the KS330233 primers in 6 of 7 fresh bone marrow biopsy specimens from patients with myeloma (Fig 2), with the exception of 1 patient in remission 2 years after autologous stem cell transplant. In 5 of these samples, marrow was also available for ISH studies. The 4 samples showing amplified PCR product were also positive by ISH whereas the sample from the transplanted patient without amplified KSHV product did not show viral staining by ISH. Using PCR, 3 normal marrows were also negative for KSHV.
DISCUSSION
Using cultured BM stromal cells from patients with multiple myeloma, we have previously shown the presence of KSHV 330233 and vIL-6 RNA transcripts in cultured dendritic cells, suggesting that the KSHV virus may play a role in supporting plasma cell proliferation in the BM and/or neoplastic transformation in these diseases.20 Since these findings were based on results from an artificial system of in vitro stromal cell cultures, the present study demonstrating the presence of KSHV in fresh BM biopsy cores from myeloma patients and AIDS patients with plasmacytosis provides further evidence relating this virus to abnormal plasma cell proliferation.
Using biotinylated probes to sense and antisense KSHV ORF 72, we selectively localized KSHV to the BM stromal cells from patients with myeloma and patients with plasmacytosis associated with the acquired immunodeficiency syndrome. One patient with aplastic anemia showed a few positive cells, while normal BMs and patients with other lymphoid proliferations and acute myelogenous leukemia were negative. Fresh BM biopsy samples from patients with myeloma were also KSHV+ by PCR, whereas normal BM specimens contained no virus. The BM stromal cell distribution of the KSHV signal by ISH corresponded to the distribution of fascin-staining cells on serial sections, suggesting that KSHV infects dendritic cells in the BM. Recent studies have shown the critical role of dendritic cells in the growth and maturation of terminally differentiated B cells.23 Thus, the presence of KSHV with its viral proteins in dendritic cells may enhance their ability to stimulate growth and possible transformation of monoclonal plasma cells. However, we cannot unequivocally prove localization in dendritic cells because attempts at dual ISH/fascin immunohistochemistry on the same sections were unsuccessful.
Although KSHV was present in all untreated myeloma patients, it was not present in BM from four treated cases by ISH. One BM biopsy specimen from a patient with myeloma was negative also by PCR, and this patient was in remission after myeloablative chemotherapy with autologous peripheral blood progenitor cell transplantation. Interestingly, another patient who was KSHV+ at diagnosis became viral negative by ISH after autologous stem cell transplant. The two other cases without virus were from a patient 1 year after autologous transplant and another patient in nearly a complete remission after VAD chemotherapy. Moreover, in the patients who were either in relapse or showed no response to conventional chemotherapy, all showed the presence of KSHV. Thus, these preliminary results suggest a correlation between the presence of KSHV in the BM of myeloma patients and clinical outcome. Larger studies will need to be performed to show this association. Importantly, staining for fascin showed that similar numbers of dendritic cells were present in the posttransplantation marrows, even though the ISH for KSHV was negative. Thus, the absence of virus did not simply result from the absence of dendritic cells in patients' BMs after myeloablative chemotherapy.
Pathogenesis of multiple myeloma appears to be a multistep process.19 IL-6 has been shown to be essential for the survival and growth of myeloma cells which also express IL-6 receptors.17 In addition to acting as a growth factor for myeloma cells, IL-6 promotes survival by preventing apoptosis.18 IL-6 has also been shown to promote bone resorption in myeloma patients.24 Murine monoclonal antibody to IL-6 has been effectively used as a therapeutic agent in the treatment of patients with plasma cell leukemia.25 Some myeloma cell lines are dependent on exogenous IL-6 for growth, and this is thought to be the most important cytokine responsible for myeloma cell proliferation in vivo.16 There is increasing evidence that IL-6 is produced by cells in the microenvironment of the BM, with this cytokine mainly produced by the adherent cells rather than the malignant plasma cells.15 Because a biologically active vIL-6 is encoded by the KSHV genome,14 and we have previously shown vIL-6 RNA transcripts in cultured infected BM dendritic cells,20 it is possible that KSHV-infected BM stromal cells may play a major role in producing paracrine stimulation of plasma cell growth in patients with myeloma.
About 15% of patients with AIDS have monoclonal gammopathy, and BM plasmacytosis and multiple myeloma may also be increased in patients with AIDS.26 The finding of KSHV-infected stromal cells by ISH in patients with AIDS and BM plasmacytosis suggests that KSHV may also be implicated in this condition. Greater numbers of BM samples from patients with AIDS-associated plasma cell dyscrasias need to be studied to confirm these observations. The presence of KSHV in rare cells from the single case of aplastic anemia is interesting and requires further investigation. Although viral etiologies have been demonstrated for some cases of aplastic anemia,27 28 this is the first time KSHV has been found in this disease.
Multiple myeloma is the second most common hematologic malignancy and accounts for about 1% of all cancer-related mortality in the West, yet its epidemiology pattern remains obscure and the etiology is unknown.19 Demonstration of KSHV by PCR and ISH in uncultured BM biopsy specimens is in concordance with previous findings of KSHV in cultured BM stromal cells from patients with myeloma, and suggests that KSHV may play an important role in the development of this B-cell malignancy. Implications include strategies for using antiviral agents and vaccines in the management of patients with myeloma.
ACKNOWLEDGMENT
The authors are grateful to Gary Schiller, Matthew Rettig, and Francisco Conde, who donated bone marrow for the study.
Supported in part by Grant No. UO1 CA 66533-02 from the National Institutes of Health and the National Cancer Institute Tissue and Biological Fluids Bank of HIV-Related Malignancies, and funds from the Jonnson Comprehensive Cancer Center at UCLA.
Address reprint requests to James R. Berenson, MD, Division of Hematology and Oncology, Veteran Affairs West Los Angeles Medical Center, 11301 Wilshire Blvd 111-H, Los Angeles, CA 90073.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal