Abstract
Gaucher disease type 1, a non-neuronopathic lysosomal storage disease, is caused by mutations at the acid β-glucosidase locus. Periodic infusions of macrophage-targeted acid β-glucosidase reverse hepatosplenomegaly, hematologic, and bony findings in many patients. Two patients receiving enzyme therapy developed neutralizing antibodies to acid β-glucosidase that were associated with a lack of improvement or progressive disease. After initial improvement, case 1 had no additional response to 2 years of high-dose (50 U/kg every 2 weeks) enzyme therapy. Similarly, case 2 initially showed a favorable response to enzyme therapy that plateaued after 1 year of treatment. Both patients developed minor allergic reactions and antibodies to acid β-glucosidase within the first 6 months of treatment. Enzyme therapy was discontinued in case 1, with resultant disease progression and need for splenectomy. An immunosuppression/tolerization protocol was initiated in case 2 because of disease progression and stable neutralizing antibody titers. The IgG neutralizing antibodies rapidly and completely inactivated the wild-type, but not the N370S, acid β-glucosidase in vitro. Antibodies to human serum albumin and chorionic gonadotropin also developed. The finding of neutralizing antibodies to acid β-glucosidase during enzyme therapy for Gaucher disease has significant implications for monitoring the therapeutic responses and for potential alternative future therapies for Gaucher disease.
GAUCHER DISEASE type 1 is a common lysosomal storage disease that results from numerous mutations at the acid β-glucosidase locus.1,2 The deficiency of acid β-glucosidase activity is present in all cells but there is a preferential accumulation of its major natural substrate, glucosylceramide, within macrophages of the liver, spleen, and bone marrow (BM).1 In addition to causing hepatosplenomegaly and pancytopenia, pathologic involvement of bones can include osteoporosis, pathologic fractures, and acute bony pain.1,2 Periodic infusions of macrophage-targeted acid β-glucosidase lead to the reversal of hepatic, splenic, and hematologic abnormalities3-7 and has positive effects on bony disease.8 9
At least 1,200 Gaucher disease type 1 patients have received enzyme therapy with resultant improvement in their disease signs and symptoms. Of these patients, about 15% develop antibodies to the administered acid β-glucosidase,4,5 and approximately half of these patients (≈7% of total patients) have allergic manifestations related to these antibodies.5 These reactions include hives and pruritus.5,10 In general, the allergic manifestations are managed with antihistamines, and only rarely do they necessitate discontinuation of therapy. In addition, substantial negative therapeutic effects have not been reported in antibody-positive patients.10 These anti–acid β-glucosidase antibodies develop in patients with Gaucher disease despite the presence of residual mutant enzyme in all their cells.1 The specificity and origin of these anti–acid β-glucosidase antibodies have not been evaluated in detail.
In this communication, we report the development of neutralizing antibody to acid β-glucosidase during the course of enzyme therapy in two patients with Gaucher disease type 1. One patient showed a lack of response to enzyme therapy, whereas the second showed progressing Gaucher disease signs and symptoms concordant with the development of the neutralizing antibody. The development of neutralizing antibodies to acid β-glucosidase has significant implications for ongoing therapeutic regimens for the treatment of Gaucher disease type 1.
MATERIALS AND METHODS
Materials
The following were from commercial sources: Ceredase (alglucerase for injection; 80 U/mL), Cerezyme (imiglucerase for injection), human serum albumin (HSA) and 4-methylumbelliferyl-β-D-glucopyranoside (4MU-Glc; Genzyme Corp, Boston, MA); human chorionic gonadotropin (HCG; National Hormone and Pituitary Program, National Institutes of Health, Bethesda, MD); 4-methylumbelliferone, Triton X-100, bovine serum albumin (BSA), and Lowry reagents (Sigma Chemical Co, St Louis, MO); SF 900 culture media, fetal calf serum (FBS), and nonimmune goat serum (GIBCO, Grand Island, NY); immobilon polyvinyl fluoride (PVDF) transfer membranes (Millipore, Bedford, MA); alkaline phosphatase (AP)-conjugated goat-antihuman IgG (Calbiochem, La Jolla, CA); AP-substrate reagents (BioRad, Hercules, CA).
Methods
Acid β-glucosidase activity.For consistency with standard medical practice, the amount of enzyme activity administered to the patients will be expressed in the units (U; μmol/min), as determined by Genzyme Corp using p-nitrophenyl β-D glucoside. The exact assay conditions have not been specified. The assays presented here use 4 methyl-umbelliferyl-β-D-glucoside as the substrate. The activity is expressed as μU.
Acid β-glucosidase neutralizing antibody assay.Serum was obtained by incubation of whole blood for 1 hour (4°C), and then centrifugation for 15 minutes at 4,000 rpm. The serum was removed and stored (−20°C). Before assay, the pH was adjusted to 5.5 by addition of 1 mol/L HEPES (pH 5.5, 10 μL) to 50 μL of serum. For titer determination, the serum was buffered with 0.1 mol/L HEPES, pH 5.5. Ceredase was diluted 1:7,000 with 0.04 mol/L citrate/phosphate, pH 5.5, containing 1% HSA. For assay, 50 μL of diluted Ceredase wase preincubated for 30 minutes (37°C) with varying aliquots of serum diluted in 0.1 mol/L HEPES, pH 5.5 (final volume = 75 μL). Acid β-glucosidase activity was determined after an additional incubation of the preincubation mixture for 30 minutes (37°C) with the substrate. The final reaction mixture included 4 mmol/L 4MU-Glc, 1% Triton X-100, 0.04 mol/L citrate/phosphate, pH 5.5, in a total volume of 200 μL. The reaction was stopped with 2.3 mL of 0.1 mol/L ethylenediamine, pH 11.0. Substrate cleavage was quantified fluorometrically.11 Remaining activity is expressed as the percent of acid β-glucosidase activity in the absence of serum. Sera from antibody-positive and -negative Gaucher disease patients, and normal individuals were used as controls.
Gel electrophoresis and immunoblots.For electrophoresis, samples were mixed 4:1 with 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (12.5% SDS, 25% β-mercaptoethanol, 0.05% bromophenol blue, 50 mmol/L Tris HCl pH 8.0, 5 mmol/L EDTA pH 8.0, and 10% glycerol), and heated for 3 minutes (95°C). Separation of the proteins in 12.5% or 20% SDS-PAGE gels and electroblotting onto PVDF membranes was performed in the Phast-System according to the manufacturer's instructions (Pharmacia Biotech, Inc, Piscataway, NJ). After separation, membranes were briefly washed in Tris-buffered saline (TBS; 50 mmol/L Tris-HCl, pH 7.5, 200 mmol/L NaCl) and dried until used. The day of the assay, membranes were prewet in methanol containing solutions and sequentially treated as follows: (1) 30-minute incubation with TBS containing 3% BSA and 3% nonimmune goat serum (TBS blocking solution); (2) 1-hour incubation with patient or control serum in TBS blocking solution (1:1,500 dilution); (3) three (10-minute) washes with T-TBS (0.05% Tween 20 in TBS); (4) 45-minute incubation with goat-antihuman-AP conjugated antibody (1:1,500 dilution); (5) two (10-minute) washes in T-TBS, one rinse with TBS; and (6) BioRad AP-developer solution was added according to manufacturer's instructions, and developed for 20 minutes. All procedures were done at room temperature.
Lymphocyte acid β-glucosidase.Mutant N370S and wild-type acid β-glucosidase were from immortalized or freshly isolated lymphocytes from Gaucher disease patients or normal individuals, respectively. The patients were shown to have one or more N370S alleles12; case 1 was homozygous for the N370S allele and case 2 had an N370S/ “unknown” genotype. The soluble lymphoid extracts were obtained by incubation of lymphocyte pellets with 5% NP40 on ice for 10 minutes, and centrifugation at 12,000g for 15 minutes (4°C). Analyses were done by SDS-PAGE.
Recombinant wild-type and mutant acid β-glucosidase.Normal and N370S mutant acid β-glucosidase cDNAs were subcloned into the EcoRI(5′)/Xba I(3′) polylinker sites of the plasmid pAC610 and were used to produce high-titer recombinant baculovirus in Sf9 insect cells by homologous recombination with wild-type baculovirus.11 Heterologous expression of human recombinant acid β-glucosidase in protein-free media (Sf900) was obtained after infection of 2 × 107Sf9 cells with enough virus inoculum to give 1 to 5 multiplicity of infection (MOI). For the analysis, cells and media containing the recombinant protein were obtained 3 days postinfection, and separated by centrifugation for 10 minutes at 800 rpm. After three washes (800 rpm; 10 minutes) with saline, the pelleted cells were stored (−20°C) until used. Both the wild-type and N370S recombinant proteins have similar biochemical and immunological properties to their natural counterparts.11
Case Reports
Case 1 is a 22-year-old Jewish man who was diagnosed with Gaucher disease due to hepatosplenomegaly at 10 years of age. The diagnosis was confirmed by demonstration of decreased acid β-glucosidase activity in lymphocytes and the identification of homozygosity for the N370S allele. His younger brother, age 20, also has Gaucher disease type 1. Case 1 and his brother have Hashimoto's thyroiditis.
At 18.5 years of age, case 1 began enzyme therapy (50 U/kg every 2 weeks), because of massive splenomegaly (≈13 fold normal) and thrombocytopenia (21,000/μL). During the first four infusions of Ceredase, he experienced sweatiness and chills during and several hours after alglucerase infusions. These symptoms subsided spontaneously. Anti–acid β-glucosidase antibody (IgG) was detected within the first 7 months. He had experienced no improvement of his Gaucher disease over 26 months of enzyme therapy. At that time he came under our care and his serum was found to inhibit acid β-glucosidase activity. Because of the lack of effectiveness of enzyme therapy and the finding of a neutralizing antibody, this treatment was discontinued and the patient was followed up. Over the next 24 months, progressive thrombocytopenia to 17,000/μL necessitated splenectomy. To date, his affected brother, treated with enzyme therapy for 4 years, remains antibody negative.
Case 2 is a 13-year-old Hispanic/German girl who was diagnosed at age 11 years with Gaucher disease due to asymptomatic splenomegaly. This diagnosis was confirmed by the deficient activity of acid β-glucosidase in lymphocytes and the presence of one N370S allele. The second allele was not identified by screening for the three other common mutations (84GG, L444P, IVS2+).13-15 She began enzyme therapy (30 U/kg every 2 weeks) due to severe fatigue, progressive thrombocytopenia, increasing bone pain (particularly in the knees), and the progressive hepatosplenomegaly. During the first 6 months of enzyme therapy she showed substantial improvement, particularly in her energy level, diminution in bone pain and partial regression of splenomegaly (Table 1). The pretreatment splenic volume was 1,620 mL (≈14 × predicted normal) and the liver volume was 1,749 mL (1.25 × predicted normal). From 6 to 12 months of enzyme therapy, the splenic volume decreased by 35% to 38%, respectively, from initial and the liver volume remained essentially normal. The improvement in energy level was quite dramatic with decreasing sleep requirements from 12 hours to 8 hours per day and participation in extracurricular school activities for the first time in several years.
Effect of Enzyme Therapy in Case 2
| Enzyme Therapy . | Liver Volume . | Platelets . | ||
|---|---|---|---|---|
| . | Splenic Volume . | . | ||
| . | Hemoglobin . | . | ||
| (mo) . | (mL/fold normal) . | (g%) . | (μL) . | |
| . | . | . | . | . |
| 30 U/kg every 2 wk | ||||
| 0 | 1,749/1.2× | 1,620/14× | 11.3 | 75,000 |
| 7 | 1,655/1.0× | 1,056/8× | 12.6 | 92,000 |
| 13 | 1,623/1.0× | 1,004/7× | 13.6 | 99,000 |
| 21 | 1,758/1.2× | 1,230/9× | 12.7 | 75,000 Stop enzyme therapy |
| 25-29 60 U/kg every wk | ||||
| 29 | 1,590/1.0× | 1,372/10× | 12.2 | 79,000 |
| 32 | 1,766/1.2× | 905/6.5× | 12.7 | 103,000 |
| Enzyme Therapy . | Liver Volume . | Platelets . | ||
|---|---|---|---|---|
| . | Splenic Volume . | . | ||
| . | Hemoglobin . | . | ||
| (mo) . | (mL/fold normal) . | (g%) . | (μL) . | |
| . | . | . | . | . |
| 30 U/kg every 2 wk | ||||
| 0 | 1,749/1.2× | 1,620/14× | 11.3 | 75,000 |
| 7 | 1,655/1.0× | 1,056/8× | 12.6 | 92,000 |
| 13 | 1,623/1.0× | 1,004/7× | 13.6 | 99,000 |
| 21 | 1,758/1.2× | 1,230/9× | 12.7 | 75,000 Stop enzyme therapy |
| 25-29 60 U/kg every wk | ||||
| 29 | 1,590/1.0× | 1,372/10× | 12.2 | 79,000 |
| 32 | 1,766/1.2× | 905/6.5× | 12.7 | 103,000 |
From 6 to 12 months after initiation of enzyme therapy, increasing fatigue, knee and ankle pain, and fullness in the left upper quadrant of the abdomen was noted by the patient. In addition, she complained of face flushing and sweatiness during the alglucerase infusions. Slowing the infusion rate provided nearly complete relief of these symptoms. Her mother also reported substantially decreased energy levels. In the next 12 months, splenomegaly increased from 1,004 mL (≈8 × normal) to 1,230 mL (≈9 × normal), or about a 17% increase. Concomitant with these changes was a return to pretreatment levels of fatigue, falling asleep during the day, and knee pain. A serum sample obtained before initiation of therapy was negative for acid β-glucosidase antibodies. Anti–acid β-glucosidase seroconversion and neutralizing antibodies were detected in a serum sample obtained at 3 months of enzyme therapy.
After 2 years, enzyme therapy was discontinued for 4 months with no major increase in hepatosplenomegaly. During that period, the fatigue continued worsening. Because of the continuing presence of inhibitory antibody, progressively increasing fatigue, and bony complaints, an immunosuppression/acid β-glucosidase tolerization schedule was instituted. This schedule included Ceredase (60 U/kg) on day 1 followed by 15 mg Cytoxan/kg body weight intravenously on days 2 and 3. Cytoxan (50 mg, orally) was administered once per day from days 4 to 16. Because of severe neutropenia (absolute neutrophil count [ANC] < 500), oral Cytoxan was discontinued on day 16. The neutropenia resolved over the next month. Transient alopecia developed over the next 2 months and began to resolve after 4 months. Ceredase (60 U/kg) was administered once per week throughout this period.
While receiving Ceredase (60 U/kg) once per week, no subjective improvement was observed after 3 months, and the fatigue and bone pain continued. Some symptoms could have been related to the emotional response to the alopecia and rejection by some of her classmates. However, the spleen decreased in volume by 34% and her platelets increased by 30%. By 6 months of enzyme therapy (60 U/kg every week), she had less fatigue and was participating is school activities.
During the first 3 months of Ceredase (60 U/kg every week) enzyme therapy, she began experiencing severe headaches, flushing, chills, chest pain, and occasional shortness of breath associated with the enzyme infusions. She was switched to the recombinant enzyme preparation, Cerezyme (60 U/kg every week), 5 months later with resolution of these symptoms and signs.
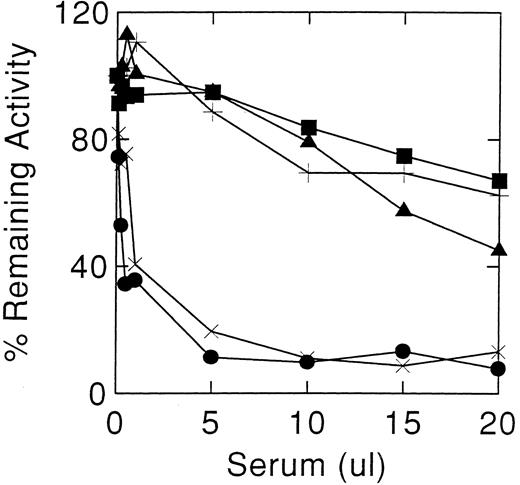
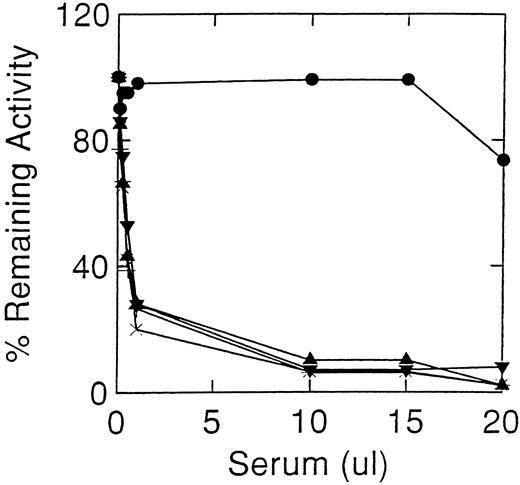
Inhibition of acid β-glucosidase activity by sera from cases 1 (×) and 2 (•). Controls sera were from normal individuals (+), and antibody positive (▪) and antibody negative (▴) Gaucher disease patients. Acid β-glucosidase, as alglucerase, was incubated with varying amounts of serum at pH 5.5 in the absence of detergents. The decrease in enzyme activity at greater than 15 μL of nonimmune serum are due to denaturation.
Inhibition of acid β-glucosidase activity by sera from cases 1 (×) and 2 (•). Controls sera were from normal individuals (+), and antibody positive (▪) and antibody negative (▴) Gaucher disease patients. Acid β-glucosidase, as alglucerase, was incubated with varying amounts of serum at pH 5.5 in the absence of detergents. The decrease in enzyme activity at greater than 15 μL of nonimmune serum are due to denaturation.
RESULTS
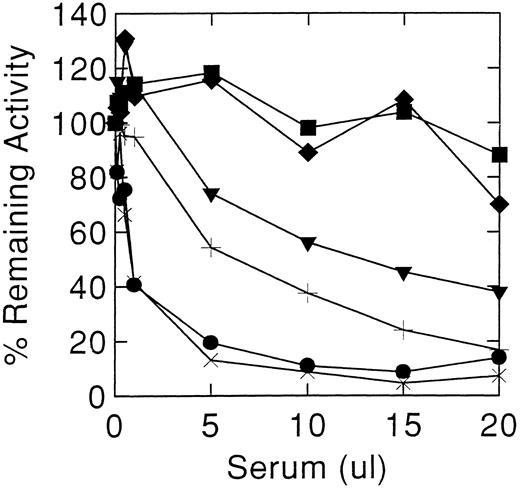
Cases 1 and 2 had high-titer antibodies that developed during enzyme therapy and inhibited acid β-glucosidase activity in vitro. To ensure that the neutralizing activity of the antibody was being tested, several controls were included: (1) sera from unaffected normal individuals, (2) pre-enzyme therapy sera from cases 1 and 2, and (3) sera from other antibody positive Gaucher patients. Since denaturation of the enzyme occurs at alkaline pH, stability of the added acid β-glucosidase in Ceredase was optimized by adjusting the serum pH to 5.5 at a final 1% Triton X-100 concentration. The initial inhibitory potencies of the serum were similar in cases 1 and 2 (Fig 1). Under optimal conditions, about 0.5 μL of antisera from either case produced a 50% inhibition of 100 μU acid β-glucosidase activity. For case 2, this titer remained unchanged throughout the time of this study (see below). However, case 1's serum showed a progressively diminishing inhibitory potency with time after discontinuing enzyme therapy. After 1 year off enzyme therapy, about 12.5 μL of serum was required for 50% inhibition of 100 μU of acid β-glucosidase (Fig 2), ie, about a 25-fold decrease in inhibitory antibody titer. An 18% to 25% decrease in activity was obtained at higher amounts of serum from other Gaucher disease patients, either anti–acid β-glucosidase antibody negative or positive, or normal individuals (Fig 1). To ensure that the Igs related to Hashimoto's thyroiditis did not have a specific effect on the enzyme, serum from case 1's affected brother (anti–acid β-glucosidase antibody negative) was shown not to inhibit acid β-glucosidase (Fig 2).
Decreasing inhibitory titres in serum from case 1 after stopping enzyme therapy. Normal control (♦), case 1's brother with Gaucher disease and Hashimoto's thyroiditis (▪), and case 1 while on enzyme therapy (•) and after stopping: 1 month (×), 8 months (+), and 12 mos (▾).
Decreasing inhibitory titres in serum from case 1 after stopping enzyme therapy. Normal control (♦), case 1's brother with Gaucher disease and Hashimoto's thyroiditis (▪), and case 1 while on enzyme therapy (•) and after stopping: 1 month (×), 8 months (+), and 12 mos (▾).
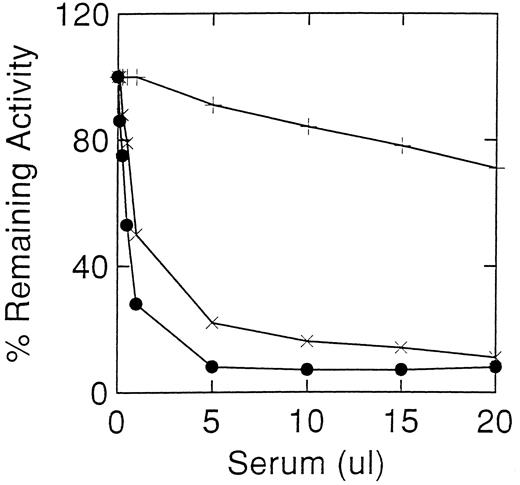
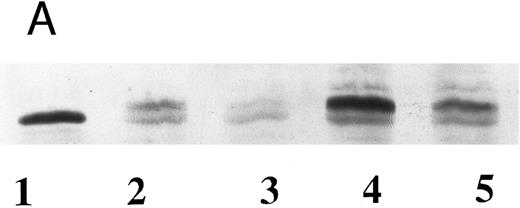
Because both patients have N370S alleles, we assessed the inhibitory effects of their sera on the N370S enzyme obtained by heterologous expression in the baculovirus system. These results showed that the N370S enzyme was poorly inhibited (maximum of 30%) by the sera from case 2 (Fig 3). The losses of activity were similar to those obtained with control sera and were probably caused by denaturation or other nonspecific effects. In comparison, Western blots (Fig 4) using inhibitory sera from both patients could detect the wild-type and N370S human enzymes expressed in the baculovirus system (Fig 4A). Also, mutant acid β-glucosidase was detected in lymphocytes from case 2 (N370S/?) or other N370S Gaucher disease patients and heterozygotes (Fig 4B).
Inhibition of acid β-glucosidase activity of recombinant normal (×) and N370S (+) enzymes expressed in the baculovirus system and alglucerase (•). Similar inhibitory profiles were obtained with the recombinant and placental acid β-glucosidase when incubated with case 2 serum. The recombinant N370S acid β-glucosidase had a similar activity profile to the normal acid β-glucosidase with non-immune sera (Fig 1).
Inhibition of acid β-glucosidase activity of recombinant normal (×) and N370S (+) enzymes expressed in the baculovirus system and alglucerase (•). Similar inhibitory profiles were obtained with the recombinant and placental acid β-glucosidase when incubated with case 2 serum. The recombinant N370S acid β-glucosidase had a similar activity profile to the normal acid β-glucosidase with non-immune sera (Fig 1).
Western blot analyses of wild-type and Gaucher disease acid β-glucosidase from recombinant (A) and natural (B) sources, using case 2 sera. (A) Immunoreactivity of recombinant wild-type and N370S enzymes expressed in the baculovirus system. Similar forms of both recombinant enzymes were detected with serum from case 2. Acid β-glucosidase (lane 1), wild-type enzyme (lanes 2 and 4), N370S enzyme (lanes 3 and 5). (B) Wild-type enzyme from an unaffected individual with normal levels of enzyme activity (lane 1), a Gaucher disease patient who was hemizygous for the N370S allele and a gene deletion (lane 2), the father of the patient in lane 2 who is hemizygous for the normal allele (lane 3), and case 2 who was heterozygous for N370S (lane 4). The three bands present in lanes 1, 3, and 4 correspond to different posttranslational glycosylation stages of acid β-glucosidase.
Western blot analyses of wild-type and Gaucher disease acid β-glucosidase from recombinant (A) and natural (B) sources, using case 2 sera. (A) Immunoreactivity of recombinant wild-type and N370S enzymes expressed in the baculovirus system. Similar forms of both recombinant enzymes were detected with serum from case 2. Acid β-glucosidase (lane 1), wild-type enzyme (lanes 2 and 4), N370S enzyme (lanes 3 and 5). (B) Wild-type enzyme from an unaffected individual with normal levels of enzyme activity (lane 1), a Gaucher disease patient who was hemizygous for the N370S allele and a gene deletion (lane 2), the father of the patient in lane 2 who is hemizygous for the normal allele (lane 3), and case 2 who was heterozygous for N370S (lane 4). The three bands present in lanes 1, 3, and 4 correspond to different posttranslational glycosylation stages of acid β-glucosidase.
Inhibitory potency of the sera from case 2 was determined every 3 months during the immunosuppression/acid β-glucosidase tolerization protocol (months 29 to 35, Fig 5). No change in in vitro inhibitory potency of the sera was detectible during this period. In comparison, simultaneous evaluation of the clinical symptoms and objective measures showed improvement (Table 1). However, the adverse effects (headaches, chills, etc) increased when on 60 U/kg every week. Some of these adverse effects may relate to the presence of anti-HSA antibodies present in case 2's serum (Fig 6). This is supported by the cessation of these adverse effects when Cerezyme was used; this recombinant enzyme that does not contain HSA.
Inhibitory titres of case 2 sera over 24 months. Pretreatment (•) and seven sera samples (other symbols) from a period of 24 months with inhibitory capacity during enzyme therapy and after discontinuation (▴). No differences in titer were appreciated.
Inhibitory titres of case 2 sera over 24 months. Pretreatment (•) and seven sera samples (other symbols) from a period of 24 months with inhibitory capacity during enzyme therapy and after discontinuation (▴). No differences in titer were appreciated.
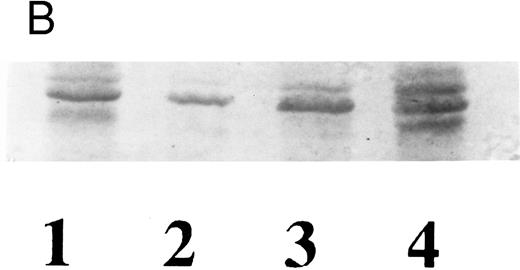
Western blot analysis of proteins present in Ceredase (lanes 1 and 2) and Cerezyme (lanes 3 and 4) using case 2 antiserum (1:1,500 dilution). Acid β-glucosidase in Ceredase [0.4 μg (lane 1) and 0.8 μg (lane 2)] and in Cerezyme [5 μg (lane 3) and 10 μg (lane 4). HSA in lanes 5 (1 μg) and 6 (2 μg).
Western blot analysis of proteins present in Ceredase (lanes 1 and 2) and Cerezyme (lanes 3 and 4) using case 2 antiserum (1:1,500 dilution). Acid β-glucosidase in Ceredase [0.4 μg (lane 1) and 0.8 μg (lane 2)] and in Cerezyme [5 μg (lane 3) and 10 μg (lane 4). HSA in lanes 5 (1 μg) and 6 (2 μg).
DISCUSSION
To date, the factors that influence the variability in response to enzyme therapy of patients with Gaucher disease type 1 have been poorly characterized. Although antibodies are present in 15% of patients receiving either natural or recombinant enzyme products, none, except those reported here, have had a substantial impact on the effectiveness of enzyme therapy. In both of our patients the development of neutralizing antibodies blunted the expected therapeutic progress by enzyme therapy even though both patients received substantial initial doses of enzyme (50 or 30 U/kg every 2 weeks). The presence of antibody positivity correlated with the development of allergic-like reactions that seem likely to be antibody mediated. However, at least in case 2, these allergic-like reactions were potentially more related to antibodies to HSA in Ceredase than to those against acid β-glucosidase. This conclusion is based on the absence of these symptoms when the recombinant enzyme, Cerezyme, was used, since this preparation does not contain HSA. However, the serum from case 2 did react with and inhibit the acid β-glucosidase, imiglucerase, in Cerezyme. Case 1 had decreasing inhibitory antibody titers during the first 8 months after discontinuation of enzyme therapy. This titer diminished an additional 50% by 12 months off therapy. This decrease in antibody titer was accompanied by increasing thrombocytopenia and by increasing splenomegaly by physical examination. Thus, his disease progressed despite the loss of neutralizing antibody. This clinical finding suggests that enzyme therapy had been controlling the rate of his disease progression, even in the presence of neutralizing antibodies.
In comparison, case 2 initially had positive responses to enzyme therapy and then her disease progressively worsened concordant with the development of neutralizing antibodies. Her improvement while on 60 U/kg every week likely relates to a large amount of free enzyme being present after all antibody in her serum was bound to enzyme. Although anti-idiotypic antibodies16 17 could have been induced by the increased enzyme dosage, these were not detected as a decreasing antibody titer by our in vitro inhibitory assay.
The development of neutralizing antibodies in these two patients highlights the need for continuing close monitoring of patients during the initial phases of therapy since most patients become antibody positive during their first 6 to 9 months.5 10 Although these two patients had antibodies that inhibited acid β-glucosidase activity, these antibodies differed somewhat in their response to enzyme therapy withdrawal.
Although the sera from cases 1 and 2 could completely neutralize acid β-glucosidase activity, other antibodies, with partial or low-level inhibitory capacity, may exist in other Gaucher disease patients. These could contribute, in part, to the less than expected responses to enzyme therapy. In our group of seven antibody-positive patients, none, other than cases 1 and 2, had inhibitory antiserum. Clearly, screening for inhibitory antibodies should be a routine component of management. The assays for effects of antibodies on acid β-glucosidase activity are not straightforward, particularly because these are done in the presence of detergents and added proteins, ie, serum and HSA, that have significant effects on activity.18 19 Based on the present cases we would recommend that all antibody-positive patients should have their antibodies tested for potential inhibitory effects. This test is available on a regular basis (Susan Richards, personal commmunication, February 1997, Genzyme Corp). Particular attention should be directed to low-level and/or partially neutralizing antibodies. Finding of such antibodies may influence dosage reductions and/or schedules due to the serum's ability to bind and remove or inhibit enzyme in vivo. The latter was suggested by the good therapeutic response of case 2 to a fourfold increase in enzyme dose that was unaccompanied by decreasing titers of antibody. It is also clear from the Western blot and neutralizing studies that several different populations of antibodies are present in the sera from cases 1 and 2. Some of these inhibit the wild type, but do not have this effect on the N370S enzymes, whereas others give immunoreactivity on Western blots.
The reactivity of the antisera from cases 1 and 2 toward the N370S mutant protein raises additional issues. The antibodies to acid β-glucosidase in cases 1 and 2 sera probably contain numerous specificities to various epitopes on the enzyme. The lack of inhibition of N370S enzyme activity by the antiserum suggests an epitope specific antibody for the region around residue N370. However, the reactivity of the antiserum from case 2 with the normal and N370S enzymes on Western blots indicates the broader interaction of the antisera than just the N370 epitope. Of potential concern is an acceleration of the disease that might follow termination of therapy due to the persistence of the reactive neutralizing antibodies within the serum. For this to occur, free antibody would need to gain access to intracellular, endogenous acid β-glucosidase. Theoretically, such interaction could worsen their disease. We did not observe this in either case. Of equal importance is the potential need to terminate therapy in some of these patients because of its lack of effect and/or its great expense, ie, for a 70-kg individual, a 60-U/kg every week treatment would represent an expense of ≈$850,000 per year. This is similar to the cost of factor VIII used to treat seroconverted hemophiliacs, ie, for a 70-kg patient with neutralizing antibodies to factor VIII, the cost would range from $210,000 to $1,540,000 per year, depending on the treatment/desensitization protocol. With time off therapy neutralizing antibodies could disappear, as in case 1, until exposure to the enzyme again elicits an anamnestic reaction. Other patients' antibodies will develop and these could be reactive to their respective variants of acid β-glucosidase. In all of these patients such antibodies also could potentially interfere with future therapeutic strategies by a BM transplantation and/or gene therapy for the treatment of their disease.
The lack of substantial decreases in titers of neutralizing antibody in case 2 during immunosuppression and high-dose tolerization indicate continuing and expensive difficulties for treatment of this patient. Thus, the inability to eliminate the reactive antibody and reinstitute effectiveness of enzyme therapy indicates the need to develop improved supportive therapy for such patients and/or the selective intermittent use of enzyme therapy for certain types of progressive involvement. Although the development of neutralizing antibodies is rare, it has had a substantial impact on the effectiveness of therapy in these two patients with Gaucher disease. It is anticipated that other patients with other lysosomal storage diseases, particularly those that are cross-reacting immunologic material negative, can also develop neutralizing antibodies or other types of adverse effects that impact the effectiveness of therapy for their diseases.
Supported by grants from the National Institutes of Health (DK 36729) and the Lucille P. Markey Charitable Trust, and by the Children's Hospital Research Foundation.
Address reprint requests to Gregory Grabowski, MD, Director, Division of Human Genetics, Children's Hospital Research Foundation, 3333 Burnet Ave, Cincinnati, OH 45229-3039

![Fig. 6. Western blot analysis of proteins present in Ceredase (lanes 1 and 2) and Cerezyme (lanes 3 and 4) using case 2 antiserum (1:1,500 dilution). Acid β-glucosidase in Ceredase [0.4 μg (lane 1) and 0.8 μg (lane 2)] and in Cerezyme [5 μg (lane 3) and 10 μg (lane 4). HSA in lanes 5 (1 μg) and 6 (2 μg).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.43/5/m_bl_0034f6.jpeg?Expires=1770966953&Signature=yQF71lIUJacxonubk~IdE~OPz-3Md8BTDewCB4rBJCPkJSgVtFtbbn4BjzFh7i7HsXyUSB~cFaLcpR4HGXZbhXwO7sYTJp9Nz2KRArx8EU7YFGP~tQGmfnltwoRoqW~8GdCNxfOq2SQVDPoKSpcLm~kJbH3RTMmeNnDyw3aN1jxWEymGf-1NTbNWvToewDRi7yh4pYHJnLD6J7br53oqHV13QZwmK6DKon9PlsEIfTzqMzjcmbykC0TLVO2yBrNilEEhCvljjTv5ask6mPpG6~TyMO-aDz-JtpNEJCTu2ZQ56Tcp4h7NaC6l8YUklxNbCLBprtAO7lUSGnEuSvdeyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal