Abstract
Primary cutaneous lymphomas represent a heterogeneous group of T- and B-cell lymphomas that show considerable variation in histology, phenotype, and prognosis. Recently, the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group has reached consensus on a new classification for this group of diseases. The EORTC classification for primary cutaneous lymphomas is based on a combination of clinical, histologic, and immunophenotypic criteria, and thus contains well-defined disease entities rather than histologic subgroups. In addition, this new classification contains a number of provisional entities, which may display characteristic histologic features, but are not yet well defined clinically. These provisional entities account for less than 5% of all primary cutaneous lymphomas. In this report the basic principles of this new classification, as well as the characteristic features of the different disease entities, are described. In addition, survival data of 626 patients with primary cutaneous lymphomas derived from the registry of the Dutch Cutaneous Lymphoma Working Group, illustrating the clinical validity of this new classification, are presented.
PRIMARY CUTANEOUS lymphomas represent a heterogeneous group of T- and B-cell lymphomas, which show considerable variation in clinical presentation, histology, immunophenotype, and prognosis. They are, after the group of primary gastrointestinal lymphomas, the second most common group of extranodal non-Hodgkin lymphomas.1 The annual incidence is estimated at 0.5 to 1/100,000.2 Until recently, distinction between primary and secondary cutaneous lymphomas was generally not made.3,4 Both groups of lymphomas were classified according to histologic classification schemes used by hematopathologists for nodal non-Hodgkin lymphomas, such as the (updated) Kiel classification5 and the Working Formulation.6 However, recent studies demonstrated that primary cutaneous lymphomas, defined as patients without concurrent extracutaneous disease at the time of diagnosis, often have highly characteristic clinical and histologic features, and a clinical behavior and prognosis, that are different from primary nodal lymphomas of the same histologic subtype, involving the skin secondarily.7-11 In addition, differences in the presence of specific translocations and in the expression of oncogenes, viral sequences, and adhesion receptors were found, suggesting that primary cutaneous lymphomas should be considered as a distinctive group, both clinically and biologically.12 New insights in the mechanisms underlying lymphocyte recirculation and organ-specific homing provided an explanation for these differences, and led to the present view that morphologically identical lymphomas arising at different sites are clonal proliferations of distinct, organ-related, lymphocyte subpopulations, which have retained many characteristics of their benign counterparts.12 13
These studies on well-defined groups of primary cutaneous lymphomas resulted not only in the delineation of several new disease entities, but also in the awareness that the classification schemes used for nodal lymphomas are inadequate to categorize primary cutaneous lymphomas in a clinically meaningful way. First, several types of primary cutaneous large cell lymphomas classified as high grade malignant lymphomas according to the updated Kiel classification and Working Formulation, were found to have an indolent clinical behavior.7-10 Second, studies on (cutaneous) T-cell lymphomas revealed that the reproducibility of the Kiel classification for this group of lymphomas is poor.14-16 Third, there is mounting evidence that primary cutaneous lymphomas cannot be defined properly by histologic criteria alone. For instance, it has been well-established that primary cutaneous large T-cell lymphomas expressing the CD30 antigen, irrespective of the histologic subtype (anaplastic or non-anaplastic), have a significantly better prognosis than primary cutaneous CD30-negative large T-cell lymphomas.14 The difficulty, or sometimes the impossibility of differentiating between primary cutaneous CD30-positive (anaplastic) large cell lymphomas and lymphomatoid papulosis (LyP), is well recognized.17,18 Epidermotropic lymphoid infiltrates containing atypical T cells with cerebriform nuclei are the hallmark of plaque stage mycosis fungoides (MF ), but can also be found in LyP type B19 and some pseudo–T-cell lymphomas.20 A definite diagnosis in these cases depends on knowledge about the clinical presentation and the results of additional immunologic and genetic investigations. These and other examples clearly illustrated that cutaneous lymphomas can only be defined by a combination of clinical, histologic, and immunologic data.
These observations resulted in several proposals for a separate classification for the group of cutaneous lymphomas.12,21,22 At three recent meetings of the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer (EORTC) these proposals have been the subject of extensive discussions that were aimed at reaching consensus on a new European classification for the group of primary cutaneous lymphomas. The EORTC classification that was agreed on is presented in Table 1. A comparison of this classification with the updated Kiel classification and the Revised European-American Lymphoma (REAL) Classification23 is given in Table 2.
EORTC Classification for Primary Cutaneous Lymphomas
| Primary CTCL . | Primary CBCL . |
|---|---|
| Indolent | Indolent |
| MF | |
| Follicle center cell lymphoma | |
| MF + follicular mucinosis | |
| Pagetoid reticulosis | |
| Immunocytoma (marginal zone B-cell lymphoma) | |
| Large cell CTCL, CD30+ | |
| Anaplastic, | |
| Immunoblastic | |
| Pleomorphic | |
| Lymphomatoid papulosis | |
| Intermediate | |
| Large B-cell lymphoma of the leg | |
| Aggressive | |
| SS | |
| Large cell CTCL, CD30− | |
| Immunoblastic, | |
| Pleomorphic | |
| Provisional | Provisional |
| Granulomatous slack skin | |
| Intravascular large B-cell lymphoma | |
| CTCL, pleomorphic small/medium-sized | Plasmacytoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Primary CTCL . | Primary CBCL . |
|---|---|
| Indolent | Indolent |
| MF | |
| Follicle center cell lymphoma | |
| MF + follicular mucinosis | |
| Pagetoid reticulosis | |
| Immunocytoma (marginal zone B-cell lymphoma) | |
| Large cell CTCL, CD30+ | |
| Anaplastic, | |
| Immunoblastic | |
| Pleomorphic | |
| Lymphomatoid papulosis | |
| Intermediate | |
| Large B-cell lymphoma of the leg | |
| Aggressive | |
| SS | |
| Large cell CTCL, CD30− | |
| Immunoblastic, | |
| Pleomorphic | |
| Provisional | Provisional |
| Granulomatous slack skin | |
| Intravascular large B-cell lymphoma | |
| CTCL, pleomorphic small/medium-sized | Plasmacytoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
Abbreviations: CTCL, cutaneous T-cell lymphoma; CBCL, cutaneous B-cell lymphoma; MF, mycosis fungoides; SS, Sezary syndrome.
EORTC Classification of Primary Cutaneous Lymphomas: Comparison With the Kiel Classification and the REAL Classification
| Kiel Classification . | EORTC Classification . | Real Classification . |
|---|---|---|
| Small cell, cerebriform | MF | MF |
| Not listed | MF-associated follicular mucinosis | Not listed |
| Not listed | Pagetoid reticulosis | Not listed |
| Not listed | Granulomatous slack skin [provisional entity] | Not listed |
| Small cell, cerebriform | SS | SS |
| Not listed | Lymphomatoid papulosis | Not listed |
| CD30-positive large T-cell lymphoma | ||
| Large cell anaplastic (CD30+) | Anaplastic | Anaplastic large cell lymphoma |
| Pleomorphic, medium-sized/large cell | Pleomorphic | Peripheral T-cell lymphoma, unspecified |
| T immunoblastic | Immunoblastic | Peripheral T-cell lymphoma, unspecified |
| CD30-negative large T-cell lymphoma | Peripheral T-cell lymphoma, unspecified | |
| Pleomorphic, medium-sized/large cell | Pleomorphic large cell | |
| T immunoblastic | Immunoblastic | |
| Pleomorphic, small cell | Pleomorphic, small/medium-sized cell [provisional] | Peripheral T-cell lymphoma, unspecified |
| Not listed | ||
| Subcutaneous panniculitis-like T-cell lymphoma [provisional entity] | ||
| Subcutaneous panniculitic T-cell lymphoma | ||
| Centroblastic/centrocytic | ||
| Centroblastic | ||
| Monomorphic | ||
| Polymorphic | ||
| Multilobated | ||
| Centrocytoid | ||
| Follicle center cell lymphoma (mainly on head and trunk) | ||
| Follicle center lymphoma | ||
| I.predominantly small cell | ||
| II.mixed small and large cell | ||
| III.predominantly large cell | ||
| Diffuse large B-cell lymphoma | ||
| Immunocytoma | ||
| Monocytoid, including marginal zone lymphoma | ||
| Immunocytoma/marginal zone B-cell lymphoma | ||
| Extranodal marginal zone B-cell lymphoma | ||
| Plasmacytoma | Plasmacytoma [provisional entity] | Plasmacytoma |
| Centroblastic lymphoma | ||
| Monomorphic | ||
| Polymorphic | ||
| Multilobated | ||
| Centrocytoid | Large B-cell lymphoma of the legs | Diffuse large B-cell lymphoma |
| B-immunoblastic lymphoma | ||
| Not listed | ||
| Intravascular large B-cell lymphoma [provisional entity] | Not listed |
| Kiel Classification . | EORTC Classification . | Real Classification . |
|---|---|---|
| Small cell, cerebriform | MF | MF |
| Not listed | MF-associated follicular mucinosis | Not listed |
| Not listed | Pagetoid reticulosis | Not listed |
| Not listed | Granulomatous slack skin [provisional entity] | Not listed |
| Small cell, cerebriform | SS | SS |
| Not listed | Lymphomatoid papulosis | Not listed |
| CD30-positive large T-cell lymphoma | ||
| Large cell anaplastic (CD30+) | Anaplastic | Anaplastic large cell lymphoma |
| Pleomorphic, medium-sized/large cell | Pleomorphic | Peripheral T-cell lymphoma, unspecified |
| T immunoblastic | Immunoblastic | Peripheral T-cell lymphoma, unspecified |
| CD30-negative large T-cell lymphoma | Peripheral T-cell lymphoma, unspecified | |
| Pleomorphic, medium-sized/large cell | Pleomorphic large cell | |
| T immunoblastic | Immunoblastic | |
| Pleomorphic, small cell | Pleomorphic, small/medium-sized cell [provisional] | Peripheral T-cell lymphoma, unspecified |
| Not listed | ||
| Subcutaneous panniculitis-like T-cell lymphoma [provisional entity] | ||
| Subcutaneous panniculitic T-cell lymphoma | ||
| Centroblastic/centrocytic | ||
| Centroblastic | ||
| Monomorphic | ||
| Polymorphic | ||
| Multilobated | ||
| Centrocytoid | ||
| Follicle center cell lymphoma (mainly on head and trunk) | ||
| Follicle center lymphoma | ||
| I.predominantly small cell | ||
| II.mixed small and large cell | ||
| III.predominantly large cell | ||
| Diffuse large B-cell lymphoma | ||
| Immunocytoma | ||
| Monocytoid, including marginal zone lymphoma | ||
| Immunocytoma/marginal zone B-cell lymphoma | ||
| Extranodal marginal zone B-cell lymphoma | ||
| Plasmacytoma | Plasmacytoma [provisional entity] | Plasmacytoma |
| Centroblastic lymphoma | ||
| Monomorphic | ||
| Polymorphic | ||
| Multilobated | ||
| Centrocytoid | Large B-cell lymphoma of the legs | Diffuse large B-cell lymphoma |
| B-immunoblastic lymphoma | ||
| Not listed | ||
| Intravascular large B-cell lymphoma [provisional entity] | Not listed |
In this report, the basic principles of this new classification, and the major clinical, histologic, immunologic, and genetic features of the different types of primary cutaneous lymphomas are presented. In addition, data on the relative frequency and 5-year survival of the different entities as well as therapeutic guidelines are included. The frequency and survival data illustrating the clinical usefulness of this new classification, are derived from the registry of the Dutch Cutaneous Lymphoma Working Group (DCLWG), and are based on 626 evaluable patients with a definite diagnosis of primary cutaneous lymphoma registered between 1986 and 1994 (Table 3). In each case the diagnosis was made by an expert panel of haematopathologists, dermatopathologists, and dermatologists during one of the quarterly meetings of the DCLWG, and is based on a combination of clinical, histologic, and immunophenotypical data. Follow-up data of all registered patients were collected each year from referring dermatologists or oncologists.
Relative Frequency and 5-Year Survival of the Main Groups of Primary Cutaneous Lymphoma Included in the EORTC Classification
| Classification . | No. . | Frequency (%) . | 5-Year Survival3-150 (%) . |
|---|---|---|---|
| MF | 278 | 44 | 87 |
| Specific variants of MF | |||
| MF + mucinosis follicularis | 30 | 4 | 70 |
| Pagetoid reticulosis | 5 | <1 | 100 |
| Granulomatous slack skin | 2 | <1 | — |
| CTCL, large cell (CD30+) | 57 | 9 | 90 |
| Lymphomatoid papulosis | 70 | 11 | 100 |
| CTCL, large cell (CD30−) | 36 | 5 | 15 |
| SS | 12 | 2 | 11 |
| CTCL, pleomorphic small/med | 18 | 3 | 62 |
| CBCL, PCFCCL3-151 | 84 | 13 | 97 |
| Immunocytoma (MZBCL) | 12 | 2 | 100 |
| LBCL of the legs | 18 | 3 | 58 |
| Intravascular B-cell lymphoma | 4 | <1 | 50 |
| Classification . | No. . | Frequency (%) . | 5-Year Survival3-150 (%) . |
|---|---|---|---|
| MF | 278 | 44 | 87 |
| Specific variants of MF | |||
| MF + mucinosis follicularis | 30 | 4 | 70 |
| Pagetoid reticulosis | 5 | <1 | 100 |
| Granulomatous slack skin | 2 | <1 | — |
| CTCL, large cell (CD30+) | 57 | 9 | 90 |
| Lymphomatoid papulosis | 70 | 11 | 100 |
| CTCL, large cell (CD30−) | 36 | 5 | 15 |
| SS | 12 | 2 | 11 |
| CTCL, pleomorphic small/med | 18 | 3 | 62 |
| CBCL, PCFCCL3-151 | 84 | 13 | 97 |
| Immunocytoma (MZBCL) | 12 | 2 | 100 |
| LBCL of the legs | 18 | 3 | 58 |
| Intravascular B-cell lymphoma | 4 | <1 | 50 |
Data are based on 626 patients with a primary cutaneous lymphoma registered at the Dutch Cutaneous Lymphoma Working Group between 1986 and 1994.
Abbreviations: CTCL, cutaneous T-cell lymphoma; CBCL, cutaneous B-cell lymphoma; PCFCCL, primary cutaneous follicle center cell lymphoma; LBCL, large B-cell lymphoma; MZBCL, marginal zone B-cell lymphoma; MF, mycosis fungoides; SS, Sezary syndrome.
The figures presented represent the disease-related 5-year survival.
Excluding follicle center cell lymphomas on the legs.
BASIC PRINCIPLES OF THE EORTC CLASSIFICATION FOR PRIMARY CUTANEOUS LYMPHOMAS
The main goal of the EORTC Cutaneous Lymphoma Study group was to constitute a clinically relevant classification. This implies that assignment of a patient to a certain category should give the clinician all essential information necessary for proper management and treatment. For that reason the EORTC classification only includes primary cutaneous lymphomas, defined as non-Hodgkin lymphomas presenting in the skin, with no evidence of extracutaneous disease at the time of diagnosis and within the first 6 months after diagnosis, as assessed by appropriate staging procedures. The 6-month interval, though arbitrary, is considered important in particular for retrospective studies. In clinical practice, the absence of extracutaneous disease at the time of diagnosis will be the decisive criterion for management and treatment. This basic principle does not apply to patients with classical MF presenting with skin and peripheral lymph node involvement, nor does it apply to Sézary syndrome patients, who have peripheral blood involvement by definition. These groups are included as well. The EORTC classification excludes malignant lymphomas with secondary skin involvement as well as malignant lymphomas in immunocompromised patients, as these lymphomas may have a different clinical behavior. Moreover, the EORTC Cutaneous Lymphoma Study Group decided not to include HTLV-1–associated adult T-cell lymphoma leukemias (ATLL), as in most cases skin lesions are a manifestation of generalized rapidly fatal disease, and ATLLs are extremely rare in Europe.
In addition, the EORTC classification for primary cutaneous lymphomas is based on a combination of clinical, histologic, immunohistochemical, and genetic criteria. The same approach has recently been used for the REAL classification.23 Thus, in contrast to the Kiel classification and earlier classifications that basically distinguish histologic subgroups, the EORTC classification principally includes distinct disease entities, with well-defined clinical and histologic features, including a predictable clinical course, response to therapy, and prognosis. Therefore, the EORTC classification does not use the terms low-grade and high-grade malignant lymphomas, which refer primarily to cell size but have no clinical significance in primary cutaneous lymphomas. Instead, distinction is made between primary cutaneous lymphomas with an indolent, intermediate, and aggressive clinical behavior. Almost 95% of all primary cutaneous lymphomas registered at the DCLWG belong to one of the disease entities included in this classification (Table 3). In addition, some provisional entities have been included. The term provisional entity refers to a group of cutaneous lymphomas, which display characteristic histologic features (eg, morphology, histologic pattern, immunophenotype), but of which a distinctive clinical presentation and/or outcome has not yet been defined.
CUTANEOUS T-CELL LYMPHOMAS WITH INDOLENT CLINICAL BEHAVIOR
Mycosis Fungoides
Definition.MF is an epidermotropic CTCL characterized by a proliferation of small or medium-sized neoplastic T lymphocytes with cerebriform nuclei. The term MF should only be used for the classical “Alibert-Bazin” type of MF characterized by the subsequent evolution of patches, plaques, and tumors, or for clinical variants with histologic features and a clinical course similar to that of classical MF.24
Clinical features.MF has an indolent clinical course with slow progression over years or sometimes decades from patches to more infiltrated plaques and eventually tumors (Fig 1A and C). In a proportion of patients lymph node and internal organs may become involved in the later stages of the disease.
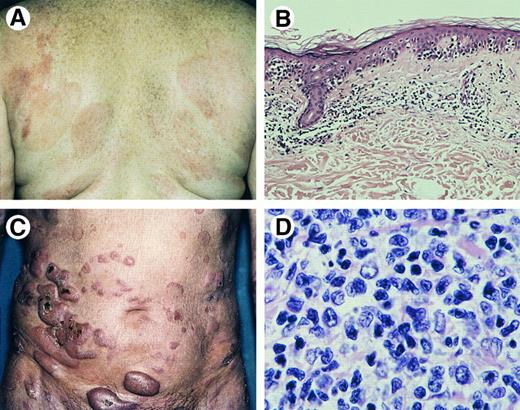
Mycosis fungoides. (A) Patches and slightly infiltrated plaques. (B) Bandlike superficial infiltrate with marked infiltration of the epidermis (Hematoxylin & Eosin (H&E), original magnification × 50). (C) Tumor stage. (D) Tumor stage: detail of dermal infiltrate showing medium-sized to large lymphoid cells with hyperchromatic nuclei and blast cells (H&E, original magnification × 480).
Mycosis fungoides. (A) Patches and slightly infiltrated plaques. (B) Bandlike superficial infiltrate with marked infiltration of the epidermis (Hematoxylin & Eosin (H&E), original magnification × 50). (C) Tumor stage. (D) Tumor stage: detail of dermal infiltrate showing medium-sized to large lymphoid cells with hyperchromatic nuclei and blast cells (H&E, original magnification × 480).
Histopathology.MF consists of epidermotropic, band-like infiltrates involving the papillary dermis containing small, medium-sized, and occasionally large mononuclear cells with hyperchromatic, indented (cerebriform) nuclei and variable numbers of admixed inflammatory cells.25-27 Colonization of the lower layers of the epidermis by single or small groups of neoplastic cells is a characteristic finding (Fig 1B). Pautrier's microabscesses are highly characteristic, but seen in only a minority of cases.27 With progression to tumor stage, the dermal infiltrates become more diffuse and the proportion of tumor cells may increase both in number and size, whereas there is a concomitant decrease in the number of reactive T cells (Fig 1D). Epidermotropism may no longer be found.
Immunophenotype.Tumor cells are CD3+, CD4+, CD45RO+, CD8−, CD30−.28 Rare cases expressing a CD3+, CD4−, CD8+ mature T-cell phenotype have been reported. In the tumor stage, an aberrant phenotype with loss of T-cell antigens is a common finding.28
Genetic features.T-cell receptor (TCR) genes are clonally rearranged in most cases, including early patch lesions.29,30 Consistent cytogenetic abnormalities have not been identified. Although an association between HTLV-1 and MF has been suggested,31 most recent studies conclude against a role for HTLV-1 in the pathogenesis of CTCL.32 33
Survival.The estimated 5-year survival of 278 patients included in the DCLWG is 87%.
Therapy.As long as the disease is confined to the skin, skin-targeted therapies as photo(chemo)therapy (eg, PUVA),34 topical application of nitrogen mustard35 or chlormustine (BCNU),36 or radiotherapy, including total skin electron beam irradiation,37 are preferred. Multiagent chemotherapy is indicated in case of unequivocal lymph node or visceral involvement, or in case of wide-spread tumor stage disease refractory to skin-targeted therapies, but should not be considered in early patch/plaque stage disease.38 Beneficial effects have also been reported of interferon α either alone or in combination with PUVA or retinoids.39
MF Specific Variants
Apart from the classical Alibert-Bazin type of MF, many clinical and/or histologic variants have been reported. Clinical variants, such as bullous and hyper- or hypopigmented MF have a clinical behavior similar to that of classical MF, and are therefore not considered separately. In contrast, MF-associated with follicular mucinosis, pagetoid reticulosis, and granulomatous slack skin (see provisional types of CTCL), which are also commonly considered as variants of MF, have distinctive clinicopathologic features, and have therefore been included as separate entities in the EORTC classification.
MF-Associated Follicular Mucinosis
Definition.It is a distinct variant of MF characterized by the presence of folliculotropic infiltrates with sparing of the epidermis, mucinous degeneration of the hair follicles, and preferential involvement of the head and neck area.
Clinical features.Indications include follicular papules, indurated plaques, and tumors usually involving the head and neck area; it is often associated with hair loss, pruritus, and secondary bacterial infections.42 43
Histopathology.Characteristic findings include the primarily perivascular and periadnexal localization of the dermal infiltrates, with marked infiltration of the follicular epithelium by medium-sized to large hyperchromatic cells with cerebriform nuclei, and sparing of the epidermis (folliculotropism instead of epidermotropism), mucinous degeneration of the follicular epithelium (follicular mucinosis), and often a considerable admixture with eosinophils and sometimes plasma cells.42,44 Similar cases, without follicular mucinosis, have been reported as follicular or pilotropic MF.45
Immunophenotype.See MF.
Genetic features.Clonal TCR gene rearrangements are found in most cases and represent a useful adjunct in the differentiation from alopecia mucinosa.46
Survival.The estimated 5-year survival of 30 patients included in the DCLWG is 70%.
Therapy.Because of the perifollicular localization of the dermal infiltrates, MF-associated FM is often less responsive to skin-targeted therapies, such as PUVA and topical nitrogen mustard than classical plaque stage MF. In such cases, total skin electron beam irradiation is the preferred mode of treatment.
Comment.Because of the absence of epidermotropism, MF-associated follicular mucinosis is often not recognized, and is classified incorrectly as a pleomorphic CTCL.
Pagetoid Reticulosis
Definition.Pagetoid reticulosis is a distinct variant of MF, characterized by the presence of localized patches or plaques with an intraepidermal proliferation of neoplastic T cells.24 47
Clinical features.It is a slowly growing psoriasiform or hyperkeratotic patch or plaque, typically affecting a distal limb (Fig 2A). Extracutaneous dissemination has not been reported.
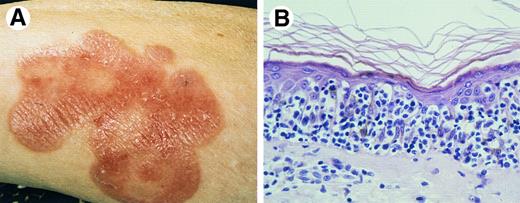
Pagetoid reticulosis. (A) Clinical presentation with solitary plaque on lower leg. (B) Proliferation of atypical lymphoid cells within the epidermis (H&E, original magnification × 300).
Pagetoid reticulosis. (A) Clinical presentation with solitary plaque on lower leg. (B) Proliferation of atypical lymphoid cells within the epidermis (H&E, original magnification × 300).
Histopathology.Acanthosis and sponge-like disaggregation of the epidermis by large atypical pagetoid cells, singly or arranged in nests or clusters are characteristic (Fig 2B). The atypical cells have medium-sized or large, sometimes hyperchromatic and cerebriform nuclei, and abundant, vacuolated cytoplasm.24
Immunophenotype.Tumor cells have a CD3+, CD4+, CD8− or a CD3+, CD4−, CD8+ phenototype. CD30 can be expressed.48 49
Genetic features.Clonal rearrangement of TCR genes have been demonstrated.50
Therapy.Radiotherapy or surgical excision is recommended.
Survival.The prognosis is excellent. Disease-related deaths have not been reported.
PRIMARY CUTANEOUS CD30-POSITIVE LYMPHOPROLIFERATIVE DISORDERS
This group includes primary cutaneous CD30-positive large T-cell lymphoma, LyP, and borderline cases. The term borderline case refers to cases in which there is a discrepancy between the clinical features and the histologic appearance.17 These include cases with the clinical presentation of a CD30-positive large T-cell lymphoma, but with histologic features suggestive of LyP, and conversely, cases with recurrent, self-healing skin lesions that on histologic examination show a rather uniform proliferation of large CD30-positive tumor cells with only few admixed inflammatory cells, characteristic of a CD30-positive large T-cell lymphoma. From a clinical and therapeutic point of view, differentiation between cutaneous CD30+ large T-cell lymphoma and LyP is important. Therefore, these borderline cases have not been included in the EORTC classification as a separate category. Distinction between LyP and primary cutaneous CD30+ large T-cell lymphomas is not always possible on the basis of histologic criteria.17,18 Therefore, the clinical appearance and course are used as a decisive criterium for the definite diagnosis and choice of treatment. The overlapping clinical, histologic and immunophenotypic features have resulted in the view that CD30+ cutaneous large T-cell lymphomas and LyP are parts of a spectrum of primary cutaneous CD30+ lymphoproliferative disorders.17,51 Rare cases of primary cutaneous Hodgkin's disease with an indolent clinical course, may also belong to this spectrum.52
CD30-Positive Cutaneous Large T-Cell Lymphoma (CTCL)
Definition.CTCL consisting of large tumor cells, the majority of which express CD30-antigen. There is no clinical evidence or history of LyP, MF, or another type of CTCL. Whereas most lymphomas in this category have the morphologic features of an anaplastic large cell lymphoma and would be classified accordingly in both the REAL and the updated Kiel classification, 20% of the lymphomas in this group do not have an anaplastic cytology.8 Previous studies demonstrated that (1) these CD30+ primary cutaneous nonanaplastic, mainly immunoblastic and large pleomorphic (updated Kiel classification), large T-cell lymphomas have an identical clinical presentation and clinical behavior similar to primary cutaneous CD30+ anaplastic large T-cell lymphomas (5-year survival 90%)8,18; and (2) primary cutaneous CD30-negative immunoblastic and pleomorphic (nonanaplastic) large T-cell lymphomas generally run an aggressive clinical course.14 53 For these reasons, the EORTC group prefers the term primary cutaneous CD30-positive large T-cell lymphoma rather than primary cutaneous anaplastic large cell lymphoma. Examination for CD30 expression is thus imperative in these primary cutaneous large T-cell lymphomas, and the best guarantee for correct classification and appropriate management and treatment.
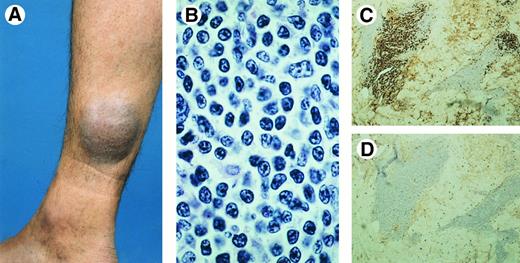
Clinical features.These lymphomas occur in adults, and rarely in children and adolescents. The male/female ratio is 3:2.8 Most cases show solitary or localized (ulcerating) nodules or tumors (Fig 3A). The prognosis is usually favorable.7,8,14,54 Complete or partial spontaneous regression may be observed in up to 25% of patients. Involvement of regional lymph nodes occurs in approximately 25% of patients, but is not necessarily associated with an unfavorable prognosis8 (see Discussion).
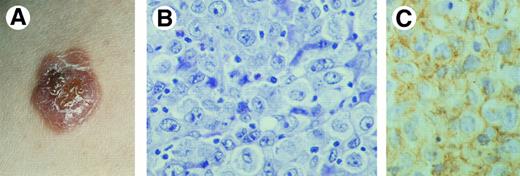
Primary cutaneous CD30+ large T-cell lymphoma. (A) Clinical presentation with solitary tumor on the right arm. (B) Diffuse proliferation of large anaplastic cells with pleomorphic nuclei, prominent nucleoli, and abundant cytoplasm (H&E, original magnification × 480). (C) CD30 expression by anaplastic T cells.
Primary cutaneous CD30+ large T-cell lymphoma. (A) Clinical presentation with solitary tumor on the right arm. (B) Diffuse proliferation of large anaplastic cells with pleomorphic nuclei, prominent nucleoli, and abundant cytoplasm (H&E, original magnification × 480). (C) CD30 expression by anaplastic T cells.
Histopathology.CTCL consisting of diffuse nonepidermotropic infiltrates with cohesive sheets of large CD30-positive tumor cells (Fig 3B and C). In most cases the tumor cells may have the characteristic morphology of anaplastic cells, showing round, oval, or irregularly shaped nuclei, prominent (eosinophilic) nucleoli, and abundant cytoplasm.7,8 Less commonly, the tumor cells have a pleomorphic or immunoblastic appearance.8,55 Reactive lymphocytes are often present at the periphery of the lesions. In some cases numerous inflammatory cells (T cells, eosinophils, neutrophils) and relatively few CD30+ cells may be observed (LyP-like histology).8 17 In such cases epidermal hyperplasia may be prominent.
Immunophenotype.The neoplastic cells often express a CD4+ T-cell phenotype with variable loss of pan-T cell antigens (CD2, CD3, CD5).7,8,51,54 Some cases (<5%) have a CD8+ T-cell phenotype. CD30 must be expressed by the majority (>75%) of neoplastic cells.14 The presence of few scattered CD30+ cells does not place a tumor in this category. Unlike primary node-based CD30-positive lymphomas, most primary cutaneous CD30+ large T-cell lymphomas do not express EMA nor CD15.17 56
Genetic features.Most cases have clonally rearranged TCR genes.54 Controversy exists whether the (2; 5) translocation, which is predominantly found in CD30+ anaplastic large cell lymphomas in children, also occurs in primary cutaneous CD30+ large T-cell lymphomas.57 58
Survival.The estimated 5-year survival of 57 patients registered at the DCLWG is 90%. The percentages for 46 anaplastic and 11 nonanaplastic CD30-positive lymphomas were 86% and 100%, respectively.
Therapy.In case of solitary or localized skin lesions, radiotherapy is the preferred mode of treatment. In case of more generalized skin lesions (cave LyP) or in case of extracutaneous dissemination, multiagent chemotherapy should be considered.8
Lymphomatoid Papulosis
Definition.Lymphomatoid papulosis is a chronic, recurrent, self-healing papulonodular skin eruption with histologic features (suggestive) of a CTCL.59
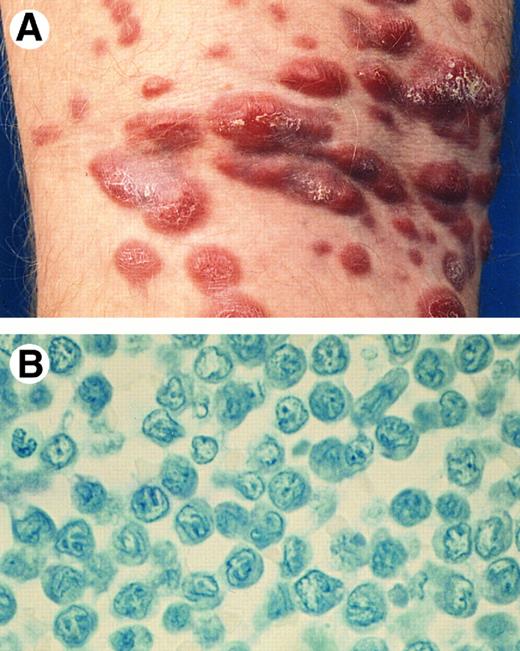
Clinical features.LyP is characterized by the presence of papular, papulonecrotic, and/or nodular skin lesions at different stages of development (Fig 4A), and runs a chronic, mostly benign clinical course (3 months to 40 years). Individual skin lesions disappear spontaneously within 3 to 6 weeks.59,60 In 10% to 20% of patients, LyP is associated with, followed by or preceded by another type of (cutaneous) lymphoma, generally, MF, a CD30+ large T-cell lymphoma or Hodgkin's disease.61
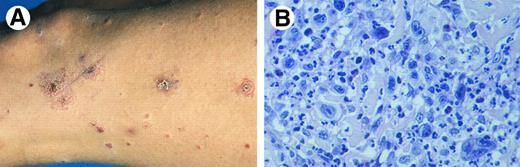
Lymphomatoid papulosis. (A) Papulonecrotic lesions in various stages of evolution. (B) LyP type A: neutrophil-rich inflammatory infiltrate with scattered large atypical (multinucleated) cells. (H&E, original magnification × 480.)
Lymphomatoid papulosis. (A) Papulonecrotic lesions in various stages of evolution. (B) LyP type A: neutrophil-rich inflammatory infiltrate with scattered large atypical (multinucleated) cells. (H&E, original magnification × 480.)
Histopathology.The histologic picture of LyP is extremely variable, which in part correlates with the age of the biopsied skin lesion.19 In addition, three histologic types of LyP are distinguished: LyP, type A, B, and C.17
LyP type A is characterized by a wedge-shaped, initially nonepidermotropic, infiltrate with scattered or small clusters of large atypical, sometimes multinucleated or Reed-Sternberg–like, CD30+ cells, interspersed in an extensive inflammatory infiltrate of histiocytes, small lymphocytes, and neutrophils and/or eosinophils17 (Fig 4B).
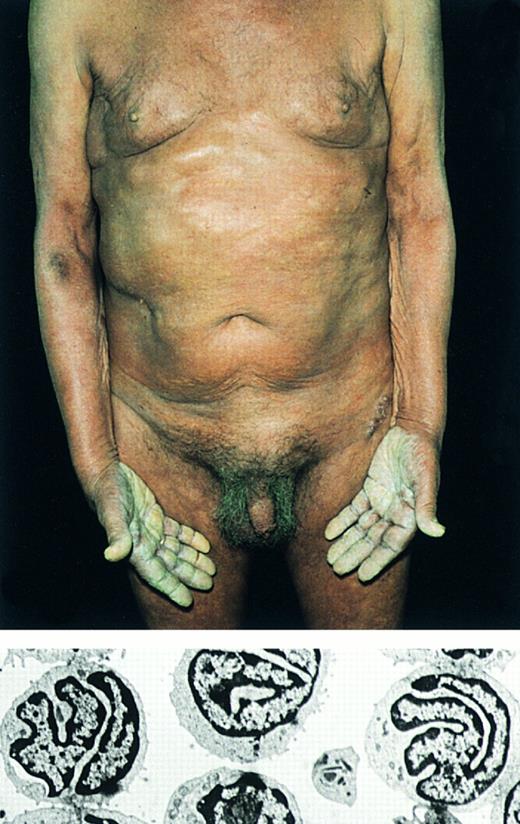
Sézary's syndrome. (Top) Erythroderma. Note hyperkeratosis of the palms. Below: Electron micrograph showing characteristic Sézary cells in the peripheral blood (original magnification × 2,600).
Sézary's syndrome. (Top) Erythroderma. Note hyperkeratosis of the palms. Below: Electron micrograph showing characteristic Sézary cells in the peripheral blood (original magnification × 2,600).
LyP, type B shows perivascular to bandlike, epidermotropic infiltrates with a predominance of small to medium-sized atypical lymphoid cells with cerebriform nuclei, simulating classical plaque stage MF.17,60 LyP type A and type B may occur in different but concurrent lesions. In addition, some LyP lesions may show histologic features of both type A and type B.17
LyP, type C has histologic features suggestive of a CD30+ large T-cell lymphoma: monotonous population or large clusters of large CD30+ T cells with relatively few admixed inflammatory cells.8 17
Immunophenotype.The large atypical cells in the LyP type A and type C lesions have the same phenotype as the tumor cells in the cutaneous CD30+ large T-cell lymphomas: CD2−/+, CD3+, CD4+/−, CD5−/+, CD8−, CD30+, CD15−, EMA−.62,63 The atypical cerebriform cells in the type B lesions have a CD3+, CD4+, CD8− phenotype and do not express CD30 antigen.60
Genetic features.Clonally rearranged TCR genes have been detected in approximately 60% of LyP lesions.51,64 Identical rearrangements have been demonstrated in LyP lesions and associated (cutaneous) lymphomas.65
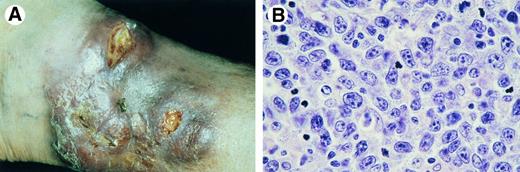
Primary cutaneous CD30− large T-cell lymphoma with (A) numerous tumorous plaques at presentation. (B) Detail of dermal infiltrate showing a monotonous proliferation of pleomorphic T cells with few admixed small T cells (Giemsa, original magnification × 600).
Primary cutaneous CD30− large T-cell lymphoma with (A) numerous tumorous plaques at presentation. (B) Detail of dermal infiltrate showing a monotonous proliferation of pleomorphic T cells with few admixed small T cells (Giemsa, original magnification × 600).
Survival.The estimated 5-year survival of 70 patients included in the DCLWG is 100% (see comments).
Therapy.There is no curative therapy available. PUVA, topical chemotherapy35 or low doses of methotrexate66 may suppress the development of new skin lesions, but after discontinuation of treatment the disease generally relapses within several months.17 60
Comment.There are few reports describing a fatal outcome in patients with LyP with or without progression into a systemic lymphoma. However, none of the 70 patients with LyP included in the registry of the DCLWG between 1986 and 1994 died of malignant lymphoma after a median follow-up of 48 months.
CUTANEOUS T-CELL LYMPHOMAS WITH AN AGGRESSIVE CLINICAL BEHAVIOR
Sézary's Syndrome
Definition.Sézary's syndrome (SS) is defined historically by the triad of erythroderma, generalized lymphadenopathy, and the presence of neoplastic T cells (Sézary cells) in skin, lymph nodes, and peripheral blood (Fig 5).67
Clinical features.SS is characterized by a pruritic erythroderma. Lymphadenopathy, alopecia, onychodystrophy, and palmoplantar hyperkeratosis are common findings.68 Bone marrow specimens may contain neoplastic cells, but real replacement of lymphoid tissue is rare. Nondiagnostic dermatitis may precede the overt clinical picture.
Histopathology.The histological features in SS may be similar to those in MF. However, the cellular infiltrates in SS are more often monotonous, and epidermotropism may sometimes be absent.69,70 Involved lymph nodes characteristically show a dense, monotonous infiltrate of Sézary cells with effacement of the normal lymph node architecture.71
Immunophenotype.Tumor cells are CD3+, CD4+, CD45RO+, CD8−, or CD30−.72
Genetic features.TCR genes are clonally rearranged in most cases. Consistent cytogenetic abnormalities have not been identified. Demonstration of clonal T cells in the peripheral blood can be considered as an important diagnostic criterion allowing differentiation from benign forms of erythroderma.73
Survival.The estimated 5-year survival of 12 cases included in the DCLWG is 11% (see comments).
Therapy.Evaluation of treatment results is hampered seriously by differences in the selection of patients. Methotrexate,74 a combination of chlorambucil and prednisone,75 and extracorporeal photopheresis, either alone or in combination with other treatment modalities,76 have been reported to be effective. Extracorporeal photopheresis has been considered as treatment of choice in SS,68 but is generally not effective in patients with CD8 levels below 15%76 (see comments).
Comments.There is at present no consensus on the diagnostic criteria of SS. Demonstration of at least 1,000 Sézary cells per mm3 is often used as a decisive criterion, but is not generally agreed on.68 Also benign forms of erythroderma may meet with this criterium. Therefore, the EORTC group suggests demonstration of clonal T cells and the presence of an expanded CD4+ T-cell population resulting in a significantly increased CD4/CD8 ratio (>10)72 in the peripheral blood as useful additional criteria. The survival data presented are based on these stricter criteria.
CD30-Negative Cutaneous Large T-Cell Lymphoma
Definition.CTCL consisting of CD30− large neoplastic cells without prior or concurrent MF. In the Kiel classification these lymphomas are classified as pleomorphic, medium-sized/large cell or immunoblastic.14 53
Clinical features.These lymphomas present with solitary, localized, or generalized plaques, nodules, or tumors (Fig 6A). Presentation with or rapid development of generalized skin lesions is more common than in CD30+ large T-cell lymphomas. Most cases have an aggressive clinical course.53
Histopathology.CTCL showing nodular or diffuse infiltrates with variable numbers of medium-sized to large pleomorphic T cells with or without cerebriform nuclei, and immunoblasts.53,77 Large neoplastic cells represent at least 30% of the total tumor cell population (Fig 6B).53 Epidermotropism may or may not be present. An angiocentric pattern may be seen.
Immunophenotype.Tumor cells often show an aberrant CD4+ T-cell phenotype with variable loss of pan-T cell antigens. CD30 staining is negative or restricted to few scattered tumor cells.53
Genetic features.Clonal rearrangements of TCR genes are present in most cases. No specific translocations have been reported.
Survival.The estimated 5-year survival of 36 patients included in the DCLWG is 15%.
Therapy.Multiagent chemotherapy is recommended. Only in patients with solitary or localized disease should radiotherapy be considered as initial treatment.
Comments.The histologic appearance of the CD30− cutaneous large T-cell lymphomas may be identical to that of classical MF undergoing transformation into a diffuse large cell lymphoma. The absence of prior or concurrent patches and plaques is used as a decisive criterion.53,77 Differentiation is important, since both conditions require a different therapeutic approach. Recent studies suggest a direct relationship between the proportion of large neoplastic cells and prognosis: lymphomas with >80% large pleomorphic cells or immunoblasts have the worst prognosis.53 Angiocentric lymphomas with a predominance of large pleomorphic CD30− T cells are included in this category78-80 (see discussion).
CUTANEOUS T-CELL LYMPHOMA: PROVISIONAL ENTITIES
Granulomatous Slack Skin [provisional entity]
Definition.A rare type of CTCL characterized by the slow development of folds of lax skin and a granulomatous infiltrate with clonal T cells.81
Clinical features.This condition shows circumscribed areas of pendulous lax skin with a predilection for the axillae and groins. Males are predominantly affected. In approximately one third of the reported patients, an association with Hodgkin's disease was observed.82 Association with classical MF has been reported as well.83
Histopathology.Histological features include dense granulomatous dermal infiltrates containing atypical T cells with cerebriform nuclei, macrophages, and often many multinucleated giant cells, as well as the destruction of elastic tissue.82
Immunophenotype.The atypical T cells have a CD3+, CD4+, CD8− phenotype.82
Genetic features.Clonal TCR gene rearrangements may be demonstrated.81-83
Survival.Most patients have an indolent clinical course.
Therapy.Radiotherapy may be effective, but experience is still limited. Rapid recurrences after surgical excision have been reported.82
Pleomorphic Small/Medium-Sized CTCL [provisional entity]
Definition.These CTCL demonstrate a neoplastic proliferation of pleomorphic small/medium-sized T cells, showing a clinical picture different from classical mycosis fungoides.53,77 84
Clinical features.One or several red-purplish nodules or tumors without patches typical of MF are evident.77 84
Histopathology.Dense, diffuse, or nodular infiltrates of small/medium pleomorphic neoplastic T cells exist within the dermis with a tendency to infiltrate the subcutis.84 Epidermotropism may be present. Differentiation between large cell pleomorphic CTCL, included in the group of CD30− large T-cell lymphomas, and these small/medium-sized pleomorphic CTCL is based on the presence of more or less than 30% large pleomorphic tumor cells.53
Immunophenotype.The neoplastic cells often express a helper T-cell phenotype with frequent loss of pan-T cell markers. Some cases may have a CD8+ phenotype84 (see comments).
Genetic features.Clonal rearrangement of TCR genes is normally present.84
Survival.A favorable prognosis has been reported in patients with a small cell pleomorphic CTCL.53,77 84 However, the number of patients studied thus far is insufficient to draw definite conclusions. The estimated 5-year survival of 18 patients with a small/medium-sized pleomorphic CTCL included in the DCLWG is 62%.
Therapy.In patients with localized skin lesions radiotherapy is the preferred mode of treatment. Cyclophosphamide as single-agent therapy and interferon α have been reported effective in patients with more generalized skin disease.84 However, the optimal treatment for this group has still to be defined.
Comments.Histologic differentiation between small/medium-sized pleomorphic CTCL and MF, in particular MF-associated follicular mucinosis, may be extremely difficult. In such cases the clinical presentation, ie, absence or presence of prior or concurrent patches and plaques, is used as a decisive criterium. Histologic differentiation between these small/medium-sized pleomorphic CTCL and pseudo–T-cell lymphomas may be equally difficult. Demonstration of an aberrant T-cell phenotype and clonality may serve as useful additional criteria for the diagnosis pleomorphic CTCL.84,85 The presence of many admixed CD8+ T cells, CD20+ B cells, and hystiocytes suggests a diagnosis of pseudo–T-cell lymphoma.85 Angiocentric lymphomas with a predominance of small and medium-sized pleomorphic T cells are included in this category78-80 (see discussion). Preliminary studies suggest that primary cutaneous pleomorphic CTCL expressing a CD8+ phenotype may have a more aggressive clinical course86 87 (see discussion).
Subcutaneous Panniculitis-Like T-Cell Lymphoma [provisional entity]
Definition.A CTCL characterized by the presence of primarily subcutaneous infiltrates of small, medium-sized or large pleomorphic T cells and many macrophages, predominantly affecting the legs, often complicated by a hemophagocytic syndrome.88 89
Clinical features.Subcutaneous nodules and plaques, mainly involving the legs, or less commonly the trunk are evident. Patients may present with systemic symptoms and signs like weight loss, fever, and fatigue due to a frequently associated hemophagocytic syndrome. The presence of a hemophagocytic syndrome is generally associated with a rapidly progressive course. Dissemination to extracutaneous sites is rare.88 89
Histopathology.Patients present with subcutaneous infiltrates simulating a panniculitis showing a mixture of neoplastic pleomorphic T cells of various sizes and benign macrophages. Tumor cell necrosis, karyorrhexis, and erythrophagocytosis are common findings.88 89
Immunophenotype.Cells have a CD3+, CD4+, CD8− or CD3+, CD4−, CD8+ mature T-cell phenotype.89 Most cases do not express CD56.90 Rare cases expressing a γ/δ phenotype have been reported.91 92
Genetic features.Clonal rearrangement of TCR genes is found in at least 50% of the cases.89 Specific genetic features have not been reported.
Therapy and survival.The prognosis is generally poor, despite aggressive chemotherapy.
Comments.Subcutaneous panniculitis-like T-cell lymphoma has recently been recognized as a distinct type of CTCL, mainly in the United States, and has therefore been included as a provisional entity. Experience among members of the EORTC group with this type of CTCL is limited. Although it cannot be excluded that similar cases have been classified as pleomorphic or angiocentric lymphoma, it is also possible that subcutaneous panniculitis-like T-cell lymphoma is indeed rare in Europe.
CUTANEOUS B-CELL LYMPHOMAS WITH AN INDOLENT CLINICAL BEHAVIOR
Primary Cutaneous Follicle Center Cell Lymphoma (PCFCCL)
Definition.PCFCCL is a tumor composed of follicle center cells, usually a mixture of centrocytes (small and large cleaved follicle center cells) and centroblasts (large follicle center cells with prominent nucleoli).93
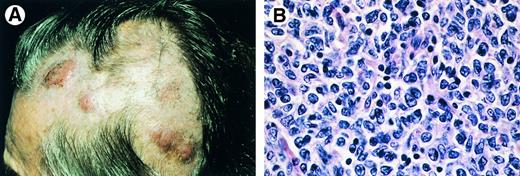
Clinical features.Patients present with nonscaling, solitary or grouped papules, plaques, and/or tumors, which may be surrounded by (annular) erythemas. In most cases the skin lesions are confined to a circumscribed area in the head and neck region or on the trunk (Fig 7A).93-96 If left untreated, the skin lesions gradually increase in size over years, but dissemination to extracutaneous sites is uncommon.93 96
Primary cutaneous follicle center cell lymphoma. (A) Characteristic clinical presentation with multiple nodules on the scalp. (B) Diffuse dermal infiltrate of (large) centrocytes and few centroblasts (H&E, original magnification × 480).
Primary cutaneous follicle center cell lymphoma. (A) Characteristic clinical presentation with multiple nodules on the scalp. (B) Diffuse dermal infiltrate of (large) centrocytes and few centroblasts (H&E, original magnification × 480).
Primary cutaneous immunocytoma (A) presenting with a deeply seated tumor on the right lower leg. (B) Diffuse dermal infiltrate of lymphocytes and lymphoplasmacytoid cells (H&E, original magnification × 750). (C-D) Monotypic lymphoplasmacytoid and plasma cells expressing lambda (C), but no kappa (D) immunoglobulin light chains.
Primary cutaneous immunocytoma (A) presenting with a deeply seated tumor on the right lower leg. (B) Diffuse dermal infiltrate of lymphocytes and lymphoplasmacytoid cells (H&E, original magnification × 750). (C-D) Monotypic lymphoplasmacytoid and plasma cells expressing lambda (C), but no kappa (D) immunoglobulin light chains.
Primary cutaneous large B-cell lymphoma of the leg. (A) Tumor mass confined to left lower leg at presentation. (B) Diffuse proliferation of centroblasts and some immunoblasts (H&E, original magnification × 750).
Primary cutaneous large B-cell lymphoma of the leg. (A) Tumor mass confined to left lower leg at presentation. (B) Diffuse proliferation of centroblasts and some immunoblasts (H&E, original magnification × 750).
Histopathology.PCFCCL show nodular or diffuse infiltrates, with the almost constant sparing of the epidermis.9,94-96 The histologic picture is variable, which relates primarily to the age and the growth rate of the biopsied skin lesion.9,96 Small and early lesions contain a mixture of centrocytes, relatively few centroblasts, and many reactive T cells, and are classified as centroblastic/centrocytic lymphoma in the Kiel classification, and as follicle center lymphoma in the REAL classification (Fig 7B). Remnants of reactive follicle centers may be observed. Neoplastic follicles are rare.94 96 With further progression to tumorous lesions, the neoplastic B cells increase both in number and size, whereas the number of tumor infiltrating T cells steadily decreases. Rapidly growing tumors often show a monotonous infiltrate of large follicle center cells with various proportions of centroblasts, large centrocytes, multilobated cells, and immunoblasts. These lesions are classified as one of the subtypes of centroblastic lymphoma in the updated Kiel classification, and as a diffuse large B-cell lymphoma in the REAL classification. Such lesions are not associated with a more unfavorable prognosis, which is probably related to the fact that dissemination to extracutaneous sites is extremely rare in these lymphomas.
Immunophenotype.The neoplastic B cells express B-cell–associated antigens (CD 19+, CD20+, CD22+, CD79a+), and show monotypic staining for sIg or lack detectable sIg, in particular in tumorous lesions.9,94-96 These PCFCCL do not express CD5 or CD10.96
Genetic features.Clonal Ig rearrangement can be demonstrated in most cases.95,97 Unlike follicular lymphomas in lymph nodes, these PCFCCL on the head and trunk are not associated with the t(14; 18) translocation, and do not or rarely express bcl-2 protein.98
Survival.The estimated 5-year survival of 84 patients included in the DCLWG is 97%.
Therapy.Radiotherapy is the preferred mode of treatment.99,100 Only in rare cases with more generalized skin lesions or in case of extracutaneous dissemination should multiagent chemotherapy be considered.100
Comments.PCFCCL is considered to be the most frequent type of cutaneous B-cell lymphoma.22 It has been suggested that a proportion of PCFCCL and immunocytomas should be classified as cutaneous marginal zone B-cell lymphomas.101 At present, there is no consensus on the criteria crucial to differentiate these different types of indolent CBCL (see discussion). CBCL showing a diffuse proliferation of large follicle center cells arising on the legs differ clinically and biologically from morphologically similar lymphomas presenting in the head and neck area or on the trunk, and are therefore considered as a separate entity (large B-cell lymphoma of the leg).102
Primary Cutaneous Immunocytoma (Marginal Zone B-Cell Lymphoma)
Definition.These lymphomas are characterized by a proliferation of small lymphocytes, lymphoplasmacytoid cells, and plasma cells, showing monotypic cIg on paraffin sections.11 In the updated Kiel classification these lymphomas are termed immunocytomas. In the REAL classification they are classified as extranodal marginal zone B-cell lymphomas or MALT type lymphomas.
Clinical features.These lymphomas present with solitary or multiple (sub)cutaneous tumors, preferentially involving the extremities (Fig 8A). They have an excellent prognosis.11
Histopathology.Characteristic findings include nodular or diffuse infiltrates composed of small lymphocytes, lymphoplasmacytoid cells, and plasma cells (Fig 8B). In some cases variable numbers of centroblasts, centrocytes, and immunoblasts are found. The monotypic lymphoplasmacytoid and/or plasma cells are usually located at the periphery of the infiltrates.11 In the central areas of the infiltrates variable numbers of reactive T cells, small CD20+ B cells, and in many cases reactive follicular structures may be observed. PAS-positive intranuclear or intracellular inclusions are frequently found.
Immunophenotype.Cells are monotypic cIg+, CD79a+, CD5−; plasma cells are CD20− 11 (Fig 8,C and D).
Genetic features.Clonal rearrangement of Ig genes can be demonstrated.97 There are no known specific translocations associated with this type of CBCL.
Survival.The estimated 5-year survival of 12 cases included in the DCLWG is 100%.
Therapy.Radiotherapy is recommended.
Comments.There is no consensus on the terminology of these lymphomas. Depending on the classification preferred, these lymphomas are designated variously as primary cutaneous immunocytoma,11,103-105 low-grade malignant B-cell lymphoma of skin-associated lymphoid tissue106 or primary cutaneous marginal zone B-cell lymphoma or MALT type lymphoma1,101,107 (see discussion). B. burgdorferi may play a role in the pathogenesis of some cutaneous immunocytomas (marginal zone B-cell lymphomas) and follicle center cell lymphomas.11,103 108
CUTANEOUS B-CELL LYMPHOMA WITH AN INTERMEDIATE CLINICAL BEHAVIOR
Large B-Cell Lymphoma of the Leg
Definition.These are CBCL with a predominance of large B cells (centroblasts and immunoblasts) presenting on and confined to the leg(s).102
Clinical features.These lymphomas predominantly affect elderly patients. In a recent study more than 80% of the patients were over 70 years of age at the time of diagnosis. Females are more often affected than males (female/male ratio: 3-4:1).102 These patients present with red or bluish nodules or tumors on one or sometimes both (lower) legs (Fig 9A). The prognosis of these lymphomas is less favorable as compared with morphologically similar large follicle center cell lymphomas on head and trunk.
Histopathology.Patients present with diffuse nonepidermotropic infiltrates predominantly composed of large B cells with variable proportions of centroblasts, large centrocytes, and immunoblasts (Fig 9B). Small cleaved cells and admixed inflammatory cells are relatively few.102
Immunophenotype.The tumor cells express monotypic sIg and/or cIg as well as CD19, CD20, CD22, and CD79a. In contrast to follicle center cell lymphomas on head and trunk, these lymphomas strongly express bcl-2 protein.109
Genetic features.Clonal rearrangement of Ig genes can be demonstrated. Expression of bcl-2 protein is not associated with t(14; 18).109
Survival.The estimated 5-year survival of 18 cases included in the DCLWG is 58%.
Therapy.In case of solitary or localized skin lesions radiotherapy is preferred; in all other cases, multiagent chemotherapy.
Comments.In most cases the majority of the neoplastic B cells have the morphologic features of large follicle center cells, and are classified as one of the subtypes of centroblastic lymphoma in the updated Kiel classification. Some cases show an almost pure population of immunoblasts (B-immunoblastic lymphoma). However, since distinction between these two groups is sometimes arbitrary, and not relevant from a clinical point of view, the unifying term large B-cell lymphoma of the leg is preferred.102 In the REAL classification these lymphomas are also classified as diffuse large B-cell lymphomas.23
CUTANEOUS B-CELL LYMPHOMA: PROVISIONAL ENTITIES
Intravascular Large B-Cell Lymphoma [provisional entity]
Definition.Intravascular CBCL, formerly considered as a vascular proliferation110 and designated as malignant angioendotheliomatosis, is characterized by an accumulation of large neoplastic B cells within blood vessels.111
Clinical features.These lymphomas present with violaceous indurated patches and plaques clinically sometimes suggestive of a panniculitis, usually on the (lower) legs or the trunk.112-114
Histopathology.Dilated blood vessels in the dermis and subcutis are filled and often extended by a proliferation of large neoplastic lymphoid cells.111-114 These cells may cause vascular occlusion of venules, capillaries, and arterioles. In 20% of the cases slight extravascular accumulation of atypical cells can be found.
Immunophenotype.Cells are CD19+, CD20+, CD22+, CD79a+, and monotypic sIg+.112-114 Note, rare cases with a T-cell phenotype have been reported.114
Genetic features.Clonal rearrangement of Ig genes can be demonstrated.114
Survival.The estimated 5-year survival of four patients included in the DCLWG is 50%.
Plasmacytoma [provisional entity]
Definition.A rare type of CBCL, plasmacytoma is characterized by a clonal proliferation of plasma cells that develops primarily in the skin (extramedullary plasmacytoma of the skin) without underlying mutiple myeloma.115-118
Clinical feature.Patients present with solitary or multiple red to violaceous (sub)cutaneous nodules with no specific site of predilection.115-118 There is an excellent prognosis after surgical excision or radiotherapy.
Histopathology.The skin lesions show nodular or diffuse dermal infiltrates that consist almost completely of mature plasma cells, including multinucleated plasma cells.115-118 Coexistent clonal lymphocytes, as in immunocytomas are lacking.
Immunophenotype.The tumor cells express monotypic cIg+ and CD38+, but are negative for CD20 and LCA.115-118
Survival.No lymphoma-related deaths have been reported.
Therapy.Radiotherapy or surgical excision is recommended.
Comment.Primary cutaneous plasmacytomas are extremely rare, representing 4% of extramedullary plasmacytomas.119 Many cases reported in the literature represent more likely immunocytoma or reactive plasma cell proliferations rather than true plasmacytomas.
DISCUSSION
Cutaneous lymphomas are generally classified according to the criteria of the updated Kiel classification, the Working Formulation, and more recently the REAL classification, and treated accordingly with treatment protocols also used for malignant lymphomas arising in lymph nodes. Since primary cutaneous lymphomas are not recognized as a distinct group, referral centers for these conditions are confronted regularly with patients with an indolent type of cutaneous lymphoma, who have been treated with unnecessarily aggressive treatment modalities. It was therefore felt essential to constitute a clinically relevant classification, which should contribute to proper diagnosis, management, and treatment of patients with a primary cutaneous lymphoma. Since primary cutaneous lymphomas cannot be defined adequately by histologic criteria alone, modification of existing histologic classification schemes as the updated Kiel classification or the Working Formulation was not considered as a realistic option. The REAL classification in its present form doesn't permit recognition of all clinically relevant types of cutaneous lymphomas (Table 2). For instance, primary cutaneous CD30-positive nonanaplastic (pleomorphic or immunoblastic) large cell lymphomas would be included in the group of peripheral T-cell lymphoma, unspecified. PCFCCL with rapidly growing tumors on the head or trunk often show a diffuse proliferation of large follicle center cells, and would thus be classified as a diffuse large B-cell lymphoma. In neither case would such a classification communicate the indolent behavior of these types of cutaneous lymphoma. For these reasons, the EORTC Cutaneous Lymphoma Study Group decided to constitute a separate classification, which, in line with recent proposals,12 22 is restricted to primary cutaneous lymphomas and includes only well-defined disease entities as well as some provisional entities.
Definition of Primary Cutaneous Lymphoma
It may sometimes be difficult to decide whether a particular lymphoma should be considered as a primary cutaneous lymphoma. It is commonly accepted that patients with classical MF, who already have lymph node involvement at the time of presentation, as well as patients with classical SS should be considered as primary cutaneous lymphomas, although they do not meet with the criteria outlined before. Moreover, cutaneous lymphomas other than MF/SS with widespread systemic and cutaneous disease at first presentation do not present with much of a problem either. Such cases should be considered as having skin localizations of a malignant lymphoma rather than a primary cutaneous lymphoma, and should be treated accordingly with standard treatment protocols for these non-Hodgkin lymphomas. However, it is yet uncertain how patients presenting with skin lesions and involvement of only draining lymph nodes should be viewed. At present, such patients are not included in the EORTC classification for primary cutaneous lymphomas. However, preliminary data of the DCLWG revealed no significant differences in the clinical behavior and prognosis between cutaneous CD30+ large T-cell lymphomas with and those without involvement of the regional lymph nodes at presentation (Geelen et al; in preparation). These data indicate that additional studies on larger groups of patients with different types of cutaneous lymphoma are required to find out whether such patients should be included in the group of primary cutaneous lymphomas.
Finally, CD30+ or CD30− cutaneous large T-cell lymphomas that have developed secondary from another type of CTCL, such as MF and SS, have not been included as a separate group in the EORTC classification. Such transformed lymphomas belong to the natural course of MF and SS, and therefore cannot be considered as a primary cutaneous lymphoma sensu strictu. It should, however, be emphasized that such cases have a different clinical behavior and require a different therapeutic approach as compared with morphologically identical cutaneous large T-cell lymphomas developing de novo in the skin.7,17 40
Relative Frequency: Common and Uncommon Types of Primary Cutaneous Lymphoma
The relative frequency and 5-year survival data of the different groups of primary cutaneous lymphomas included in the EORTC classification are presented in Table 3 and Fig 10. The EORTC classification covers over 97% of all primary cutaneous lymphoma; approximately 2% to 3% of patients registered at the DCLWG are considered unclassified. The data presented in Table 3 indicate that MF, including MF-associated follicular mucinosis, is by far the most common group, including approximately 50% of all primary cutaneous lymphomas. Primary cutaneous CD30+ lymphoproliferations, including CD30+ large T-cell lymphomas and LyP, account for approximately 20%. Other types of cutaneous large cell lymphomas, including CD30− large T-cell lymphomas, most follicle center cell lymphomas, and the large B-cell lymphomas of the legs, represent another 20%. Thus, if one includes CD30+ large T-cell lymphomas, approximately 30% of all primary cutaneous lymphomas represent large cell lymphomas developing de novo in the skin.
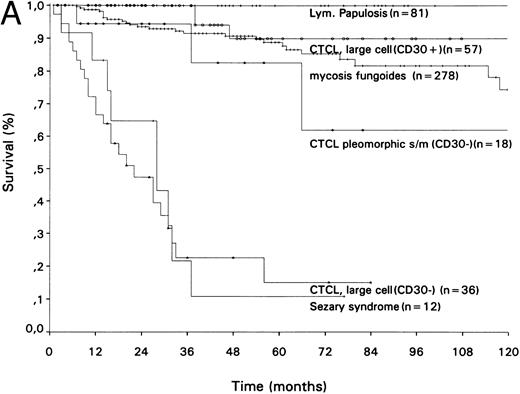
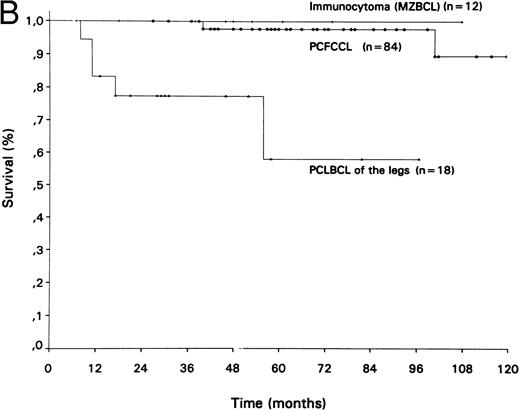
Survival curves of different groups of (A) primary cutaneous T-cell lymphomas and (B) primary cutaneous B-cell lymphomas.
Survival curves of different groups of (A) primary cutaneous T-cell lymphomas and (B) primary cutaneous B-cell lymphomas.
There are several subtypes of cutaneous lymphomas that have been reported in literature, but are not (yet) included in the EORTC classification, or have been included in other categories. Examples are cutaneous angiocentric lymphomas, CD8+ CTCL, CTCL expressing the γ/δ T-cell receptor, and mantle cell lymphomas. An angiocentric growth pattern with dermal infiltrates localized preferentially around and within blood vessels may be observed in CTCL, in particular pleomorphic CTCL. However, unlike angiocentric lymphomas (natural killer/T-cell lymphomas) involving the upper respiratory tract with or without secondary cutaneous involvement, primary cutaneous angiocentric lymphomas generally have a CD3+, CD4+ T-cell phenotype,79,80 they uncommonly express CD8 or CD56 antigens,80,120 are much less frequently associated with Epstein-Barr virus sequences,80 and may have a more favorable prognosis.77,79 80 Clinically, these primary cutaneous angiocentric lymphomas do not differ significantly from other pleomorphic T-cell lymphomas. In the EORTC classification these lymphomas are therefore classified as CTCL, pleomorphic small/medium, or as a CD30− large T-cell lymphoma, dependent on the proportion of large tumor cells.
Whereas most CTCL have a CD4+ phenotype, CTCL expressing a CD8+ phenotype have been described as well. These include approximately 50% of patients with pagetoid reticulosis,48,49 and rare cases of MF28 and CD30+ large T-cell lymphomas.8 In all these cases no difference in clinical presentation and clinical course between CD4+ and CD8+ has been found. However, there is also a group of CD8+ CTCL with rather distinctive clinical and histologic features not consistent with any of the other types of CTCL. Clinically, these CD8+ cases are characterized by the presence of eruptive papules, nodules, and tumors showing central ulceration and necrosis, and often an aggressive clinical course.86,87 Histologically, these lymphomas demonstrate strongly epidermotropic, band-like or nodular infiltrates of either small and medium-sized or large pleomorphic T cells with a CD3+, CD4−, CD7+/− CD8+, CD45RA+ phenotype. CD7 expression has been associated with a more aggressive clinical course.86 However, the number of patients reported thus far is still too few to be included as a (provisional) entity. Similarly, expression of a γ/δ phenotype has been observed in rare cases of CTCL with the clinical and/or histologic features of MF,121 disseminated pagetoid reticulosis,122 or a subcutaneous panniculitis-like T-cell lymphoma.91,92 Although CTCL expressing a γ/δ phenotype cannot be considered as a distinct disease entity, they are often associated with an aggressive clinical behavior and a poor prognosis.91,92 122-124
Mantle cell lymphomas represent a group of small cell B-cell lymphomas characterized by expression of CD5 antigen and cyclin D1 protein, which almost without exception involve the skin secondarily, and for that reason have not been included.125 Only one report on a primary cutaneous mantle cell lymphoma has been published.126
Recently, there has been considerable controversy regarding the terminology of the “low-grade malignant” CBCL. In the EORTC classification these lymphomas are designated primarily as PCFCCL or primary cutaneous immunocytoma. In the REAL classification23 and other recent publications,1,101,127 extranodal imunocytomas, as defined in the updated Kiel classification,5 have been included in the group of extranodal marginal zone B-cell lymphomas or MALT-type lymphomas. It is therefore not surprising that the clinical and histologic features of the primary cutaneous immunocytomas, as described herein, are very similar to those of primary cutaneous marginal zone B-cell lymphomas or MALT-type lymphomas in other publications.1,101,106,107 Consequently, one might consider to adopt the term marginal zone B-cell lymphoma for the group of primary cutaneous immunocytomas. However, there is at present no consensus among pathologists regarding the definition of the term marginal zone B-cell lymphoma.1,23,101,128,129 By emphasizing histologic heterogeneity as a characteristic feature of these lymphomas, the ends of this spectrum of marginal zone B-cell lymphomas are open for discussion, and marginal zone B-cell lymphoma is likely to become an encompassing term for all or most primary CBCL. This is illustrated by a recent editorial, which suggested that not only the primary cutaneous immunocytomas, but also over 90% of the PCFCCL are derived from marginal zone B cells, and should be classified accordingly.101 This suggestion was based on the observation that most primary CBCL, as the MALT lymphomas, but unlike follicular lymphomas in lymph nodes, do not express CD5 and CD10 antigens, and are not associated with the interchromosomal 14;18 translocation.101 For similar reasons others proposed the encompassing term skin-associated lymphoid tissue (SALT)-related B-cell lymphoma for the whole group of primary CBCL.96 Following the suggestion that most primary CBCL are marginal zone B-cell lymphomas,101 early lesions in PCFCCL would most probably be classified as marginal zone B-cell lymphoma, whereas PCFCCL with rapidly growing tumors, having the same excellent prognosis, would be classified as diffuse large B-cell lymphoma. It would be highly unfortunate, if by this purely histologic approach, this well-defined entity would fall apart in different categories. Moreover, it should be emphasized that specific phenotypic or genotypic markers, which might prove a marginal zone B-cell origin, and might indicate more precisely which small B-cell lymphomas should be included in this group, are not available.130 As long as differentiation between marginal zone B-cell lymphomas and other types of small B-cell lymphomas is controversial, the EORTC group prefers to use the terms PCFCCL and primary cutaneous immunocytomas, as they refer to well-defined disease entities.
In conclusion, in the last decade many studies demonstrated that primary cutaneous and primary nodal non-Hodgkin lymphomas, although morphologically identical, should be considered as distinct groups of diseases, both clinically and biologically. The EORTC classification for primary cutaneous lymphomas presented herein aims to contribute to a more uniform diagnosis, management, and treatment of patients with a cutaneous lymphoma. Awareness of the special clinical behavior of many types of cutaneous lymphomas should prevent unnecessarily aggressive treatment in these patients. Recognition of the different types of CTCL, which show considerable variation in clinical and histologic features, including clinical behavior and prognosis, underscores that the common usage of the term CTCL without further specification is obsolete, and should be discouraged strongly. This classification also offers the opportunity to perform clinical trials as well as fundamental studies aimed at unravelling the molecular mechanisms underlying cutaneous lymphoma development and progression in well-defined groups of patients with a CTCL or a CBCL. Finally, the EORTC classification is designed in such a way it can be incorporated easily in an updated REAL classification or the forthcoming WHO classification.
Address reprint requests to R. Willemze, MD, Department of Dermatology, Free University Hospital, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal