Abstract
Cytarabine (araC) is converted to araC 5′-triphosphate after entering leukemia cells as a substrate for nucleoside transport processes. This study tested the relationship between araC cytotoxicity, measured in an in vitro tetrazolium dye reduction assay of cell viability, and the cellular abundance of es nucleoside transport elements, assayed by a flow cytometric method that used the es-specific stain, 5-(SAENTA-x8)-fluorescein (5-(Sx8)-F), in cultured leukemia cells and in myeloblasts and lymphoblasts (blasts) from leukemia patients. Cellular es site abundance (Bmax value for 5-(Sx8)-F binding) varied sixfold among nine leukemic myeloblast samples from patients. In cultured OCI/AML-2 myeloblasts and CCRF-CEM T-lymphoblasts, and in fresh leukemic blasts, es sites were fractionally blocked by treatment with graded concentrations of nitrobenzylthioinosine (NBMPR), an inhibitory es site ligand, to simulate the variation in es expression found in leukemic blasts from patients with acute myeloid leukemia. When the cytotoxicity of a single concentration of araC was determined in NBMPR-treated leukemia cells, cell kill correlated closely with the intensity of 5-(Sx8)-F fluorescence (r = .92 to .99), a measure of the cell surface abundance of functional es nucleoside transporter sites. Concentrations of NBMPR that achieved half-maximal reduction (4.3 to 12 nmol/L) of cellular 5-(Sx8)-F fluorescence (measured by flow cytometry) approximated IC50 values (1 to 10 nmol/L) previously found for inhibition by NBMPR of es-mediated nucleoside fluxes in several cell types, supporting the view that 5-(Sx8)-F interacted with the es transporter. The correlation of araC cytotoxicity and the Bmax for 5-(Sx8)-F binding to es sites in cultured leukemia cells and in leukemic blasts from acute leukemia patients (r = .95) suggests that the flow cytometry assay of es capacity may be useful in predicting clinical response to araC.
CYTARABINE (araC, cytosine arabinoside) is an important agent in the treatment of acute myeloid leukemia (AML),1 and is a component of some induction and consolidation regimens used in treatment of acute lymphoblastic leukemia (ALL).2 After entering cells primarily as a substrate for nucleoside transport (NT) systems,3,4 araC is converted to the active metabolite, the 5′-triphosphate ester, through sequential phosphorylation steps catalyzed by deoxycytidine kinase and pyrimidine nucleotide kinases.5 The cytotoxicity of araC has been attributed to incorporation of the 5′-triphosphate metabolite into nascent DNA strands, with the consequences of slowing and eventually terminating strand elongation.6
Nucleoside transport processes are determinants of the accumulation and retention of several cytotoxic nucleosides in leukemia cells.7-10 Nucleoside-specific membrane transport processes have been identified in many animal cell types and comprise a diverse array of NT systems (transporters). Two equilibrative, facilitated diffusion NT systems, termed es and ei, differ in their sensitivity to nitrobenzylthioinosine (NBMPR), and five Na+-dependent, concentrative transporters are distinguishable on the bases of substrate specificity and inhibitor sensitivity.11,12 Two or more NT systems may be coexpressed in a single cell type.11 Although four of the concentrative transporters are thought to be insensitive to NT inhibitors, one, termed cs, which has been recognized in fresh leukemia cells from patients, is inhibited by NBMPR and by two other NT inhibitors, dilazep and dipyridamole.13
NBMPR is a tightly bound, inhibitory ligand of the es nucleoside transporter,14 and [3H]NBMPR has served as a probe for the es transporter in many cell types.15,16 Wiley et al17 used [3H]NBMPR in equilibrium binding assays to enumerate transporter sites in leukemic myeloblasts from AML patients, the abundance of which ranged from 500 to 27,600 NBMPR binding sites per cell. The wide variation in NBMPR site abundance among blast samples from acute leukemia patients suggests that this cellular characteristic might contribute to differences in clinical response to treatment with araC. The role of the membrane transport step in araC activation was examined in earlier studies that showed a correlation between the cellular content of [3H]NBMPR sites and araC influx18 and araCTP formation19 in blasts from acute leukemia patients. The transport step was rate-limiting in the uptake of araC at concentrations below 1 μmol/L in blasts from acute leukemia patients,9 that is, at araC concentrations achieved in plasma during araC therapy at conventional doses,1,20 whereas at concentrations exceeding 10 μmol/L, the phosphorylation capacity of cells determined the net rate of araC uptake.9 Wiley et al18 reported that a low inward flux of araC characterized the leukemic lymphoblasts from a subset of acute leukemia patients, including some who failed induction therapy with araC; however, that study did not show a correlation between nucleoside transporter expression and clinical response to araC.
The recent synthesis of SAENTA (5′-S-(2-aminoethyl)-N6-(4-nitrobenzyl)-5′-thioadenosine), a derivatizable analog of NBMPR,21 has enabled the development of a sensitive, nonisotopic method of determining cellular nucleoside transporter site abundance. Conjugates of SAENTA in which the nucleoside analog is linked to fluorescent reporting moieties have proven to be specific stains for cellular NBMPR binding sites.22-24 Thus, the SAENTA-fluoresceins allow the measurement of the es nucleoside transporter site content of cells by flow cytometry,22-24 in a procedure that requires 10-fold fewer cells than the [3H]NBMPR binding assay, and provides rapid and sensitive measurement of es sites in leukemia cells from patients.23,25 26
The present study tested the hypothesis that the cytotoxicity of araC in leukemia cells is a correlate of the cellular abundance of es nucleoside transporters in leukemic blasts from AML and ALL patients, and in established human cell lines derived originally from AML and ALL patient samples. The NT site content of cells was determined in a flow cytometry assay that measured site-specific binding of 5-(SAENTA-x8)-fluorescein (5-(Sx8)-F) from graded concentrations of that ligand under equilibrium conditions, allowing a determination of the maximum 5-(Sx8)-F binding capacity of cells (Bmax). Sensitivity of cells to araC was determined in an in vitro tetrazolium dye reduction assay,27 which has been shown by others to predict the attainment of remission in AML patients treated with araC.28 A goal of the present study was to determine if the flow cytometric determination of es transporter sites in SAENTA-fluorescein–stained cells might predict the sensitivity or resistance of cells to araC. This was approached by exposing samples of cells to graded concentrations of NBMPR to yield cell subpopulations in which es sites were fractionally occupied with NBMPR, thereby simulating the variation in es site abundance in cells from acute leukemia patients, encountered in the present study and recognized by others.17 23 The cytotoxicity of araC toward the NBMPR-treated cells correlated closely with es site abundance measured by the flow cytometric method. AraC cytotoxicity and Bmax values for site-specific binding of the SAENTA-fluorescein stain in leukemic blasts from patients were also correlated, suggesting clinical utility of the flow cytometric assay for es site content.
MATERIALS AND METHODS
Chemicals.5-(Sx8)-F and NBMPR were provided by Alberta Nucleoside Therapeutics Laboratories, Department of Pharmacology, University of Alberta. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and araC were from Sigma Chemical Co (St Louis, MO). Eagle's minimal essential medium (MEM) and the α-modification (α-MEM, with nucleosides), RPMI 1640 medium, and fetal bovine serum (FBS), were from GIBCO Laboratories (Mississauga, Ontario, Canada).
Cell culture.Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air. Stock cultures of OCI/AML-2 cells29 (provided by Dr David W. Hedley, Ontario Cancer Institute, Toronto, Ontario, Canada) were maintained in α-MEM containing 10% FBS. The cell population doubling time under these conditions was about 31 hours. For experiments, OCI/AML-2 cultures were expanded in MEM with 10% FBS; MEM medium lacks ascorbic acid, a component of α-MEM that interferes with the measurement of cell-mediated reduction of the MTT dye. That modification in culture conditions increased the population doubling time of the OCI/AML-2 cells to about 41 hours. CCRF-CEM cells30 (hereafter, CEM cells) were maintained in RPMI 1640 medium containing 5% or 10% FBS, with a population doubling time of 20 hours.
Patient specimens.Peripheral blood samples were obtained from newly diagnosed adult AML and ALL patients with informed consent, and were collected into heparin-containing Vacutainer tubes, which were kept at 20°C to 25°C. Within 24 hours of collection, the samples were diluted in phosphate-buffered saline (PBS) of this composition: 137 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4 , and 1.1 mmol/L KH2PO4 (pH 7.4) or RPMI 1640 culture medium, and layered over a cushion of Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden) for the isolation of mononuclear cells by density gradient centrifugation.31 The lymphocyte/monocyte band was collected from the cushion surface, that is, from the sample/Ficoll-Paque interface, washed in PBS or RPMI 1640 medium and resuspended in PBS at a cell concentration of 6 × 106 cells/mL for staining with 5-(Sx8)-F, or in RPMI 1640 medium with 2 mmol/L N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) buffer (pH 7.4) and 10% FBS at a cell concentration of 107 cells/mL for the chemosensitivity assay.
Flow cytometry.Cell suspensions were diluted to 3 × 106 cells/mL in PBS (for assay of total binding of the 5-(Sx8)-F probe), or in PBS containing 5 μmol/L NBMPR (for nonspecific binding of the probe) and kept at 22°C for 30 minutes. These suspensions were then added to graded concentrations of 5-(Sx8)-F (0.5 to 30 nmol/L) in PBS in the absence or presence of 5 μmol/L NBMPR. The equilibration of 5-(Sx8)-F with cellular binding sites was allowed to proceed for at least 15 minutes before measurement of cell-associated fluorescence. Flow cytometry was performed with a Becton-Dickinson FACScan instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with an argon laser (excitation at 488 nm); fluorescence signals from 10,000 cells for each condition were analyzed with Lysis II software (Becton Dickinson Immunocytometry Systems) to determine mean relative fluorescence intensity values (RFI) for the fluorescence histograms. Es-specific binding of 5-(Sx8)-F was calculated as the difference between total and nonspecific binding. In mass law calculations with 5-(Sx8)-F binding data, initial concentrations were used in place of the equilibrium concentrations of the free ligand.
Measurement of the cellular es site content in cell preparations in which the functional es NT site content was pharmacologically adjusted with NBMPR was performed by a modification of the described procedure. Cell suspensions (3 × 106 cells/mL in PBS) were exposed to graded concentrations of NBMPR (see Fig 4) for 30 minutes at 22°C, then added to solutions containing a single, es site-saturating concentration of 5-(Sx8)-F in the presence of the same graded concentrations of NBMPR. After a further incubation period of 15 minutes at 22°C, cell-associated fluorescence was determined by flow cytometry as described.
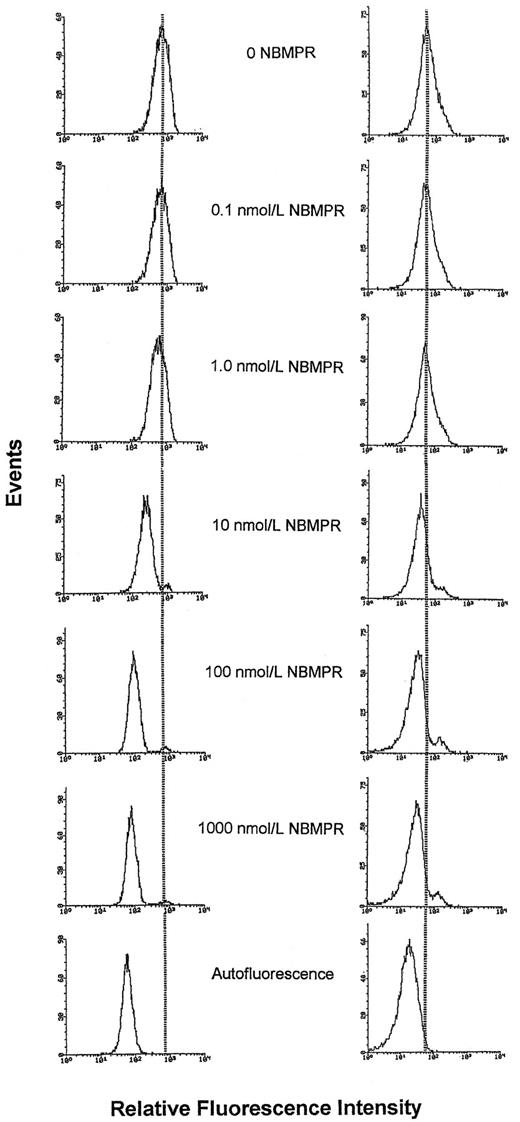
Fluorescence histograms of CEM T-lymphoblasts and myeloblasts from AML patient H.M. stained with 5-(Sx8)-F in the presence of graded concentrations of NBMPR. Cells were treated with NBMPR at the concentrations shown before staining with 80 nmol/L 5-(Sx8)-F (CCRF-CEM cells) or 20 nmol/L 5-(Sx8)-F (myeloblasts from patient H.M.) and analysis by flow cytometry. Each fluorescence histogram represents the analysis of 10,000 cells. With increasing concentrations of NBMPR, the decrease in fluorescence indicates a progressive blockade of 5-(Sx8)-F sites on the es transporter. Left series, CEM cells; right series, myeloblasts from patient H.M.
Fluorescence histograms of CEM T-lymphoblasts and myeloblasts from AML patient H.M. stained with 5-(Sx8)-F in the presence of graded concentrations of NBMPR. Cells were treated with NBMPR at the concentrations shown before staining with 80 nmol/L 5-(Sx8)-F (CCRF-CEM cells) or 20 nmol/L 5-(Sx8)-F (myeloblasts from patient H.M.) and analysis by flow cytometry. Each fluorescence histogram represents the analysis of 10,000 cells. With increasing concentrations of NBMPR, the decrease in fluorescence indicates a progressive blockade of 5-(Sx8)-F sites on the es transporter. Left series, CEM cells; right series, myeloblasts from patient H.M.
Chemosensitivity assay.The MTT assay of Mosmann,31 as modified by Alley et al,27 was used to measure the cytotoxicity of araC in cell lines and fresh leukemic blasts. Briefly, cell suspensions were diluted with media containing graded concentrations of araC; the media were (1) MEM containing 2 mmol/L HEPES buffer (pH 7.4) and 10% FBS (for OCI/AML-2 cells), or (2) RPMI 1640 medium containing 2 mmol/L HEPES buffer (pH 7.4) and 10% FBS (for CEM cells and leukemic blasts). Final cell suspensions were prepared in replicate wells of 96-well microtiter plates (total volume, 200 μL/well). The microcultures contained 8 × 103 cells per well (OCI/AML-2 cells and CEM cells), or 106 cells per well (fresh leukemic blasts). After a 72-hour incubation interval, 30 μL MTT solution (30 mg/mL) was added to each well, and the incubation continued for 2 more hours. The plates were centrifuged (82g, 7 minutes), supernatants were aspirated and 150 μL of dimethyl sulfoxide (spectrophotometric grade, Aldrich) was added to dissolve the precipitated MTT formazan reduction product. The absorbance of each microwell at 540 nm (A540), which was proportional to cell viability, was determined with a Titertek Multiskan Plus, Model MKII microplate reader (ICN Biomedicals, Mississauga, Ontario, Canada). A540 values in test wells were expressed as a percentage of that in control wells, which lacked araC. IC50 values for araC cytotoxicity were determined by using a computer program (Origin, version 3.5; Microcal Software, Inc, Northampton, MA) to fit the following four-parameter logistic equation to plots of A540 (% of control) versus the log of the concentration of araC:
where y is the response (A540 , % of control), A1 and A2 are, respectively, the extrapolated upper and lower limits for y, x is the araC concentration, IC50 is the concentration of araC that produces a half-maximal response, and b is a constant related to the steepness of the curve.
To measure the relationship between es transporter site abundance and araC cytotoxicity, replicate microcultures of leukemia cells were exposed to graded concentrations of NBMPR in the absence or presence of a single concentration of araC, which was sufficient to reduce cell viability to less than or equal to 50% of that of drug-free cultures. Control cultures contained no drugs (drug-free control), or contained araC alone (NBMPR-free control). After a 96-hour incubation period, MTT was added to determine cell viability as described. Concentration-effect curves for the protection by NBMPR against araC cytotoxicity were fitted to plots of the A540 (% of NBMPR-free control) versus the log of the concentration of NBMPR, by using the logistic equation described above.
RESULTS
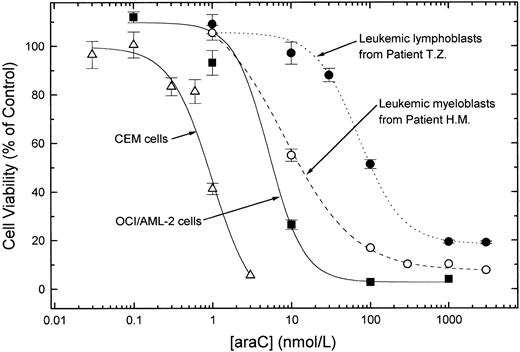
AraC sensitivity of cultured cells and fresh leukemic blasts.To establish conditions for measuring the cytotoxic effects of araC in leukemia cells with the MTT assay, concentration-effect curves were constructed for experiments with OCI/AML-2 cells (a continuous line of AML myeloblasts established in culture29 ), CEM cells (a human T-lymphoblast cell line30 ), and fresh blasts from two acute leukemia patients, one AML and the other ALL. Separate experiments (not shown) demonstrated the linearity of the MTT response over wide ranges of viable cell numbers when OCI/AML-2 cells (1 × 103 to 6.4 × 104 cells per well), CEM cells (1 × 104 to 1.2 × 105 cells per well), and freshly isolated leukemic blasts from AML and ALL patients (5 × 104 to 1 × 106 cells per well) were tested. After a 72-hour exposure of cells to araC (Fig 1), the IC50 values for OCI/AML-2 myeloblasts and myeloblasts from AML patient H.M. were similar (5.2 nmol/L and 8.2 nmol/L, respectively). The araC IC50 values for CEM cells (980 pmol/L) and for lymphoblasts from B-precursor ALL patient T.Z. (72 nmol/L) differed by about two orders of magnitude. CEM cells were the most sensitive to araC of the cell types tested in this study. All IC50 values determined in the experiments of Fig 1 were lower than the steady state araC concentrations (about 300 nmol/L) attained in plasma in humans during the administration of araC at conventional doses.20
Sensitivity to araC of acute leukemia cell lines and fresh blasts from patients. Cells were incubated for 72 hours in culture media containing graded concentrations of araC. After the incubation period, cell viability was determined by the MTT assay. The data are mean ± SEM of 12 replicate measurements at each araC concentration. IC50 values were 980 pmol/L (CEM), 5.2 nmol/L (OCI/AML-2), 8.2 nmol/L (AML patient H.M.), and 72 nmol/L (ALL patient T.Z.).
Sensitivity to araC of acute leukemia cell lines and fresh blasts from patients. Cells were incubated for 72 hours in culture media containing graded concentrations of araC. After the incubation period, cell viability was determined by the MTT assay. The data are mean ± SEM of 12 replicate measurements at each araC concentration. IC50 values were 980 pmol/L (CEM), 5.2 nmol/L (OCI/AML-2), 8.2 nmol/L (AML patient H.M.), and 72 nmol/L (ALL patient T.Z.).
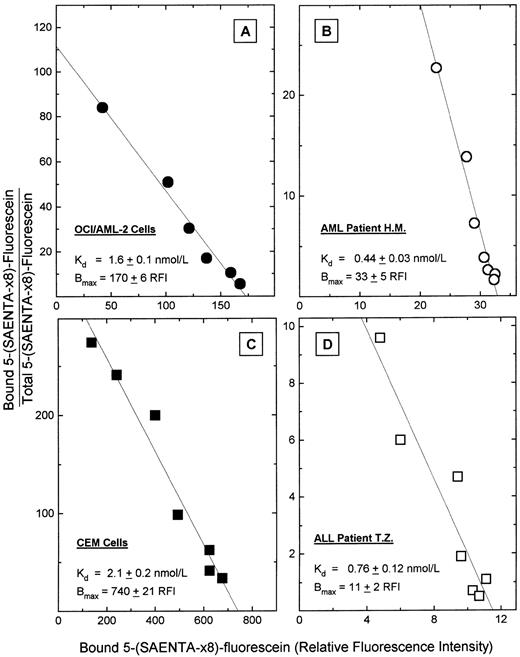
Measurement of es nucleoside transporter expression by flow cytometric analysis of 5-(Sx8)-F–stained cells.Using the cell types of Fig 1, the cellular content of es nucleoside transporter binding sites was determined in equilibrium binding assays that used 5-(Sx8)-F in a flow cytometry procedure. Cells were stained with graded concentrations of 5-(Sx8)-F, the mean fluorescence intensity of cell samples was measured at each fluor concentration, and the data subjected to mass law analysis by the method of Scatchard, yielding the plots of Fig 2. Nonspecific binding of 5-(Sx8)-F was 6.2% to 36% of total binding in these cell types in the presence of 20 nmol/L 5-(Sx8)-F. That range of relative nonspecific binding was largely accounted for by the wide range of total binding values among the cell samples. The Scatchard plots showed specific binding of 5-(Sx8)-F at a single population of high-affinity sites on these cells, with Kd values ranging from 0.44 to 2.1 nmol/L, values which were similar to Kd values (0.1 to 1 nmol/L) for NBMPR binding at es sites in several cell types.14 15 The cellular abundance of es sites (Bmax) differed considerably among the four cell types of Fig 2, with values of 11, 33, 170, and 740 mean RFI units/cell. Those values correspond approximately to 2,200, 6,600, 34,000, and 150,000 NBMPR binding sites per cell for lymphoblasts from patient T.Z., myeloblasts from patient H.M., OCI/AML-2 cells, and CEM cells, respectively, based on an approximate factor (200) for converting RFI values to NBMPR site content per cell. To determine that factor, Scatchard plots of es-specific 5-(Sx8)-F fluorescence and of [3H]NBMPR binding were constructed to measure Bmax values with units of RFI and sites per cell, respectively, in leukemia cell lines (Gati WP, Paterson ARP, and Belch AR, unpublished results, 1996). A comparison of Bmax values determined by the two methods yielded a conversion factor that was used to express Bmax values as NBMPR sites per cell, from measurements of site-bound 5-(Sx8)-F in the cell types of Fig 2.
Equilibrium binding of 5-(Sx8)-F in cultured acute leukemia cells and in blasts from two acute leukemia patients. Cells were incubated with graded concentrations of 5-(Sx8)-F (0.5 to 30 nmol/L), and then cell-associated 5-(Sx8)-F was measured by flow cytometry, as the mean fluorescence intensity in each sample determined by flow analysis of 10,000 cells. Nonspecific binding of 5-(Sx8)-F was determined in a parallel set of samples that were pretreated with 5 μmol/L NBMPR to occupy es transporter sites. The data were subjected to mass law analysis by the method of Scatchard to yield the Kd and Bmax values (±SD) shown. (A) OCI/AML-2 cells; (B) blasts from AML patient H.M.; (C) CEM cells; (D) blasts from ALL patient T.Z.
Equilibrium binding of 5-(Sx8)-F in cultured acute leukemia cells and in blasts from two acute leukemia patients. Cells were incubated with graded concentrations of 5-(Sx8)-F (0.5 to 30 nmol/L), and then cell-associated 5-(Sx8)-F was measured by flow cytometry, as the mean fluorescence intensity in each sample determined by flow analysis of 10,000 cells. Nonspecific binding of 5-(Sx8)-F was determined in a parallel set of samples that were pretreated with 5 μmol/L NBMPR to occupy es transporter sites. The data were subjected to mass law analysis by the method of Scatchard to yield the Kd and Bmax values (±SD) shown. (A) OCI/AML-2 cells; (B) blasts from AML patient H.M.; (C) CEM cells; (D) blasts from ALL patient T.Z.
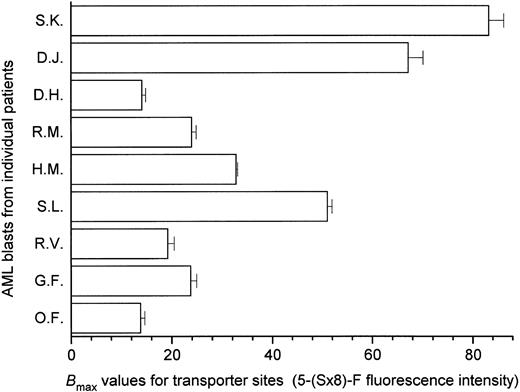
Interpatient variation in es nucleoside transporter expression in AML blasts.When the es site content was measured in leukemic myeloblasts from nine AML patients using 5-(Sx8)-F in the flow cytometry assay, Bmax values ranged from 14 to 83 RFI units/cell (Fig 3), representing a sixfold variation in es transporter expression among the AML patients in that group. These results are consistent with a report of diversity in [3H]NBMPR binding site numbers in AML blasts from patients, which ranged from 500 to 27,600 NBMPR sites per cell.17
Interpatient variation in es nucleoside transporter expression in leukemic blasts from nine AML patients. The es transporter site content of fresh leukemic blasts was measured by flow cytometry in an equilibrium binding assay that used 5-(Sx8)-F, as in Fig 2. In these experiments, the equilibrium binding constants were obtained by fitting hyperbolas to the untransformed specific binding data. The histograms show Bmax values ± SE for es site-specific binding of 5-(Sx8)-F in each blast sample, indicating sixfold variation among the patient samples examined.
Interpatient variation in es nucleoside transporter expression in leukemic blasts from nine AML patients. The es transporter site content of fresh leukemic blasts was measured by flow cytometry in an equilibrium binding assay that used 5-(Sx8)-F, as in Fig 2. In these experiments, the equilibrium binding constants were obtained by fitting hyperbolas to the untransformed specific binding data. The histograms show Bmax values ± SE for es site-specific binding of 5-(Sx8)-F in each blast sample, indicating sixfold variation among the patient samples examined.
Correlation of araC sensitivity with es transporter site content in leukemia cells after pharmacological manipulation of transporter sites with NBMPR.To mimic the variation in es site abundance recognized in leukemic myeloblasts from patients (Fig 3), a set of cell subpopulations was prepared from each of the four cell types used in the experiments of Figs 1 and 2; in each set, es sites were fractionally occupied by exposure of cells to graded concentrations of NBMPR. When the cellular content of unblocked es sites in each subpopulation was measured by flow cytometric analysis of cells stained with 5-(Sx8)-F, an NBMPR concentration-dependent reduction in the mean value of fluorescence histograms was apparent, as shown in Fig 4, which shows changes in fluorescence associated with the progressive blockade of es sites in leukemic myeloblasts from patient H.M. and in CEM cells. At the highest NBMPR concentration tested (1 μmol/L), fluorescence histograms were similar to histograms of cellular autofluorescence, indicating that 1 μmol/L NBMPR blocked virtually all es sites. The histograms of Fig 4 illustrate the use of the flow cytometry assay to measure the pharmacologically-altered es site content of NBMPR-treated cells.
In the flow cytometry data given in Fig 5, cellular 5-(Sx8)-F fluorescence is plotted as a function of NBMPR concentration, and is presented together with plots of coordinate changes in the viability of parallel cell populations that were exposed to araC in the presence of the same graded concentrations of NBMPR. It is seen that 5-(Sx8)-F fluorescence decreased as NBMPR levels were increased, apparently because NT sites were preempted by NBMPR. NBMPR alone, at the concentrations tested, did not affect cell viability (not shown). The araC concentrations that were used in these experiments were expected to reduce cell viability to 10% to 20% of control values. The results of Fig 5 show that the cellular abundance of functional es nucleoside transporters was a determinant of the cytotoxicity of araC in both leukemic myeloblasts and lymphoblasts. The logistic equation that was fitted to the data of Fig 5 yielded IC50 values for the NBMPR blockade of 5-(Sx8)-F sites: 5.3 nmol/L (OCI/AML-2 cells), 7.8 nmol/L (myeloblasts from patient H.M.), 4.3 nmol/L (CEM cells), and 12 nmol/L (lymphoblasts from patient T.Z.). These values were similar to IC50 values (1 to 10 nmol/L) reported for the inhibition by NBMPR of es-mediated nucleoside fluxes in several cell types,32,33 supporting the view that 5-(Sx8)-F binds at a site on the es nucleoside transporter.24
The coordinate decrease in (a) es-specific 5-(Sx8)-F fluorescence and (b) increase in viability of araC-treated cells, in the presence of graded concentrations of NBMPR. Es transporter sites were fractionally occupied by treatment of cells with graded concentrations of NBMPR, before staining with 5-(Sx8)-F and analysis by flow cytometry, as described in the legend to Fig 4. 5-(Sx8)-F concentrations, known to be sufficient to saturate es transporter sites, were 30 nmol/L (OCI/AML-2), 20 nmol/L (patient H.M.), 80 nmol/L (CEM), and 20 nmol/L (patient T.Z.). Es-specific staining with 5-(Sx8)-F is shown (open symbols), as is the viability of cells treated with NBMPR at the same, graded concentrations, measured with the MTT assay after a 96-hour exposure to araC (closed symbols). AraC concentrations were 8 nmol/L (OCI/AML-2 cells, [A]), 300 nmol/L (blasts from AML patient H.M., [B]), 1 nmol/L (CEM cells, [C]), and 100 nmol/L (blasts from ALL patient T.Z., [D]). Those concentrations reduced cell viability to 3.8%, 17%, 2.1%, and 50% of controls in the absence of NBMPR, respectively, in the four cell types. In each panel, flow cytometry data are means ± SD of duplicate determinations, and cytotoxicity values are means ± SEM of 6 to 12 replicates.
The coordinate decrease in (a) es-specific 5-(Sx8)-F fluorescence and (b) increase in viability of araC-treated cells, in the presence of graded concentrations of NBMPR. Es transporter sites were fractionally occupied by treatment of cells with graded concentrations of NBMPR, before staining with 5-(Sx8)-F and analysis by flow cytometry, as described in the legend to Fig 4. 5-(Sx8)-F concentrations, known to be sufficient to saturate es transporter sites, were 30 nmol/L (OCI/AML-2), 20 nmol/L (patient H.M.), 80 nmol/L (CEM), and 20 nmol/L (patient T.Z.). Es-specific staining with 5-(Sx8)-F is shown (open symbols), as is the viability of cells treated with NBMPR at the same, graded concentrations, measured with the MTT assay after a 96-hour exposure to araC (closed symbols). AraC concentrations were 8 nmol/L (OCI/AML-2 cells, [A]), 300 nmol/L (blasts from AML patient H.M., [B]), 1 nmol/L (CEM cells, [C]), and 100 nmol/L (blasts from ALL patient T.Z., [D]). Those concentrations reduced cell viability to 3.8%, 17%, 2.1%, and 50% of controls in the absence of NBMPR, respectively, in the four cell types. In each panel, flow cytometry data are means ± SD of duplicate determinations, and cytotoxicity values are means ± SEM of 6 to 12 replicates.
The chemosensitivity experiments (Fig 5) also showed that 1 μmol/L NBMPR protected CEM cells and lymphoblasts from patient T.Z. against araC cytotoxicity (evaluated by the MTT method); viability of cells exposed to the araC/NBMPR combination was 85% of the viability of drug-free controls in CEM cells and 110% of the controls in lymphoblasts from patient T.Z. In contrast, protection by 1 μmol/L NBMPR in myeloblasts from patient H.M. and in OCI/AML-2 cells under similar circumstances was 34% and 48%, respectively.
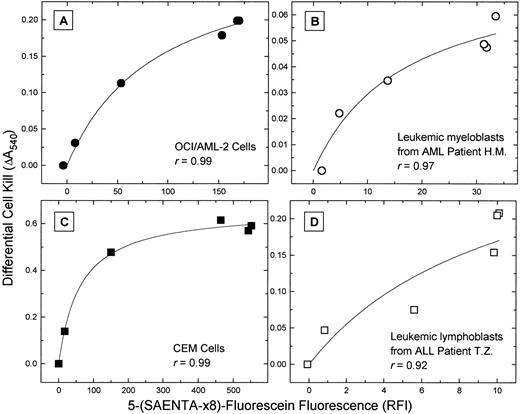
When the data of Fig 5 were plotted as the NBMPR-dependent change in cell kill by araC versus the es-specific 5-(Sx8)-F fluorescence, a measure of the cellular content of unblocked es transporters, a close correlation was evident (Fig 6). In the four cell types, these two parameters appeared to be related by a hyperbolic function, yielding correlation coefficients (r) ranging from .92 to .99.
Correlation of the cytotoxicity of araC and the cell content of es nucleoside transporters. The data of Fig 5 are plotted as the differential cell kill by araC (the difference between cell survival (a) in the presence of the 1 μmol/L NBMPR/araC combination and (b) in the presence of lower, graded NBMPR concentrations with araC) versus the es-specific 5-(Sx8)-F fluorescence for each NBMPR-treated subpopulation of cells. The hyperbolic function fitted to the data shows a close correlation of cytotoxicity and es site content. (A) OCI/AML-2 cells; (B) blasts from AML patient H.M.; (C) CEM cells; (D) blasts from ALL patient T.Z.
Correlation of the cytotoxicity of araC and the cell content of es nucleoside transporters. The data of Fig 5 are plotted as the differential cell kill by araC (the difference between cell survival (a) in the presence of the 1 μmol/L NBMPR/araC combination and (b) in the presence of lower, graded NBMPR concentrations with araC) versus the es-specific 5-(Sx8)-F fluorescence for each NBMPR-treated subpopulation of cells. The hyperbolic function fitted to the data shows a close correlation of cytotoxicity and es site content. (A) OCI/AML-2 cells; (B) blasts from AML patient H.M.; (C) CEM cells; (D) blasts from ALL patient T.Z.
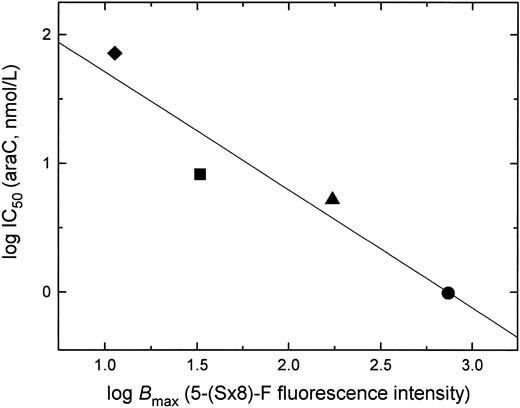
Correlation of araC sensitivity and es transporter expression (Bmax) in acute leukemia cells.Figure 7 shows a logarithmic plot of IC50 values for araC cytotoxicity (Fig 1) and the Bmax for 5-(Sx8)-F binding (Fig 2) in the two cell lines and two samples of leukemic blasts from patients examined in this study. The plot shows a positive correlation between sensitivity to araC and es nucleoside transporter expression in these cell types (r = .95, P = .047), and illustrates the potential of the flow cytometry assay with 5-(Sx8)-F as a predictor of cellular resistance to araC.
Correlation of es nucleoside transporter expression and the sensitivity of cells to araC. Shown is a plot of data obtained with leukemia cells from AML patient H.M. (▪), ALL patient T.Z. (♦), and acute leukemia cell lines OCI/AML-2 (▴) and CEM (•). Chemosensitivity is shown as the log IC50 for araC in a 72-hour MTT assay (Fig 1), and es expression as the log Bmax for 5-(Sx8)-F binding, determined from flow cytometric measurements (Fig 2). P = .047; r = .95.
Correlation of es nucleoside transporter expression and the sensitivity of cells to araC. Shown is a plot of data obtained with leukemia cells from AML patient H.M. (▪), ALL patient T.Z. (♦), and acute leukemia cell lines OCI/AML-2 (▴) and CEM (•). Chemosensitivity is shown as the log IC50 for araC in a 72-hour MTT assay (Fig 1), and es expression as the log Bmax for 5-(Sx8)-F binding, determined from flow cytometric measurements (Fig 2). P = .047; r = .95.
DISCUSSION
Despite the major impact of araC in the treatment of acute leukemia during the past 3 decades, failure to achieve durable remissions remains a significant therapeutic problem. Studies with bone marrow cells from patients with acute leukemia have implicated decreases in deoxycytidine kinase activity,34 increases in cytidine deaminase activity,35 and a reduction in the abundance of nucleoside transport sites19 as determinants of the antileukemic activity of araC. However, consistent correlations of those factors with response to araC have not come forth, and the properties of leukemic blasts that underlie clinical resistance to araC remain to be clearly identified. The present study has further defined the role of nucleoside transporters in araC resistance by using a flow cytometry assay of es nucleoside transporter expression in experiments that coordinately measured araC sensitivity in blasts from acute leukemia patients.
This study has shown that sensitivity of leukemic blasts to araC is a correlate of the cellular abundance of functional es nucleoside transporters. Because of the low accrual at this time of patient samples in which both chemosensitivity and transporter site abundance were measured, the es nucleoside transporter site content was pharmacologically manipulated with NBMPR in cell samples from two patients and in the two cell lines, to simulate the variation in es expression recognized in leukemic blasts from AML patients.
The present study showed that NBMPR, through blockade of es transporter sites, protected cells against the cytotoxicity of araC, supporting the view that membrane transport is a determinant of araC efficacy under these conditions of araC exposure. The diversity of that protection among the several cell types studied may possibly be explained by differences between them in nucleoside transporter activity. In fresh leukemic lymphocytes from chronic lymphocytic leukemia patients, remarkable interpatient differences in the expression of es and cs transporters and of NBMPR-insensitive transporters have been found in this laboratory.10 The high level of protection by NBMPR against araC cytotoxicity in CEM cells seen in the present study, in which 1 μmol/L NBMPR prevented araC toxicity in 85% of the cell population (Fig 5), is consistent with an NT phenotype in which only the es transporter is expressed.11 The similarly high degree of protection afforded by NBMPR in B-precursor lymphoblasts of patient T.Z. (Fig 5) suggests also that only NBMPR-sensitive nucleoside transporter(s) were expressed in those cells. In contrast, partial protection by NBMPR against araC cytotoxicity in the myeloblasts of patient H.M. and in OCI/AML-2 cells (48% and 34%, respectively, Fig 5) suggests that NBMPR-insensitive nucleoside transporters, such as the equilibrative ei, or concentrative cit transporters, may contribute significantly to the inward flux of araC in these cells.11 Nevertheless, the correlation of araC sensitivity and es transporter site abundance across a range of site abundances within these cell populations indicates that the es transporter is a significant factor in the uptake and activation of araC.
Although the view has been taken in this report that 5-(Sx8)-F binding is es-specific, the present results do not exclude the possibility that the concentrative, cs nucleoside transporter, which is sensitive to NBMPR,13 may be expressed in some cell types and might possibly bind 5-(Sx8)-F, contributing to the fluorescence signals associated with 5-(Sx8)-F–stained cells. In such instances, the cs transporter might also contribute to the inward flux of araC. A rigorous determination of the inhibitor binding properties of the cs transporter awaits the identification of a cell line in which the expression of cs is substantial. Thus, it may eventually be possible to correlate also cs transporter–associated 5-(Sx8)-F fluorescence and sensitivity to araC.
The correlation of es expression and chemosensitivity that is apparent in the data of Fig 7 may be confounded in leukemia cell samples in which resistance to araC is attributable to mechanisms other than a deficiency in nucleoside transporter expression. Thus, the flow cytometry assay, in recognizing but one of several mechanisms of araC resistance, may be predictive of resistance to araC, but it is not evident that sensitivity to araC is forecast. Thus, one might expect that mutant cells deficient in deoxycytidine kinase would be resistant to araC in the MTT assay, despite high Bmax values for es transporter expression. The identification of such anomalies in separate assays that can evaluate the expression of alternative determinants of araC resistance may overcome such difficulties. AraC-sensitive cell samples that have a low es site content would not be expected because an es-deficiency would limit araC activation at the level of membrane transport, as the present data suggest.
This study provides strong support for the view that nucleoside transporters are a limiting determinant of araC efficacy, and that a deficiency in transporter expression may be the basis of cellular resistance to araC under some conditions. The flow cytometry assay for nucleoside transporter expression in patient samples here used may be useful in predicting resistance to araC and likely to other cytotoxic nucleoside drugs that is attributable to es transporter deficiencies in acute leukemia cells. The assay is not only rapid and facile, but requires substantially fewer cells than do assays of drug sensitivity, such as the MTT assay. Furthermore, the assay allows, through the analysis of 5-(Sx8)-F–fluorescence histograms, the recognition of heterogeneity in transporter binding site expression within a cell population, a characteristic that will not be evident in radioligand binding assays of transporter site abundance. The results of this study suggest that cell samples exhibiting heterogeneity in es site expression [for example, a broad flow cytometric histogram with 5-(S × 8)-F–stained cells] would probably respond to araC as a population of cells with mixed araC sensitivities, that is, with a gradual araC concentration-effect plot rather than a steep plot.
Rigorous evaluation of the flow cytometry assay as a predictor of clinical response awaits larger studies in which patient accrual is sufficient for statistical analysis of the correlation of cellular transporter site content with chemosensitivity. Such a study, in which the es transporter site content of AML blasts will be correlated with in vitro sensitivity to araC and with clinical response to araC, is currently in progress.36
ACKNOWLEDGMENT
We thank Dawn Kieller and Ying Cao for excellent technical assistance. The assistance of Dr Lanny Xue with flow cytometry assays is gratefully acknowledged.
Supported by the Alberta Cancer Board and the Alberta Heritage Foundation for Medical Research.
Address reprint requests to Wendy P. Gati, PhD, Department of Pharmacology, University of Alberta, Edmonton, Alberta, Canada T6G 2H7.

![Fig. 5. The coordinate decrease in (a) es-specific 5-(Sx8)-F fluorescence and (b) increase in viability of araC-treated cells, in the presence of graded concentrations of NBMPR. Es transporter sites were fractionally occupied by treatment of cells with graded concentrations of NBMPR, before staining with 5-(Sx8)-F and analysis by flow cytometry, as described in the legend to Fig 4. 5-(Sx8)-F concentrations, known to be sufficient to saturate es transporter sites, were 30 nmol/L (OCI/AML-2), 20 nmol/L (patient H.M.), 80 nmol/L (CEM), and 20 nmol/L (patient T.Z.). Es-specific staining with 5-(Sx8)-F is shown (open symbols), as is the viability of cells treated with NBMPR at the same, graded concentrations, measured with the MTT assay after a 96-hour exposure to araC (closed symbols). AraC concentrations were 8 nmol/L (OCI/AML-2 cells, [A]), 300 nmol/L (blasts from AML patient H.M., [B]), 1 nmol/L (CEM cells, [C]), and 100 nmol/L (blasts from ALL patient T.Z., [D]). Those concentrations reduced cell viability to 3.8%, 17%, 2.1%, and 50% of controls in the absence of NBMPR, respectively, in the four cell types. In each panel, flow cytometry data are means ± SD of duplicate determinations, and cytotoxicity values are means ± SEM of 6 to 12 replicates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.346/5/m_bl_0048f5.jpeg?Expires=1769088438&Signature=kgiKb6h6l6YpZq4-Yq0ot91-dXkBoc5fQCU8t3qiiGyKqCb8TtZIXqdUICB~LwPzTNGFCxzhMWss~CdGntu8hfEDP4MHHgkrzDGEXAJhRHhxoCoUVsDqChX24XkZpE8jqA5v7VnkXGk6BS81K6n~0jbxIKdPEhkNLtvkg2tAARdgpECG1Xu93FZt6ZS5gCxyoGnT3NOL0NHM1etAf8LWQ3sUo6IWExj1oKrsw0jviFH0vXgYpddr6eNk-nRNEQj38HuCbWn~~sijaJDq~SXgznmzL0j8AFmsdMMPJ80mdV15XHj84VDNwUWTDohbOqz~Pwku3rEfHCugF5Nz1RJONw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal