Abstract
Destruction of immune cells in peripheral lymphoid tissues plays presumably a pivotal role in acquired immune deficiency syndrome pathogenesis. We found that cell suspensions obtained from lymph nodes of eight human immunodeficiency virus (HIV)-infected individuals contained variable proportions (2.1% to 18.3%, median 11.2%) of dead lymphocytes permeable to supravital dyes, represented by CD4+, CD8+, and B cells. The frequency of dead cells correlated directly (R = 0.847) with the amount of HIV provirus in the cell populations, and HIV provirus was enriched in the dead cell fractions. Similar proportions of dead cells were observed in cell suspensions from lymphadenopathic lymph nodes of HIV− donors, but not from small resting HIV− lymph nodes. Electron microscopic and flow cytometric analyses revealed that most dead cells from HIV+ lymph nodes lacked internucleosomal DNA fragmentation but displayed combined features of apoptosis and necrosis, eg, chromatin condensation and mitochondrial swelling. Cells with similar morphology were readily identified in lymph node tissue sections, and marked mitochondrial swelling could be occasionally observed in cells with otherwise normal morphology. Our findings have two major implications. One is that the in vivo cell death in HIV-infected lymph nodes occurs predominantly through a novel pathway, related to but distinct from classical apoptosis and characterised by early and severe mitochondrial damage. The second implication is that HIV-related lymphadenopathy is accompanied in vivo by massive destruction of uninfected lymph node cells. Comparable levels of cell death were observed in other inflammatory lymphadenopathies not related to HIV; however, the uniquely endless and generalized nature of HIV lymphadenopathy might render this “inflammatory” cell destruction a powerful pathogenetic mechanism, accounting for the progressive disruption and depletion of lymphoid tissues seen in HIV infection.
A NUMBER OF IN VITRO studies have suggested that apoptosis, a form of “programmed cell death” characterized by typical morphological alterations and by internucleosomal DNA fragmentation,1-4 may be a central mechanism of lymphocyte depletion in human immunodeficiency virus (HIV) infection.5 Recent in situ studies6-8 in lymph nodes, the major sites of virus accumulation,9-11 showed that apoptotic lymphocytes, detected by DNA fragmentation, were more abundant in HIV-infected patients than in uninfected controls and, consistently with previous in vitro studies,12-16 were predominantly represented by uninfected cells. Apoptosis was observed both in germinal centers and in the paracortical areas in HIV-infected subjects but only in germinal centers in uninfected controls; in one study,6 but not in another,7 the extent of in situ apoptosis correlated with the local viral burden. However, a semiquantitative morphometric estimate7 showed that apoptotic cells averaged 10 cells per mm2 of tissue section, thus representing a minimal fraction of total lymph node cells. The discrepancy between this figure and those predicted by studies on lymphocyte turnover in HIV infection17,18 could be due to the fact that the phase of detectable DNA fragmentation is relatively short19 and apoptotic cells are rapidly removed by phagocytes.2,3 Alternatively, part of the cells undergoing “programmed death” in vivo in HIV-infected lymph nodes could not be typical apoptotic cells with internucleosomally fragmented DNA.1
To address the above issues we exploited flow cytometry,20-23 electron microscopy and other assays for apoptosis to further characterise cell death in lymph nodes from HIV-infected donors. We observed that cell suspensions from HIV+ lymph nodes contained variable proportions of abnormally small lymphocytes permeable to supravital dyes, which were represented by CD4, CD8, and B cells. The frequency of these dead cells correlated with the local viral burden, and they were enriched of HIV DNA compared to viable cells. These dead cells did not contain fragmented DNA nor appeared hypodiploid on flow cytometry, although many of them displayed apoptotic-like chromatin condensation associated with necrotic-like mitochondrial abnormalities. Cells with identical ultrastructure could be readily identified in tissue sections. Similar proportions of dead cells were observed in cell suspensions from HIV− lymphadenopathic lymph nodes but not from small resting lymph nodes. Taken together, our findings suggest that lymph node cell death during HIV infection occurs in vivo predominantly through a pathway related to but distinct from apoptosis, and that inflammatory lymphadenopathy per se could sustain the massive death of uninfected lymph node cells. The possible role of this “inflammatory” damage to lymphoid tissues in HIV pathogenesis will be discussed.
PATIENTS AND METHODS
Tissue and cell preparations.Cervical or axillary lymph node biopsies were performed for diagnostic purposes in HIV-infected subjects without clinical or laboratory evidence of opportunistic infections. Inguinal or cholecystic lymph nodes from HIV− subjects were obtained during routine surgery for benign noninflammatory gallbladder disease or for varicose vein stripping. All patients gave their informed consent.
Great care was put in handling the tissues, which were constantly kept in an ice bath and processed within 15 minutes after surgical removal. Squeezing with tweezers was strictly avoided. Lymph nodes were transversally dissected to obtain fragments representative of the whole lymph node structure. Tissue fragments were immediately processed for histopathology, transmission electron microscopy and for the preparation of cell suspensions.
In preliminary experiments, we sought to determine the best way to obtain lymph node cell suspensions. We used three different methods: (1) lymph node fragments were extensively, but extremely gently, grinded using a steel screen (Sigma, St Louis, MO); (2) lymph node tissues were punched and perfused with 15 to 20 mL of culture medium through a 23-gauge needle inserted in the tissue and delicately shifted through it; (3) small tissue fragments were vigorously vortexed in 5 mL of culture medium in a 50-mL polypropilene tube. After dissociation, cells were kept in ice and immediately processed for flow cytometry and other assays without further treatments. In some experiments, viable and dead cells from lymph node cell suspensions were enriched by centrifugation onto a Ficoll-Hypaque density gradient as previously described.22 Viable cell fractions were >98% pure, while dead cell fractions contained 10-15% of normal-sized lymphocytes not permeable to 7-AAD. Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation of EDTA-treated venous blood onto Ficoll-Hypaque. The culture conditions for the “spontaneous” in vitro apoptosis of lymphocytes from HIV-infected patients have been described.16 22
Flow cytometry and measurement of apoptosis.The method for the identification and phenotypic characterization of apoptotic lymphocytes in mixed cell populations by multiparameter analysis of light scatters and plasma membrane characteristics has been previously described in detail.22 All monoclonal antibodies used were from Becton Dickinson (Mountain View, CA); propidium iodide (PI) and 7-amino-actinomycin D (7-AAD) were from Sigma. Cells were examined using a FACScan flow cytometer (Becton Dickinson), with light scatter detection parameters appropriate for the identification of apoptotic lymphocytes.22 Cells with hypodiploid (sub-G0/G1) DNA content were detected by flow cytometry with the method of Telford et al.21 Apoptotic ladder-like internucleosomal fragmentation was detected by gel electrophoresis as described.22 The TUNEL assay was performed on paraffin-embedded tissue sections using the APOPTAG kit (Oncor, Gaithesburg, MD) according to manufacturer's instructions.
Measurement of viral burden.Quantitation of HIV genomes in dissociated lymph node cells and in PBMC was done by the polymerase chain reaction (PCR) as described.24 Briefly, DNA was extracted by directly boiling cells for 10′ in double-distilled water. Primers were SK38/SK39 (0.25 μmol/L) for the gag region of HIV-1, and PC04/GH20 (0.08 mmol/L) for human β-globin (Perkin-Elmer Cetus). The thermal cycle was 94°20″, 59°20″, 72°30″ for 40 cycles followed by 72° for 5′. Fivefold dilutions of patients' cells (from 5 to 0.04 × 103) were assayed in parallel with a reference fivefold dilution curve of a mixture of cloned HIV-1 genomes (clone HIVZ6, from Perkin-Elmer Cetus, from 100 to 0.8 copies) with DNA extracted from human uninfected lymphocytes (from 5 to 0.04 × 103). The relative amounts of amplification products were evaluated by densitometric scanning of ethidium bromide-stained gels. The numbers of HIV-1 genomes per 100 patients' cells were calculated by comparing the relative amounts of HIV-specific and β-globin–specific products in the experimental samples with those in the reference curves. For each sample we ran at least three separate experiments, which gave consistent results.
RESULTS
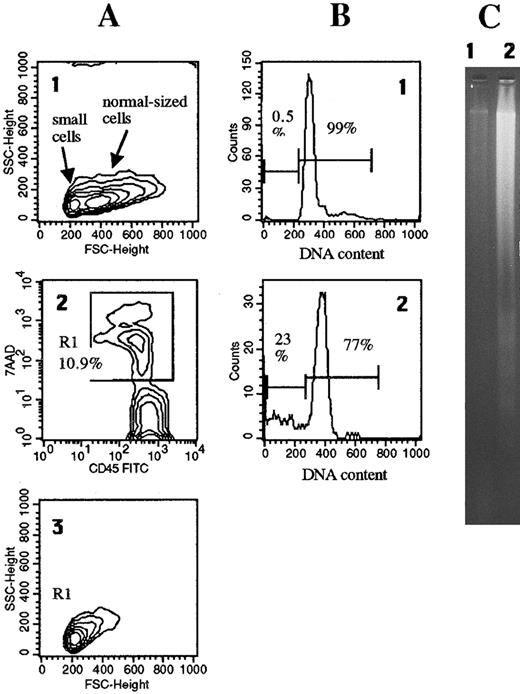
Nonapoptotic cell death in lymph node cell suspensions: Correlation with HIV burden.Cell suspensions obtained from lymph node tissues of HIV-infected individuals contained variable proportions of dead cells which, on flow cytometry, appeared as abnormally small (low forward scatter) lymphocytes, stainable with antibodies to CD45 and to other surface antigens, and highly permeable to PI (not shown) and to 7-AAD (Fig 1A). These characteristics are similar to those of apoptotic lymphocytes, which however differ for having sligthly higher side scatter, reduced expression of surface CD45, and lower plasma membrane permeability to a low-penetrating dye such as 7-AAD.22
Dead cells in freshly isolated lymph node cell suspensions lack apoptotic DNA fragmentation and hypodiploidy. A single-cell suspension was obtained from a lymph node (patient #2) by the grinding technique described in Materials and Methods. (A1) Forward scatter (FSC) analysis revealed two distinct cell populations, one composed of abnormally small lymphocytes with a size comparable to that of apoptotic cells,10 and one of normal-sized lymphocytes. (A2) The cell suspension contained 10.9% dead cells, gated in R1, permeable to 7-AAD and stainable with anti-CD45. (A3) Electronic gating on these cells revealed that they corresponded to the population of abnormally small apoptotic-like cells. Only 0.5% of these freshly isolated lymph node cells had an hypodiploid DNA content (B1) and no fragmented DNA was detected by gel electrophoresis (2 × 106 cells) (C1). By contrast, after 24-hour in vitro culture 23% of lymph node cells were hypodiploid (B2) and fragmented DNA could be revealed (C2).
Dead cells in freshly isolated lymph node cell suspensions lack apoptotic DNA fragmentation and hypodiploidy. A single-cell suspension was obtained from a lymph node (patient #2) by the grinding technique described in Materials and Methods. (A1) Forward scatter (FSC) analysis revealed two distinct cell populations, one composed of abnormally small lymphocytes with a size comparable to that of apoptotic cells,10 and one of normal-sized lymphocytes. (A2) The cell suspension contained 10.9% dead cells, gated in R1, permeable to 7-AAD and stainable with anti-CD45. (A3) Electronic gating on these cells revealed that they corresponded to the population of abnormally small apoptotic-like cells. Only 0.5% of these freshly isolated lymph node cells had an hypodiploid DNA content (B1) and no fragmented DNA was detected by gel electrophoresis (2 × 106 cells) (C1). By contrast, after 24-hour in vitro culture 23% of lymph node cells were hypodiploid (B2) and fragmented DNA could be revealed (C2).
To determine to which extent mechanical damage inflicted during the procedures of tissue dissociation could be responsible for the observed cell death we compared different methods for obtaining lymph node cell suspensions. Roughly similar proportions of dead cells with the above flow cytometric features were recovered when fragments from the same lymph nodes were either grinded or were perfused with culture medium as described in Materials and Methods. By contrast, a technique of dissociation that inflicted a severe mechanical damage, such as the extensive vortexing of small tissue fragments, determined cell swelling and fragmentation which resulted in a wide dispersions of the events throughout the light scattergram (not shown). These observations suggest that the small-sized dead cells observed in suspensions obtained by grinding or by perfusion were, at least in large part, preexisting in tissues and were not generated by manipulation. The data presented throughout the report refer to cell preparations obtained by the grinding technique.
Clinical and virological data and lymph node cell mortality rates in eight HIV-infected subjects are shown in Table 1. The percentages of dead lymphocytes in lymph node cell suspensions ranged between 2.1% and 18.3%. CD3+, CD4+, CD8+, CD19+, and activated (HLA-DR–expressing) T cells were roughly equally represented among viable and dead cells (data not shown). We also examined cell suspensions from lymph nodes of four HIV− subjects. Three macroscopically enlarged inguinal lymph nodes, with morphological aspects similar to those observed in lymph nodes of HIV patients, contained respectively 8.2%, 8.6%, and 11.1% dead lymphocytes with flow cytometric features similar to those observed in HIV-infected lymph nodes. By contrast, two small (less than 2-mm diameter) cholecystic lymph nodes contained 3.2% and 3.9% of these cells, respectively. These data suggest that cell suspensions derived from lymphadenopathic (HIV-related and -unrelated) lymph nodes contain increased proportions of dead cells compared to cell suspensions from small resting lymph nodes.
Clinical, Immunological and Virological Data, and Lymph Node Cell Mortality in Patients With HIV Infection
| Patient No. . | Age (yr)/sex/Time From Diagnosis (mo) . | CDC Stage . | Antiviral Therapy . | CD4/cmm . | HIV DNA Genomes/100 Cells . | Percent Dead Lymph Node Cells* . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | PBMC . | Lymph Node . | . |
| 1 | 24/M/6 | III | None | 610 | ND | ND | 13.8 |
| 2 | 33/M/6 | III | None | 591 | 0.5 | 2.5 | 10.9 |
| 3 | 29/M/5 | III | None | 560 | 0.08 | 0.05 | 2.1 |
| 4 | 25/M/2 | III | Zidovudine | 495 | 0.04 | 0.6 | 7.9 |
| 5 | 30/F/26 | IV | None | 261 | 0.5 | 4.8 | 18.3 |
| 6 | 28/F/36 | IV | Zidovudine | 150 | 0.08 | 1.3 | 11.5 |
| 7 | 32/M/84 | IV | None | 105 | 2.5 | 3.7 | 14.2 |
| 8 | 27/F/15 | III | Zidovudine | 268 | <0.01 | 0.02 | 10.5 |
| Patient No. . | Age (yr)/sex/Time From Diagnosis (mo) . | CDC Stage . | Antiviral Therapy . | CD4/cmm . | HIV DNA Genomes/100 Cells . | Percent Dead Lymph Node Cells* . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | PBMC . | Lymph Node . | . |
| 1 | 24/M/6 | III | None | 610 | ND | ND | 13.8 |
| 2 | 33/M/6 | III | None | 591 | 0.5 | 2.5 | 10.9 |
| 3 | 29/M/5 | III | None | 560 | 0.08 | 0.05 | 2.1 |
| 4 | 25/M/2 | III | Zidovudine | 495 | 0.04 | 0.6 | 7.9 |
| 5 | 30/F/26 | IV | None | 261 | 0.5 | 4.8 | 18.3 |
| 6 | 28/F/36 | IV | Zidovudine | 150 | 0.08 | 1.3 | 11.5 |
| 7 | 32/M/84 | IV | None | 105 | 2.5 | 3.7 | 14.2 |
| 8 | 27/F/15 | III | Zidovudine | 268 | <0.01 | 0.02 | 10.5 |
CD45+ cells permeable to 7-AAD in lymph node cell suspensions.
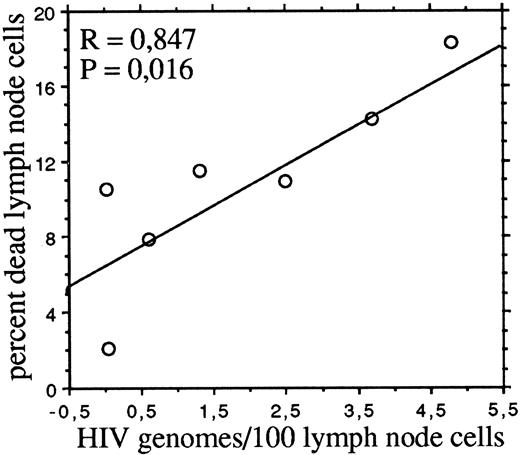
Consistently with previous reports9-11 we found that, with the exception of one patient, lymph node cells contained 1.5 to 16 times more HIV provirus than autologous PBMC (Table 1). The proportions of dead cells (ie, permeable to 7-AAD) in freshly isolated lymph node cell suspensions significantly correlated (P = .016) by linear regression analysis with the number of HIV genomes per 100 cells (Fig 2). No correlations were instead observed between dead cells and the absolute numbers of circulating CD4 or CD8 cells, the percentage of activated (HLA-DR–expressing) T cells in lymph node cell suspensions, or the HIV burden in PBMC.
The frequency of dead cells correlates with the HIV DNA burden of lymph node cells. The percentages of dead cells and the numbers of HIV genomes per 100 cells are as in Table 1.
The frequency of dead cells correlates with the HIV DNA burden of lymph node cells. The percentages of dead cells and the numbers of HIV genomes per 100 cells are as in Table 1.
Viable and dead cells were enriched by density gradient centrifugation from lymph node cell suspensions, and HIV DNA was measured in each population. The amounts of HIV provirus in the dead cell fractions from two patients were, respectively, ∼5 and >30 times higher than in their viable counterparts (Fig 3). This finding suggests that the death rate of HIV-infected lymph node cells is accelerated in vivo. However, the data also indicate that the majority of dead cells in HIV+ lymph nodes were indeed uninfected. In fact, in the two cases (no. 5 and 7) where viral genomes were directly measured in dead cells, HIV-infected cells accounted for ∼20% and ∼25% of dead cells, respectively. Furthermore, also in the other cases it can be indirectly argued that infected cells represented only a minority of dead lymph node cells. In fact, assuming that in these samples all viral genomes were sequestered in the dead cell fractions, one can calculate that infected cells represented from 0.2% (case 8) to 23% (case 2) (median 7.6%) of dead cells.
Viral DNA is enriched in dead cells from HIV-infected lymph nodes. Viable (V) and dead (D) cells were purified by density gradient centrifugation as described in Materials and Methods. HIV and β-globin DNA sequences were co-amplified by PCR in 1,000 cells from each population. Upper panels show ethidium bromide-stained amplification products separated in agarose gel. Comparison of the relative intensities of β-globin–specific and HIV-specific bands by densitometric scanning (lower panels) revealed that HIV genomes in the dead cell populations from patients 5 and 7 were, respectively, ∼5 and <30 times more abundant than in the corresponding viable populations.
Viral DNA is enriched in dead cells from HIV-infected lymph nodes. Viable (V) and dead (D) cells were purified by density gradient centrifugation as described in Materials and Methods. HIV and β-globin DNA sequences were co-amplified by PCR in 1,000 cells from each population. Upper panels show ethidium bromide-stained amplification products separated in agarose gel. Comparison of the relative intensities of β-globin–specific and HIV-specific bands by densitometric scanning (lower panels) revealed that HIV genomes in the dead cell populations from patients 5 and 7 were, respectively, ∼5 and <30 times more abundant than in the corresponding viable populations.
A mean value of only about 10 apoptotic cells per mm2 of tissue section has been observed in HIV+ lymph nodes using the in situ TUNEL assay.7 In the HIV+ lymph node specimens studied by us TUNEL+ cells were also quite rare, averaging 0.05% to 0.1% of total cells. TUNEL+ cells were mainly localized in the germinal centers inside the tingible body macrophages, and scattered TUNEL+ cells with lymphoid morphology were observed in the T-dependent paracortical areas (not shown).
To confirm the paucity of cells with DNA changes typical of apoptosis we evaluated the proportions of cells with an hypodiploid DNA content in lymph node cell suspensions. We found that hypodiploid lymphocytes accounted for a minimal proportion of cells, much lower than that represented in the same cell suspensions by 7-AAD–permeable dead cells. In a representative patient (Fig 1) less than 0.5% of total lymph node cells had an hypodiploid DNA content, compared to 10.8% dead cells. Gel electrophoretic analysis of DNA extracted from freshly isolated lymph node cells confirmed the absence of fragmented DNA.
When lymph node cells from HIV patients were cultured in vitro for 48 hours most dead cells displayed the typical features of apoptosis on flow cytometry, ie, hypodiploidy and decreased expression of surface CD45,22 and ladder-like fragmented DNA was generated (see Fig 1 for a representative case). Although it cannot be excluded that a small proportion of the dead cells found after in vitro culture had the atypical features observed in fresh cells (ie, lack of DNA fragmentation, high permeability to dyes and the ultrastructural features described below), the percentages of dead cells, as evaluated by flow cytometry,22 were very close to the percentages of cells with hypodiploid nuclei, indicating that the large majority of dead cells in cultures were classically apoptotic.
The levels of culture-induced apoptosis of lymph node cells and of PBMC were compared in three HIV-infected patients and in one HIV− subject with lymphadenopathy. In two of the HIV-infected patients apoptosis was higher with lymph node cells than with PBMC (patient no. 1, lymph node 45% and PBMC 37%; patient no. 2, lymph node 23% and PBMC 14%), while in the third case apoptosis was slightly higher with PBMC (patient no. 5, lymph node 54% and PBMC 61%). In the HIV− subject the levels of culture-induced apoptosis were 18% with lymph node cells and 16% with PBMC (baseline mortality among freshly isolated lymph node cells was 11.1%). In another HIV− subject with lymphadenopathy culture-induced apoptosis of lymph node cells was 6% (baseline mortality 8.2%).
Ultrastructure of dead cells in HIV-infected lymph nodes.A proportion of lymphocytes present in freshly isolated cell suspensions from HIV+ lymph nodes had peculiar ultrastructural changes, resembling those observed in discrete stages of apoptosis.2 These cells were characterized by reduced size, different degrees of chromatin condensation ranging from clumping to apoptotic-like condensation, and reduction of cytoplasm up to the apparent fusion of nuclear and plasma membranes. However, unlike classical apoptotic cells1-4 they presented marked mitochondrial swelling (Fig 4A). Cells with similar ultrastructure, either free or engulfed by macrophages, could be readily identified in different areas of lymph node tissues (Fig 4B and C). Thus, morphological data indicate that a large part of lymphocytes dying in HIV-infected lymphoid tissues undergo a process reminiscent of apoptosis, but differing from it for being accompanied by profound mitochondrial damage. Mitochondrial swelling did not appear to be related to treatment with zidovudine, which is known to affect mitochondrial function,25 since it was observed both in treated and in untreated subjects. Severe mitochondrial abnormalities could be detected in cells with normal nuclear morphology (Fig 4D). This latter finding indicates that mitochondrial damage occurred early in dying cells, and rules out that it could be due to “secondary necrosis” taking place in late apoptotic cells.4
Apoptotic-like nuclear morphology and mitochondrial damage in lymphocytes of an HIV-infected lymph node. (A) One normal lymphocyte and one lymphocyte with initial chromatin condensation and mitochondrial swelling (arrow) in a cell suspension obtained from the lymph node (original magnification [OM] × 9,000). (B) A tissue section from the same lymph node showing one cell with similar nuclear and mitochondrial abnormalities and partial fusion of nuclear and plasma membranes (arrow) (OM × 8,000). (C) A tissue section showing two cells in which the chromatin has collapsed down along the nuclear envelope. One of the cells (arrow) is engulfed by a macrophage and presents swollen and tightly packed mitochondria; the other cell (arrowhead) is free, and presents condensation of nucleolus and extreme reduction of cytoplasm with loss of intercellular contacts (OM × 4,900). (D) A tissue section showing several cells with initial chromatin condensation (arrows) and one cell with normal nuclear morphology (arrowhead), all displaying mitochondrial swelling (OM × 3,300). Note in all micrographs the intact mitochondria of neighboring cells.
Apoptotic-like nuclear morphology and mitochondrial damage in lymphocytes of an HIV-infected lymph node. (A) One normal lymphocyte and one lymphocyte with initial chromatin condensation and mitochondrial swelling (arrow) in a cell suspension obtained from the lymph node (original magnification [OM] × 9,000). (B) A tissue section from the same lymph node showing one cell with similar nuclear and mitochondrial abnormalities and partial fusion of nuclear and plasma membranes (arrow) (OM × 8,000). (C) A tissue section showing two cells in which the chromatin has collapsed down along the nuclear envelope. One of the cells (arrow) is engulfed by a macrophage and presents swollen and tightly packed mitochondria; the other cell (arrowhead) is free, and presents condensation of nucleolus and extreme reduction of cytoplasm with loss of intercellular contacts (OM × 4,900). (D) A tissue section showing several cells with initial chromatin condensation (arrows) and one cell with normal nuclear morphology (arrowhead), all displaying mitochondrial swelling (OM × 3,300). Note in all micrographs the intact mitochondria of neighboring cells.
DISCUSSION
We report here that, in addition to a relatively scarce population of classic apoptotic lymphocytes with hypodiploid DNA content, freshly isolated cell suspensions from HIV-infected lymph nodes contained abundant dead cells permeable to supravital dyes. These cells were not hypodiploid nor displayed apoptotic internucleosomal DNA cleavage, although many of them had nuclear features typical of apoptosis associated with mithocondrial swelling indicative of necrosis. The fact that these cells preexisted in vivo and were not simply the consequence of tissue manipulation is indicated by several considerations. First, apoptotic-like chromatin condensation could hardly be attributed to mechanical damage, especially taking into account that cells were examined immediately after extraction. Furthermore, dead cells were much less numerous in small resting HIV− lymph nodes than in inflammatory lymph nodes. In this regard, it could be argued that cells in inflammatory lymph nodes may be more fragile than those in normal lymph nodes and, therefore, that manipulations could have induced or enhanced some of the observed features (eg, permeability to dyes); however, cell fragility per se can reasonably be viewed as an indicator of severe metabolic damage. Finally, a specific link with HIV infection was indicated by the observations that the frequency of these dead cells correlated with the local viral burden and that HIV provirus was enriched in the dead cell fraction.
Our findings have two major implications. One is that the predominant form of in vivo cell death in HIV-infected lymph nodes is related to but distinct from classical apoptosis. The second is that HIV lymphadenopathy is associated with massive bystander cell destruction, whose possible pathogenetic implications will be discussed.
Electron microscopy revealed in many of the dead cells extracted from HIV lymph nodes mitochondrial swelling associated with typical apoptotic-like chromatin condensation, while flow cytometry and gel electrophoresis failed to reveal apoptotic DNA fragmentation. It is highly unlikely that these cells were at an early apoptotic stage preceding DNA digestion, since they were highly permeable to PI and to 7-AAD, a characteristic of late apoptosis,23,26 and presented morphological alterations of mitochondria which do not occur in classical apoptosis until very late stages.1-4 Rather, our findings suggest that the above described features reflect a novel cell death pathway related to but distinct from apoptosis and resembling an admixture of apoptosis and necrosis.
Cell death accompanied by apoptotic chromatin condensation but without DNA fragmentation is not unprecedented. In vitro, this pattern of cell death could be induced by treatment with the K+ ionophor valinomycin (which also determined mitochondrial swelling),27 the protein phosphatase inhibitor okadaic acid,28 transforming growth factor β1,29 or by ligation of CD45.30 Complement-mediated “necrotic” cell death may also present some features of apoptosis.31 The in vitro model most closely resembling the pattern described here is the death of MOLT-4 cells induced by low-dose irradiation, which is characterized by apoptotic-like chromatin condensation without DNA cleavage, necrotic-like mitochondrial swelling and high membrane permeability to dyes.32 In vivo, Tidball et al33 have observed both apoptosis and necrosis in degenerating dystrophic muscle cells.
Electron microscopic examination of tissue sections showed that severely damaged mitochondria (markedly swollen and with completely extralucent matrix) were typically seen in cells with peripheral chromatin condensation. However, mitochondrial abnormalities were occasionally observed in situ in lymphocytes with otherwise normal nuclear structure. This latter finding indicates that morphological alterations of mitochondria are an early event in the cell death pathway described here. Mitochondrial damage may promote apoptotic-like nuclear changes through a mechanism involving loss of the permeability barrier of mitochondria.34,35 Thus, the mitochondrial swelling associated with a partial apoptotic phenotype (nuclear morphological changes without DNA fragmentation) observed in lymph node cells could be interpreted as the result of a mitochondrial insult, on apoptotically committed cells, of such severity to determine metabolic breakdown and cell death before the completion of the apoptotic program. It is worth noting in this regard that tumor necrosis factor (TNF ), whose toxicity is mediated by early damage of mitochondrial function,36 and reactive oxygen species can induce either apoptosis or necrosis depending on the intensity of the damage.37-40 Mitochondrial dysfunctions have been observed in circulating lymphocytes from HIV carriers,41 and HIV RNA has been shown to selectively accumulate in the mitochondria of infected cells, possibly reducing mitochondrial viability.42 Furthermore, soluble HIV tat protein suppresses the expression of mitochondrial Mn-dependent superoxide dismutase by uninfected cells rendering them hypersensitive to oxidative stress and to TNF-mediated cytotoxicity.43 Tat released locally might therefore enhance the susceptibility of bystander cells to the cytopathic effects of TNF and oxidants overproduced within HIV-infected lymph nodes,44-47 with mitochondria as primary targets for such cytotoxicity.
The second issue raised by our findings concerns the pathogenetic significance of the pattern of diffuse cell death observed in HIV-infected lymph nodes. The mechanisms of immunodeficiency caused by HIV infection are still incompletely understood,48 and theories involving solely CD4 cell depletion are insufficient to explain the array of immunological abnormalities and the histologic changes observed in lymphoid tissues.49-51 Alternative pathogenetic models have therefore been proposed.52,53 Taken together, our findings suggest the coexistence of three distinct cell death mechanisms in HIV infection: viral cytopathicity, the priming to apoptosis, and the destruction of uninfected cells by a novel necrotic/apoptotic pathway. In vivo killing of infected cells by viral cytopathicity was highlighted by the accumulation of HIV provirus in dead lymph node cells, in agreement with studies17 18 on virus and lymphocyte turnover indicating that productive infection per se is directly involved in CD4 cell destruction.
Priming of uninfected cells to apoptosis, which is executed when cells are cultured in vitro, has been widely described with peripheral blood lymphocytes from HIV-infected donors,5,12-16 and the present report extends to lymph node cells the occurrence of this phenomenon. The triggering mechanisms and the possible relevance to HIV pathogenesis of the apoptotic commitment of lymphocytes, which is also observed in other viral infections,54 55 remain to be elucidated.
Taken overall, our data suggest the following chain of events resulting in the atypical apoptotic/necrotic features of dead cells observed in vivo in lymphoid tissues of HIV-infected patients. A proportion of lymphocytes present in lymph nodes are primed to apoptosis, and they execute the entire apoptotic program when maintained under ex vivo conditions. By contrast, within the lymph node environment these cells initiate apoptosis (ie, they shrink and condense their chromatin) but cannot progress to the later stage of DNA digestion because local factor(s) determine a drastic mitochondrial damage which rapidly kills cells by “necrosis.”4
Our observations indicate that this pattern of diffuse death of lymph node cells is not unique to HIV infection but is also observed in other inflammatory lymphadenopathies. Thus, this “lymphadenopathic” cell death could be brought about by proinflammatory cytokines and reactive oxigen species generated within the milieu of lymphadenopathic lymph nodes. In the case of HIV, cytopathicity might be enhanced through cell sensitization to TNF and oxidants by tat.43 However, in pathogenetic terms it is much more important to take into account that HIV lymphadenopathy has the unique feature of being of unlimited duration and generalized to most or all lymph nodes.50 In plain words, it is conceivable that any endless and generalized inflammatory lymphadenopathy would eventually lead to an extensive damage of lymphoid tissues. A similar parallel has been proposed to explain by a common mechanism mediated by proinflammatory cytokines the wasting and cachexia seen in AIDS and in other inflammatory conditions.56 A pathogenetic model of chronic inflammatory damage may help to explain the histologic changes of HIV-infected lymph nodes, whose structure becomes progressively subverted and depleted of lymphocytes and of other cell types (eg, follicular dendritic cells) until the almost complete loss of cellularity and replacement by fibrotic tissue.49 51 Accordingly, measures aimed at suppressing inflammation could perhaps be helpful in reducing immunological damage in HIV infection.
Supported by grants from the Italian Ministry of Health, VIII Progetto AIDS, and from the Istituto Pasteur-Fondazione Cenci Bolognetti, Rome (to M.F ). M.Ci. was supported by a postdoctoral fellowship from the Italian Ministry of Health.
Address reprint requests to Massimo Fiorilli, MD, Department of Clinical Medicine, University of Rome ‘La Sapienza,’ Viale dell'Università 37, 00185 Rome, Italy.


![Fig. 4. Apoptotic-like nuclear morphology and mitochondrial damage in lymphocytes of an HIV-infected lymph node. (A) One normal lymphocyte and one lymphocyte with initial chromatin condensation and mitochondrial swelling (arrow) in a cell suspension obtained from the lymph node (original magnification [OM] × 9,000). (B) A tissue section from the same lymph node showing one cell with similar nuclear and mitochondrial abnormalities and partial fusion of nuclear and plasma membranes (arrow) (OM × 8,000). (C) A tissue section showing two cells in which the chromatin has collapsed down along the nuclear envelope. One of the cells (arrow) is engulfed by a macrophage and presents swollen and tightly packed mitochondria; the other cell (arrowhead) is free, and presents condensation of nucleolus and extreme reduction of cytoplasm with loss of intercellular contacts (OM × 4,900). (D) A tissue section showing several cells with initial chromatin condensation (arrows) and one cell with normal nuclear morphology (arrowhead), all displaying mitochondrial swelling (OM × 3,300). Note in all micrographs the intact mitochondria of neighboring cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.209/5/m_bl_0014f4.jpeg?Expires=1763569141&Signature=Df5ALpyLlrprdbbizjjxhQQDQmw8F4S~4qX1ltGed3kFpiaUooAWGXOBPXh0YtmdpoirMauN4ALBZzKktZMHEcbIFY8lUvdEJuc3vu9ro9JnODf9qL2-BvaROjvWU0wpOyQjkQNXT8KYow4i5VKdu8mAac-lhgXv8KG4sfo8EjxvDtEeIH-tvKBCXEhpOCoYIb~0U7Z0QRy4sMCUu6dFL0YUDg9cAoCgMs5mTB0UEA8fkwS-qoC4l6OTtNGjBrIVvcrsXbHsqHMqZ1ruSQiuMcGxRcctcWGqjSdDfh4CgfcCCunWFh8YldaxoSV2XPb6a--Jv5cUsz97t~Q9YMQSVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal